Carbon Nanomaterial-Based Electrochemical Biosensors for Alzheimer’s Disease Biomarkers: Progress, Challenges, and Future Perspectives
Abstract
1. Introduction
2. Carbon Nanomaterials
2.1. Graphene-Based Nanomaterials
2.2. Carbon Nanotubes
2.3. Graphitic Carbon Nitride
2.4. Carbon Black
2.5. Fullerenes
2.6. Carbon Dots
3. Carbon-Based Electrochemical Sensors for Alzheimer’s Disease Biomarker Detection
3.1. Graphene-Based Electrochemical Sensors
3.2. CNTs-Based Electrochemical Sensors
3.3. Hybrid Carbon-Based Electrochemical Sensors Involving Graphene
3.4. Carbon Nitride (g-C3N4)-Based Electrochemical Sensors
3.5. Other Carbon-Based Electrochemical Sensors
4. Comparative Analysis of Recognition Elements and Targeted Biomarkers
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025; World Health Organization: Geneva, Switzerland, 2017.
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 28 September 2025).
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013, 9, 63–75.e62. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.S.; Cardoso, A.R.; Sales, M.G.F. Electrochemical biosensor-based detection assays for early diagnosis of neurodegenerative disorders. In Smart Diagnostics for Neurodegenerative Disorders; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2024; pp. 155–177. [Google Scholar]
- Kim, K.; Kim, M.J.; Kim, D.W.; Kim, S.Y.; Park, S.; Park, C.B. Clinically accurate diagnosis of Alzheimer’s disease via multiplexed sensing of core biomarkers in human plasma. Nat. Commun. 2020, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Mulumba, J.; Duan, R.; Luo, B.; Wu, J.; Sulaiman, M.; Wang, F.; Yang, Y. The role of neuroimaging in Alzheimer’s disease: Implications for the diagnosis, monitoring disease progression, and treatment. Explor. Neurosci. 2025, 4, 100675. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, Q.; Yang, M.; Lin, G.; Yao, K.; Wu, Z.; Xu, D.; Zhou, H.; Chen, B.; Shi, H.; et al. Plasma p-tau217 and p-tau217/Abeta1-42 are effective biomarkers for identifying CSF- and PET imaging-diagnosed Alzheimer’s disease: Insights for research and clinical practice. Alzheimers Dement. 2025, 21, e14536. [Google Scholar] [CrossRef]
- Dague, C. Fujirebio Receives Marketing Clearance for Lumipulse® G pTau 217/β-Amyloid 1-42 Plasma Ratio In-Vitro Diagnostic Test As An Aid To Identify Patients with Amyloid Pathology Associated with Alzheimer’s Disease. Available online: https://www.fujirebio.com/en-us/news-events/fujirebio-receives-marketing-clearance-for-lumipulser-g-ptau-217bamyloid-142-plasma-0 (accessed on 28 September 2025).
- Evtugyn, G.; Porfireva, A.; Shamagsumova, R.; Hianik, T. Advances in Electrochemical Aptasensors Based on Carbon Nanomaterials. Chemosensors 2020, 8, 96. [Google Scholar] [CrossRef]
- Wu, H.; Duan, Y.; Jiang, L.; Cao, X.; Xie, Z.; Quan, Y.; Ren, M.X.; Wu, S.; Zhang, N.; Yang, Z.; et al. Label-Free Analysis of Protein Biomarkers Using Pattern-Optimized Graphene-Nanopyramid SERS for the Rapid Diagnosis of Alzheimer’s Disease. ACS Appl. Nano Mater. 2024, 7, 9167–9175. [Google Scholar] [CrossRef]
- Park, J.S.; Ahmad, W.; Choe, K.; Ahmad, R.; Park, T.J.; Kim, M.O. Biosensors and biomarkers: A dynamic duo towards Alzheimer’s disease detection. Sens. Bio-Sens. Res. 2024, 46, 100704. [Google Scholar] [CrossRef]
- Mikula, E. Recent Advancements in Electrochemical Biosensors for Alzheimer’s Disease Biomarkers Detection. Curr. Med. Chem. 2021, 28, 4049–4073. [Google Scholar] [CrossRef]
- Valkova, P.; Pohanka, M. Novel Trends in Electrochemical Biosensors for Early Diagnosis of Alzheimer’s Disease. Int. J. Anal. Chem. 2021, 2021, 9984876. [Google Scholar] [CrossRef]
- Bousiakou, L.; Al-Dosary, O.; Economou, A.; Subjakova, V.; Hianik, T. Current Trends in the Use of Semiconducting Materials for Electrochemical Aptasensing. Chemosensors 2023, 11, 438. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Carbon-Nanotube Based Electrochemical Biosensors: A Review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Gui, R.; Jin, H.; Xia, Y. Carbon nanomaterials-based electrochemical aptasensors. Biosens. Bioelectron. 2016, 79, 136–149. [Google Scholar] [CrossRef]
- Douaki, A.; Demelash Abera, B.; Cantarella, G.; Shkodra, B.; Mushtaq, A.; Ibba, P.; Inam, A.S.; Petti, L.; Lugli, P. Flexible Screen Printed Aptasensor for Rapid Detection of Furaneol: A Comparison of CNTs and AgNPs Effect on Aptasensor Performance. Nanomaterials 2020, 10, 1167. [Google Scholar] [CrossRef]
- Fenta, E.W.; Mebratie, B.A. Advancements in carbon nanotube-polymer composites: Enhancing properties and applications through advanced manufacturing techniques. Heliyon 2024, 10, e36490. [Google Scholar] [CrossRef]
- Pimpilova, M. A brief review on methods and materials for electrode modification: Electroanalytical applications towards biologically relevant compounds. Discov. Electrochem. 2024, 1, 12. [Google Scholar] [CrossRef]
- Eres, G.; Geohegan, D.B.; Puretzky, A.A.; Rouleau, C.M. All Carbon Nanotubes Are Not Created Equal. In Nanotechnology for Electronics, Photonics, and Renewable Energy; Korkin, A., Krstić, P.S., Wells, J.C., Eds.; Springer: New York, NY, USA, 2010; pp. 131–152. [Google Scholar]
- Dugeč, J.; Škugor Rončević, I.; Vladislavić, N.; Radić, J.; Buljac, M.; Buzuk, M. The Interpretation of Carbon Nanotubes’ Electrochemistry: Electrocatalysis and Mass Transport Regime in the Apparent Promotion of Electron Transfer. Biosensors 2025, 15, 89. [Google Scholar] [CrossRef]
- Kaliyaraj Selva Kumar, A.; Lu, Y.; Compton, R.G. Voltammetry of Carbon Nanotubes and the Limitations of Particle-Modified Electrodes: Are Carbon Nanotubes Electrocatalytic? J. Phys. Chem. Lett. 2022, 13, 8699–8710. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation of 18 October 2011 on the Definition of a Nanomaterial (2011/696/EU); European Commission: Brussels, Belgium, 2011; p. 2.
- ISO 80004-1:2023; Nanotechnologies—Vocabulary—Part 1: Core Vocabulary. International Organization for Standardization: Geneva, Switzerland, 2023; pp. 1–11.
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Bhushan, B. Springer Handbook of Nanotechnology, 4th ed.; Springer: Berlin, Heidelberg, 2017; pp. 2–5. [Google Scholar]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Barhoum, A.; Shalan, A.E.; El-Hout, S.I.; Ali, G.A.M.; Abdelbasir, S.M.; Abu Serea, E.S.; Ibrahim, A.H.; Pal, K. A Broad Family of Carbon Nanomaterials: Classification, Properties, Synthesis, and Emerging Applications. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–40. [Google Scholar]
- Abraham, J.; Thomas, S.; Kalarikkal, N. Handbook of Carbon Nanotubes; Abraham, J., Thomas, S., Kalarikkal, N., Eds.; Springer Nature: Cham, Switzerland, 2022. [Google Scholar]
- Raman, A.; Appukuttan, S.; George, G.; Wilson, R.; Joseph, K. General introduction to zero-dimensional carbon nanomaterials and their properties and applications. In Zero-Dimensional Carbon Nanomaterials; Woodhead Publishing (Elsevier): Cambridge, MA, USA, 2024; pp. 1–16. [Google Scholar]
- Wang, Z.; Tian, M.; Yu, J.; Jiao, J.; Yang, C.; Pei, L.; Yan, C.; Fang, C. Recent advances of 3D compressible carbon assemblies: A review of synthesis, properties and applications in energy and environment. J. Environ. Chem. Eng. 2021, 9, 106269. [Google Scholar] [CrossRef]
- Lalwani, G.; Patel, S.C.; Sitharaman, B. Two- and Three-Dimensional All-Carbon Nanomaterial Assemblies for Tissue Engineering and Regenerative Medicine. Ann. Biomed. Eng. 2016, 44, 2020–2035. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, L.; Cheon, D.; Yoo, S.H. Carbon-Based Nanomaterials for Catalytic Wastewater Treatment: A Review. Molecules 2023, 28, 1805. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Ahmadi, N.; Ramazani, A. Graphene quantum dots: Syntheses, properties, and applications. In Zero-Dimensional Carbon Nanomaterials; Woodhead Publishing (Elsevier): Cambridge, MA, USA, 2024; pp. 83–106. [Google Scholar]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on gas impermeability of graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Sajith, V.; Sarathchandran, C. Graphene: The magic material. In Handbook of Carbon-Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 517–549. [Google Scholar]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Rohrl, J.; et al. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nat. Mater. 2009, 8, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Zhai, Y.; Zhai, J.; Ren, W.; Wang, F.; Dong, S. Controlled synthesis of large-area and patterned electrochemically reduced graphene oxide films. Chemistry 2009, 15, 6116–6120. [Google Scholar] [CrossRef]
- Singh, S.; Hasan, M.R.; Sharma, P.; Narang, J. Graphene nanomaterials: The wondering material from synthesis to applications. Sens. Int. 2022, 3, 100190. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Bellier, N.; Baipaywad, P.; Ryu, N.; Lee, J.Y.; Park, H. Recent biomedical advancements in graphene oxide- and reduced graphene oxide-based nanocomposite nanocarriers. Biomater. Res. 2022, 26, 65. [Google Scholar] [CrossRef] [PubMed]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Shariatinia, Z. Applications of carbon nanotubes. In Handbook of Carbon-Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 321–364. [Google Scholar]
- Wu, K.; Wang, B.; Niu, Y.; Wang, W.; Wu, C.; Zhou, T.; Chen, L.; Zhan, X.; Wan, Z.; Wang, S.; et al. Carbon nanotube fibers with excellent mechanical and electrical properties by structural realigning and densification. Nano Res. 2023, 16, 12762–12771. [Google Scholar] [CrossRef]
- Bandaru, P.R. Electronic Transport and Electrical Properties of Carbon Nanotubes. In Handbook of Carbon Nanotubes; Abraham, J., Thomas, S., Kalarikkal, N., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–39. [Google Scholar]
- Sánchez-Romate, X.F.; Jiménez-Suárez, A.; Ureña, A. Electrical Properties of Carbon Nanotubes. In Handbook of Carbon Nanotubes; Abraham, J., Thomas, S., Kalarikkal, N., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–35. [Google Scholar]
- Avouris, P.; Chen, Z.; Perebeinos, V. Carbon-based electronics. Nat. Nanotechnol. 2007, 2, 605–615. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes--the route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Maruyama, T. Carbon nanotubes. In Handbook of Carbon-Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 299–319. [Google Scholar]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.H.; Kim, S.G.; Rinzler, A.G.; et al. Crystalline Ropes of Metallic Carbon Nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef]
- Hafner, J.H.; Bronikowski, M.J.; Azamian, B.R.; Nikolaev, P.; Rinzler, A.G.; Colbert, D.T.; Smith, K.A.; Smalley, R.E. Catalytic growth of single-wall carbon nanotubes from metal particles. Chem. Phys. Lett. 1998, 296, 195–202. [Google Scholar] [CrossRef]
- Zhao, T.; Ji, X.; Jin, W.; Yang, W.; Zhao, X.; Dang, A.; Li, H.; Li, T. In situ synthesis of semiconducting single-walled carbon nanotubes by modified arc discharging method. Appl. Phys. A 2017, 123, 132. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled nanotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [Google Scholar] [CrossRef]
- Choudhary, N.; Hwang, S.; Choi, W. Carbon Nanomaterials: A Review. In Handbook of Nanomaterials Properties; Springer: Berlin, Heidelberg, 2014; pp. 709–769. [Google Scholar]
- Kramberger, C. One dimensionality and spectroscopy in carbon nanotubes. Mater. Sci.-Pol. 2013, 31, 338–342. [Google Scholar] [CrossRef]
- Javey, A. Carbon Nanotube Field-Effect Transistors. In Carbon Nanotube Electronics; Javey, A., Kong, J., Eds.; Integrated Circuits and Systems; Springer: New York, NY, USA, 2009; pp. 63–86. [Google Scholar]
- Kim, S.N.; Rusling, J.F.; Papadimitrakopoulos, F. Carbon Nanotubes for Electronic and Electrochemical Detection of Biomolecules. Adv. Mater. 2007, 19, 3214–3228. [Google Scholar] [CrossRef] [PubMed]
- Thostenson, E.T.; Ren, Z.; Chou, T.-W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Odom, T.W.; Huang, J.-L.; Kim, P.; Lieber, C.M. Structure and Electronic Properties of Carbon Nanotubes. J. Phys. Chem. B 2000, 104, 2794–2809. [Google Scholar] [CrossRef]
- Zhang, F.; Hou, P.-X.; Liu, C.; Cheng, H.-M. Epitaxial growth of single-wall carbon nanotubes. Carbon 2016, 102, 181–197. [Google Scholar] [CrossRef]
- Monthioux, M.; Flahaut, E.; Laurent, C.; Escoffier, W.; Raquet, B.; Bacsa, W.; Puech, P.; Machado, B.; Serp, P. Properties of Carbon Nanotubes. In Handbook of Nanomaterials Properties; Springer: Berlin, Heidelberg, 2014; pp. 1–49. [Google Scholar]
- Berzelius, J.J. Untersuchung über die Eigenschaften des Tellurs. Ann. Phys. 1834, 108, 577–627. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, M.L. Prediction of new low compressibility solids. Science 1989, 245, 841–842. [Google Scholar] [CrossRef]
- Cuomo, J.J.; Leary, P.A.; Yu, D.; Reuter, W.; Frisch, M. Reactive Sputtering of Carbon and Carbide Targets in Nitrogen. J. Vac. Sci. Technol. 1979, 16, 299–302. [Google Scholar] [CrossRef]
- Bhanderi, D.; Lakhani, P.; Modi, C.K. Graphitic carbon nitride (g-C3N4) as an emerging photocatalyst for sustainable environmental applications: A comprehensive review. RSC Sustain. 2024, 2, 265–287. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Acharya, L.; Biswal, L.; Priyadarshini, P.; Parida, K. Recent advancements in graphitic carbon nitride based direct Z- and S-scheme heterostructures for photocatalytic H2O2 production. Inorg. Chem. Front. 2024, 11, 4914–4973. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Zhang, Y.; Mori, T.; Ye, J.; Antonietti, M. Phosphorus-doped carbon nitride solid: Enhanced electrical conductivity and photocurrent generation. J. Am. Chem. Soc. 2010, 132, 6294–6295. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Khaneghah, M.H.; Sohouli, E. Graphitic carbon nitride: Synthesis and characterization. In Handbook of Carbon-Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 573–590. [Google Scholar]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic carbon nitrides (g-C3N4) with comparative discussion to carbon materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Chubenko, E.B.; Kovalchuk, N.G.; Komissarov, I.V.; Borisenko, V.E. Chemical Vapor Deposition of 2D Crystallized g-C3N4 Layered Films. J. Phys. Chem. C 2022, 126, 4710–4714. [Google Scholar] [CrossRef]
- Abdullahi, M.T.; Ali, M.; Farooq, W.; Khan, M.; Younas, M.; Tahir, M.N. Solvothermal synthesis of carbon nitride (g-C3N4): Bandgap engineering for improved photocatalytic performance. Sustain. Energy Fuels 2025, 9, 1109–1119. [Google Scholar] [CrossRef]
- Li, L.; Wei, N.; Guo, Y.; Zhu, X.; Wang, L.; Zhu, Y.; Fang, K.; Ma, S.; Zhang, Y.; Zhang, Y.; et al. Detection of Abeta40 in cerebrospinal fluid and plasma of Alzheimer’s disease patients using photoelectrochemical biosensors. Mikrochim. Acta 2024, 192, 5. [Google Scholar] [CrossRef]
- Yadav, S.K.; Yadav, A.K.; Kaushik, A.; Solanki, P.R. Functionalized graphitic carbon nitride as an efficient electro-analytical platform for the label-free electrochemical sensing of interleukin-8 in saliva samples. Nanoscale 2025, 17, 7926–7944. [Google Scholar] [CrossRef]
- Liu, C.-C.; Walters, A.B.; Vannice, M.A. Measurement of electrical properties of a carbon black. Carbon 1995, 33, 1699–1708. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Fulvio, P.F.; Andreoli, E. Carbon black reborn: Structure and chemistry for renewable energy harnessing. Carbon 2020, 162, 604–649. [Google Scholar] [CrossRef]
- Wang, M.J.; Gray, C.A.; Reznek, S.A.; Mahmud, K.; Kutsovsky, Y. Carbon Black. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Arduini, F.; Cinti, S.; Mazzaracchio, V.; Scognamiglio, V.; Amine, A.; Moscone, D. Carbon black as an outstanding and affordable nanomaterial for electrochemical (bio)sensor design. Biosens. Bioelectron. 2020, 156, 112033. [Google Scholar] [CrossRef] [PubMed]
- Smalley, R.E. Discovering the fullerenes. Rev. Mod. Phys. 1997, 69, 723–730. [Google Scholar] [CrossRef]
- Ha, T.-J.; Hedau, B.; Park, S.-J. Electronic properties of zero-dimensional carbon–based nanomaterials. In Zero-Dimensional Carbon Nanomaterials; Woodhead Publishing (Elsevier): Cambridge, MA, USA, 2024; pp. 185–248. [Google Scholar]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, G.; Zhang, W.; Lin, T.; Xie, H.; Liu, Q.; Zhang, H. Synthesis of fullerene by pyrolysis of acetylene in thermal HF-Plasma. J. Wuhan. Univ. Technol.-Mater. Sci. Ed. 2007, 22, 94–97. [Google Scholar] [CrossRef]
- Taylor, R.; Langley, G.J.; Kroto, H.W.; Walton, D.R.M. Formation of C60 by pyrolysis of naphthalene. Nature 1993, 366, 728–731. [Google Scholar] [CrossRef]
- Saunders, M.; Jiménez-Vázquez, H.A.; Cross, R.J.; Mroczkowski, S.; Freedberg, D.I.; Anet, F.A.L. Probing the interior of fullerenes by 3He NMR spectroscopy of endohedral 3He@C60 and 3He@C70. Nature 1994, 367, 256–258. [Google Scholar] [CrossRef]
- Komatsu, K.; Murata, M.; Murata, Y. Encapsulation of molecular hydrogen in fullerene C60 by organic synthesis. Science 2005, 307, 238–240. [Google Scholar] [CrossRef]
- Shinohara, H. Endohedral metallofullerenes. Rep. Progress. Phys. 2000, 63, 843–892. [Google Scholar] [CrossRef]
- Yang, S.; Chen, C.; Liu, F.; Xie, Y.; Li, F.; Jiao, M.; Suzuki, M.; Wei, T.; Wang, S.; Chen, Z.; et al. An improbable monometallic cluster entrapped in a popular fullerene cage: YCN@C(s)(6)-C82. Sci. Rep. 2013, 3, 1487. [Google Scholar] [CrossRef]
- Lu, X.; Akasaka, T.; Slanina, Z. Handbook of Fullerene Science and Technology; Springer Nature Singapore Pte Ltd.: Singapore, 2022. [Google Scholar]
- Taouri, L.; Bourouina, M.; Bourouina-Bacha, S.; Hauchard, D. Fullerene-MWCNT nanostructured-based electrochemical sensor for the detection of Vanillin as food additive. J. Food Compos. Anal. 2021, 100, 103811. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Palanisamy, S.; Chen, S.M.; Lou, B.S. Preparation of highly stable fullerene C60 decorated graphene oxide nanocomposite and its sensitive electrochemical detection of dopamine in rat brain and pharmaceutical samples. J. Colloid. Interface Sci. 2016, 462, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-R.; Fernandez-Delgado, O.; Echegoyen, L. Fullerenes and their applications. In Handbook of Carbon-Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 19–158. [Google Scholar]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Bose, A. Carbon dots: A review of innovations, applications, challenges, and future prospects. Inorg. Chem. Commun. 2025, 173, 113852. [Google Scholar] [CrossRef]
- Madhavikutti, A.S.; Subramaniam, M.P.; Jacob, G.V.; Jayan, J.S. Advances in the synthesis approaches of carbon and graphene quantum dots. In Zero-Dimensional Carbon Nanomaterials; Woodhead Publishing (Elsevier): Cambridge, MA, USA, 2024; pp. 17–59. [Google Scholar]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrión, C.; Valcárcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Anagnostou, P.; Constantinou, I.; Dakidi, K.; Tolia, E.; Skolariki, T.; Stalikas, C.D.; Chatzimitakos, T. Optical properties and applications of zero-dimensional carbon nanomaterials. In Zero-Dimensional Carbon Nanomaterials; Woodhead Publishing (Elsevier): Cambridge, MA, USA, 2024; pp. 153–183. [Google Scholar]
- Carbonaro, C.M.; Engelbrecht, L.; Olla, C.; Cappai, A.; Casula, M.F.; Melis, C.; Stagi, L.; Laaksonen, A.; Mocci, F. Graphene quantum dots and carbon nanodots: Modeling of zero-dimensional carbon nanomaterials. In Zero-Dimensional Carbon Nanomaterials; Woodhead Publishing (Elsevier): Cambridge, MA, USA, 2024; pp. 411–482. [Google Scholar]
- Modi, P.D.; Mehta, V.N.; Prajapati, V.S.; Patel, S.; Rohit, J.V. Bottom-up approaches for the preparation of carbon dots. In Carbon Dots in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 15–29. [Google Scholar]
- Ikebukuro, K.; Kiyohara, C.; Sode, K. Electrochemical Detection of Protein Using a Double Aptamer Sandwich. Anal. Lett. 2004, 37, 2901–2909. [Google Scholar] [CrossRef]
- Tayfour Ahmed, A.E.; Dhahi, T.S.; Attia, T.A.; Elhassan Ali, F.A.; Elobaid, M.E.; Adam, T.; Gopinath, S.C.B. AI-optimized electrochemical aptasensors for stable, reproducible detection of neurodegenerative diseases, cancer, and coronavirus. Heliyon 2025, 11, e41338. [Google Scholar] [CrossRef]
- Sethi, J.; Van Bulck, M.; Suhail, A.; Safarzadeh, M.; Perez-Castillo, A.; Pan, G. A label-free biosensor based on graphene and reduced graphene oxide dual-layer for electrochemical determination of beta-amyloid biomarkers. Mikrochim. Acta 2020, 187, 288. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liu, X.; Feng, R.; Zhang, N.; Fan, D.; Ding, C.; Zhao, H.; Du, Y.; Wei, Q.; et al. Bifunctional pd-decorated polysulfide nanoparticle of Co9S8 supported on graphene oxide: A new and efficient label-free immunosensor for amyloid β-protein detection. Sens. Actuators B Chem. 2020, 304, 127413. [Google Scholar] [CrossRef]
- Sonuc Karaboga, M.N.; Sezginturk, M.K. Analysis of Tau-441 protein in clinical samples using rGO/AuNP nanocomposite-supported disposable impedimetric neuro-biosensing platform: Towards Alzheimer’s disease detection. Talanta 2020, 219, 121257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lv, Y.; Dong, H.; Liu, L.; Mao, G.; Zhang, Y.; Xu, M. Ultrasensitive assay of amyloid-beta oligomers using Au-vertical graphene/carbon cloth electrode based on poly(thymine)-templated copper nanoparticles as probes. Sens. Actuators B Chem. 2021, 331, 129429. [Google Scholar] [CrossRef]
- Gallo-Orive, A.; Moreno-Guzman, M.; Sanchez-Paniagua, M.; Montero-Calle, A.; Barderas, R.; Escarpa, A. Gold Nanoparticle-Decorated Catalytic Micromotor-Based Aptassay for Rapid Electrochemical Label-Free Amyloid-beta42 Oligomer Determination in Clinical Samples from Alzheimer’s Patients. Anal. Chem. 2024, 96, 5509–5518. [Google Scholar] [CrossRef] [PubMed]
- Vajedi, F.S.; Rasoolzadeh, R.; Angnes, L.; Santos, E.C.S.; Silva, L.P.C. Ultrasensitive Aptasensing Platform for the Detection of beta-Amyloid-42 Peptide Based on MOF Containing Bimetallic Porphyrin Graphene Oxide and Gold Nanoparticles. ACS Appl. Bio Mater. 2024, 7, 2218–2239. [Google Scholar] [CrossRef]
- Fan, Z.; Yuan, L.; Xia, T.; Zhao, C.; Guo, W.; Ibrahim, A.A.; Ansari, S.A.; Umar, A. Enhanced Alzheimer’s biomarker detection using a ternary composite electrochemical aptasensor. Mikrochim. Acta 2025, 192, 167. [Google Scholar] [CrossRef]
- Liu, L.; Bai, T.; Chi, Q.; Wang, Z.; Xu, S.; Liu, Q.; Wang, Q. How to Make a Fast, Efficient Bubble-Driven Micromotor: A Mechanical View. Micromachines 2017, 8, 267. [Google Scholar] [CrossRef]
- Fernández-Medina, M.; Ramos-Docampo, M.A.; Hovorka, O.; Salgueiriño, V.; Städler, B. Recent Advances in Nano- and Micromotors. Adv. Funct. Mater. 2020, 30, 1908283. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, H.; Cai, Y.; Jurado-Sánchez, B.; Dong, R. Recent Advances in One-Dimensional Micro/Nanomotors: Fabrication, Propulsion and Application. Nano-Micro Lett. 2022, 15, 20. [Google Scholar] [CrossRef]
- Özcan, N.; Medetalibeyoglu, H.; Akyıldırım, O.; Atar, N.; Yola, M.L. Electrochemical detection of amyloid-β protein by delaminated titanium carbide MXene/multi-walled carbon nanotubes composite with molecularly imprinted polymer. Mater. Today Commun. 2020, 23, 101097. [Google Scholar] [CrossRef]
- Yin, M.; Xu, D.; Yu, J.; Huang, S.; Gopinath, S.C.B.; Kang, P. Impedance spectroscopy for identifying tau protein to monitor anesthesia-based issues. Biotechnol. Appl. Biochem. 2022, 69, 1805–1811. [Google Scholar] [CrossRef]
- Eduarda Schneider, M.; Guillade, L.; Correa-Duarte, M.A.; Moreira, F.T.C. Development of a biosensor for phosphorylated Tau 181 protein detection in Early-Stage Alzheimer’s disease. Bioelectrochemistry 2022, 145, 108057. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiao, M.; He, J.; Zhang, Y.; Liang, Y.; Liu, H.; Zhang, Z. Aptamer-Functionalized Carbon Nanotube Field-Effect Transistor Biosensors for Alzheimer’s Disease Serum Biomarker Detection. ACS Sens. 2022, 7, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, L.; Zhao, L.; Zuo, Y.; Gao, S.; Gu, H.; Wang, Y.; Yu, Y. Multiplex paper-based electrochemical immunosensor for the simultaneous monitoring of blood biomarkers in Alzheimer’s disease. Sens. Actuators B Chem. 2024, 406, 135456. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, X.; Liu, T.; Zhang, W.; Dong, H.; Chu, Z. Freestanding Nanofiber-Assembled Aptasensor for Precisely and Ultrafast Electrochemical Detection of Alzheimer’s Disease Biomarkers. Adv. Healthc. Mater. 2024, 13, e2304355. [Google Scholar] [CrossRef]
- Chen, M.; Man, Y.; Xu, S.; Wu, H.; Ling, P.; Gao, F. A label-free dually-amplified aptamer sensor for the specific detection of amyloid-beta peptide oligomers in cerebrospinal fluids. Anal. Chim. Acta 2023, 1266, 341298. [Google Scholar] [CrossRef]
- Li, X.; Jiang, M.; Cheng, J.; Ye, M.; Zhang, W.; Jaffrezic-Renault, N.; Guo, Z. Signal multi-amplified electrochemical biosensor for voltammetric determination of tau-441 protein in biological samples using carbon nanomaterials and gold nanoparticles to hint dementia. Mikrochim. Acta 2020, 187, 302. [Google Scholar] [CrossRef]
- Tao, D.; Xie, C.; Fu, S.; Rong, S.; Song, S.; Ye, H.; Jaffrezic-Renault, N.; Guo, Z. Thionine-functionalized three-dimensional carbon nanomaterial-based aptasensor for analysis of Abeta oligomers in serum. Anal. Chim. Acta 2021, 1183, 338990. [Google Scholar] [CrossRef]
- Negahdary, M.; Buoro, R.M.; Bacil, R.P.; Santos, B.G.; Angnes, L. Design of an electrochemical aptasensor in the presence of an array of gold nanostructure and a GO-MWCNTs nanocomposite: Application in diagnosis of Alzheimer’s disease. Mikrochim. Acta 2023, 190, 409. [Google Scholar] [CrossRef]
- Pakapongpan, S.; Poo-arporn, Y.; Ninket, S.; Poo-arporn, R.P. A disposable electrochemical sensor for amyloid-β42 protein based on molecular imprinted polymers with nitrogen doped carbon dots-graphene nanohybrid. Microchem. J. 2024, 206, 111559. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Qin, H.; Yang, X.; Cao, W.; Liu, S. g-C3N4-heme bound to amyloid β peptides: In-situ generation of the secondary co-reactant for dual-enhanced electrochemiluminescence assay of amyloid β detection. Electrochim. Acta 2020, 361, 137096. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Gao, Y.; Yan, J.; Song, W. Integrating CuO/g-C(3)N(4) p-n heterojunctioned photocathode with MoS(2) QDs@Cu NWs multifunctional signal amplifier for ultrasensitive detection of AbetaO. Biosens. Bioelectron. 2021, 176, 112945. [Google Scholar] [CrossRef]
- Pereira, M.V.; Marques, A.C.; Oliveira, D.; Martins, R.; Moreira, F.T.C.; Sales, M.G.F.; Fortunato, E. Paper-Based Platform with an In Situ Molecularly Imprinted Polymer for beta-Amyloid. ACS Omega 2020, 5, 12057–12066. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Q.; Zhang, Y.; Ren, B.; Huang, L.; Cai, H.; Xu, T.; Liu, Q.; Zhang, X. An electrochemical aptasensor based on AuPt alloy nanoparticles for ultrasensitive detection of amyloid-beta oligomers. Talanta 2021, 231, 122360. [Google Scholar] [CrossRef]
- Ren, Z.; Guo, W.; Umar, A.; Zhao, C.; Wang, L.; Ibrahim, A.A.; Alkhanjaf, A.A.M.; Baskoutas, S.; Pei, M.; Zhang, X. An electrochemical aptasensor based on stimulus response and signal amplification strategy for the detection of Amyloid-β oligomers. Microchem. J. 2023, 195, 109377. [Google Scholar] [CrossRef]
- Qin, Y.; Ouyang, X.; Lv, Y.; Liu, W.; Liu, Q.; Wang, S. A Review of Carbon-Based Conductive Inks and Their Printing Technologies for Integrated Circuits. Coatings 2023, 13, 1769. [Google Scholar] [CrossRef]

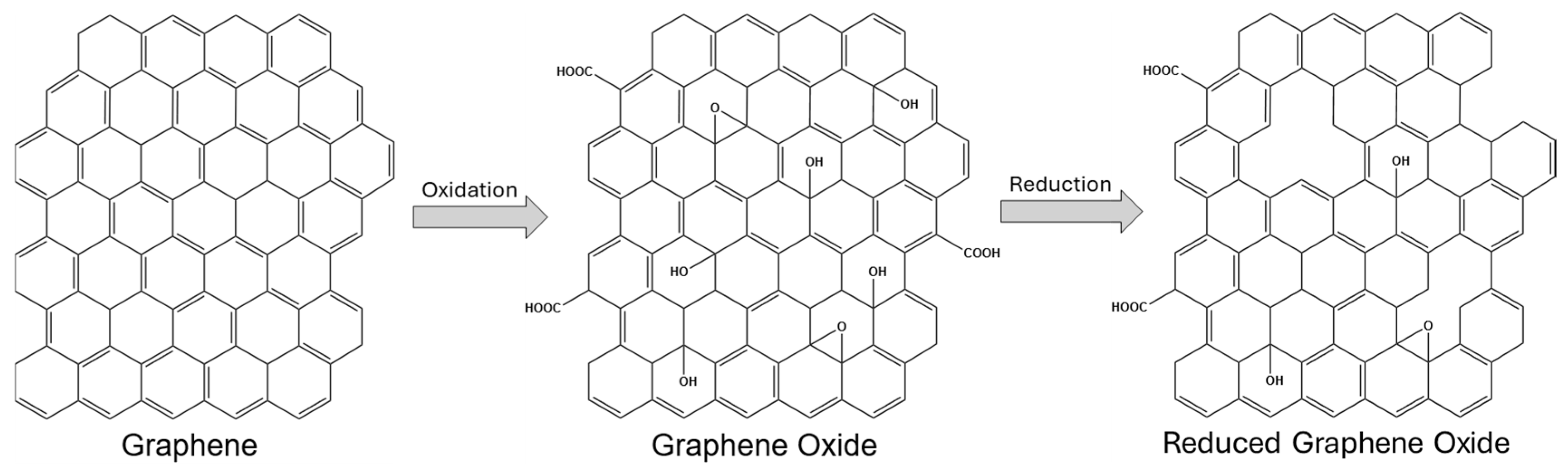
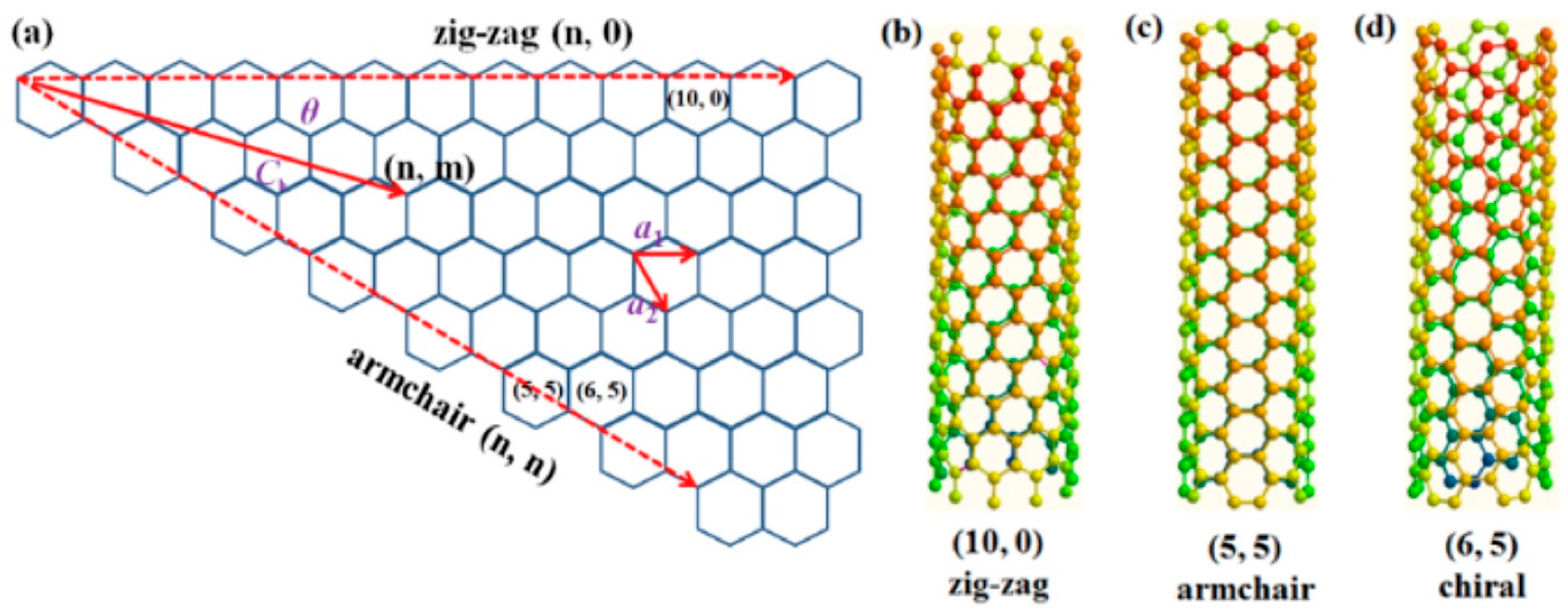
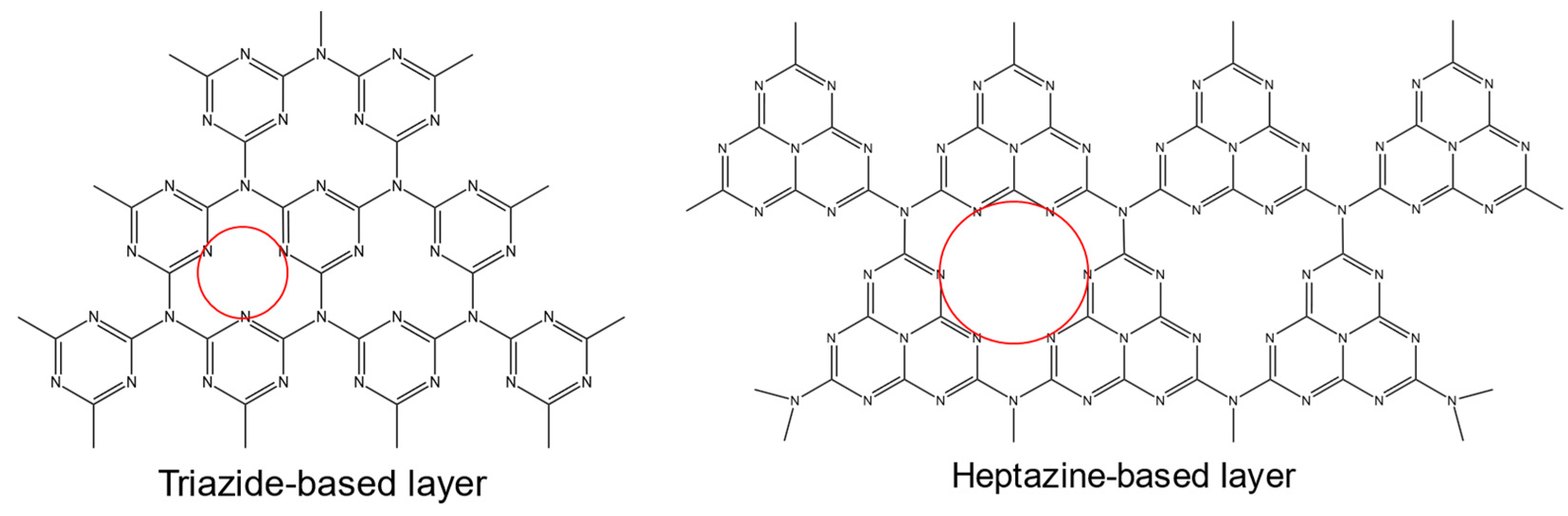
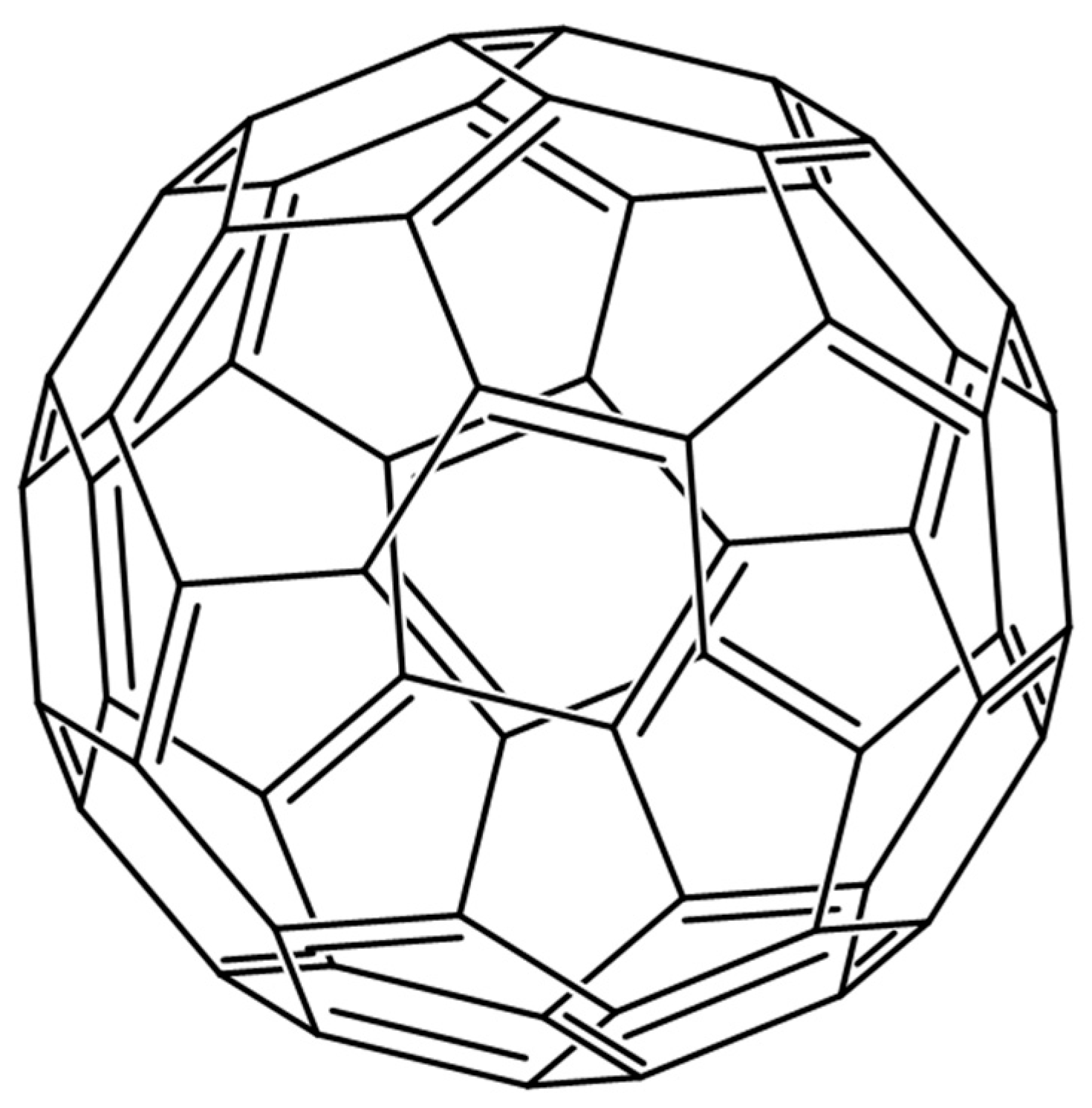
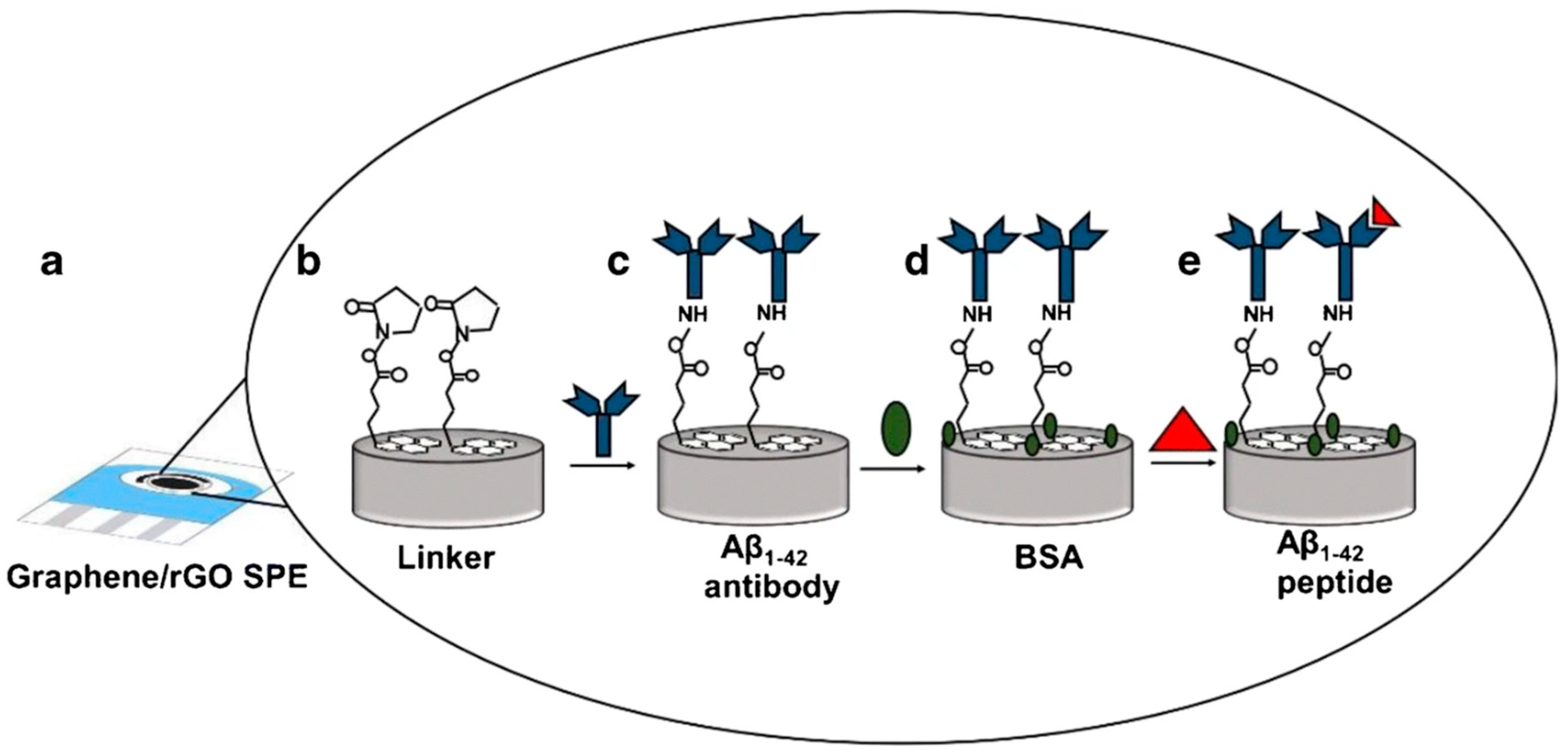
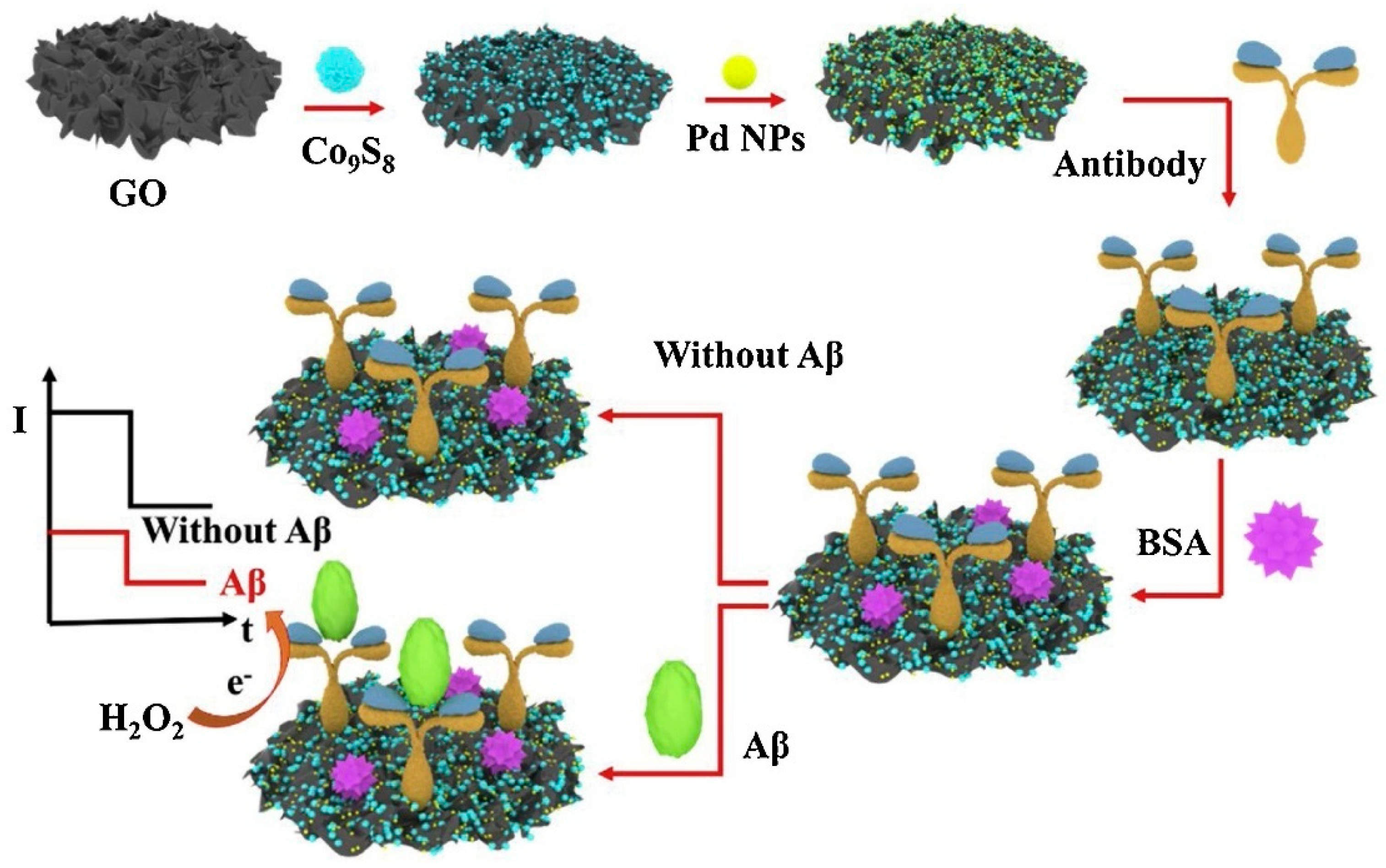
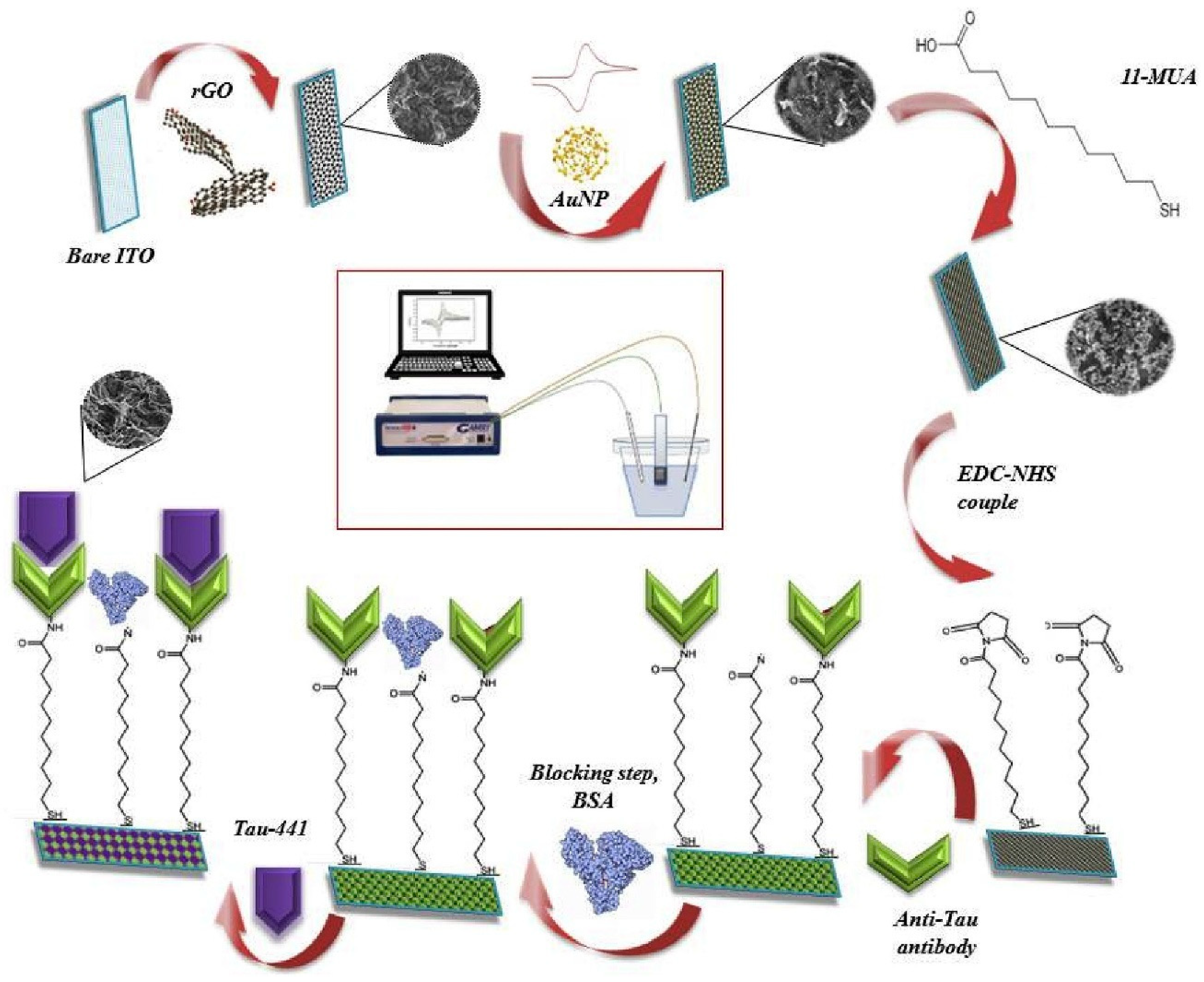
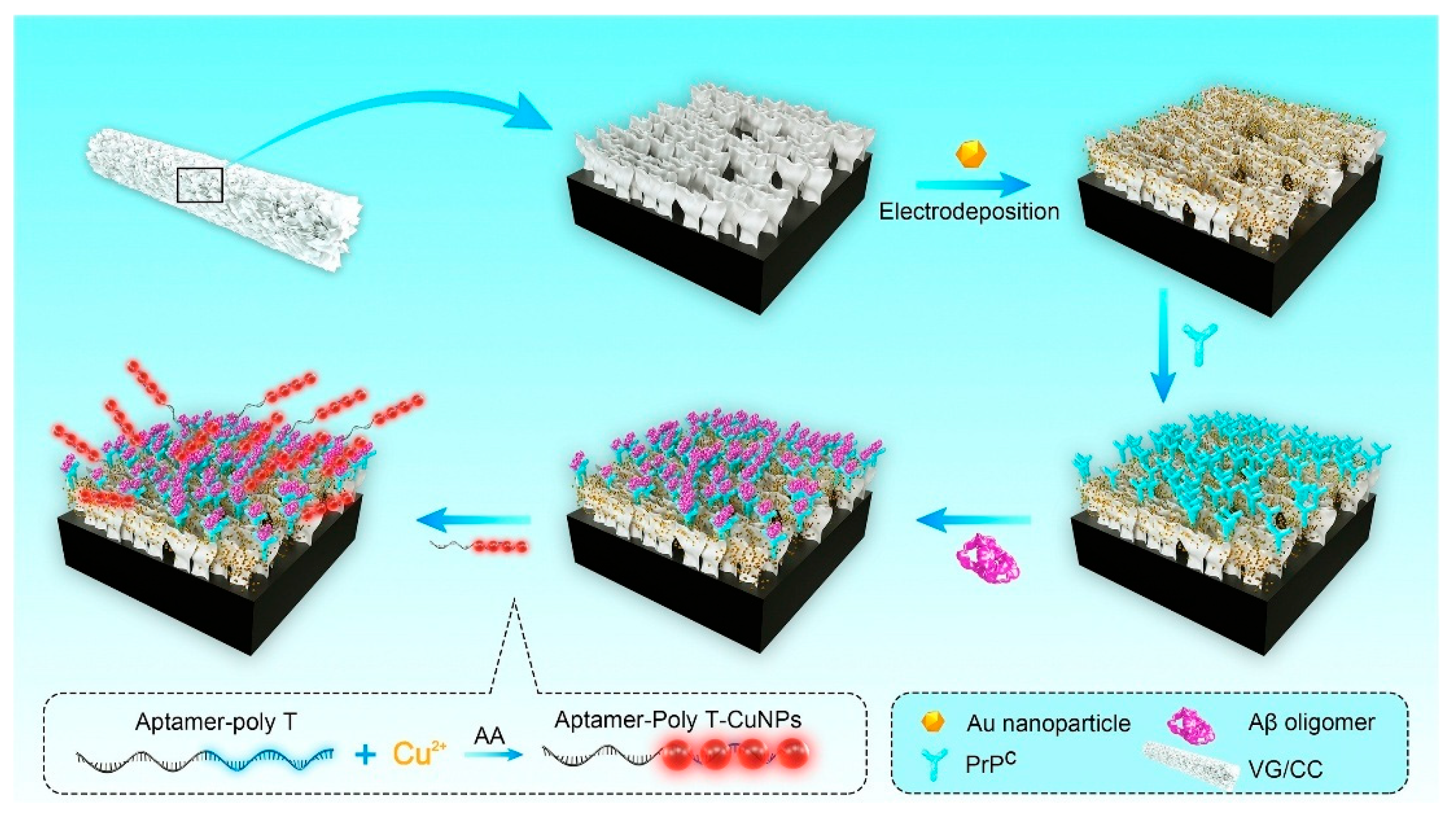

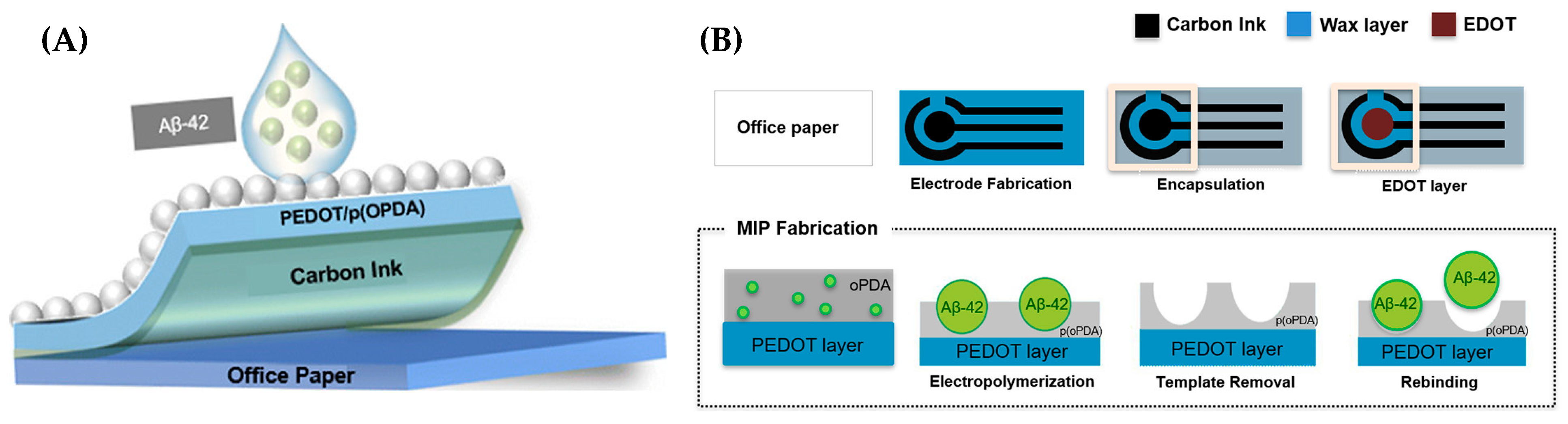
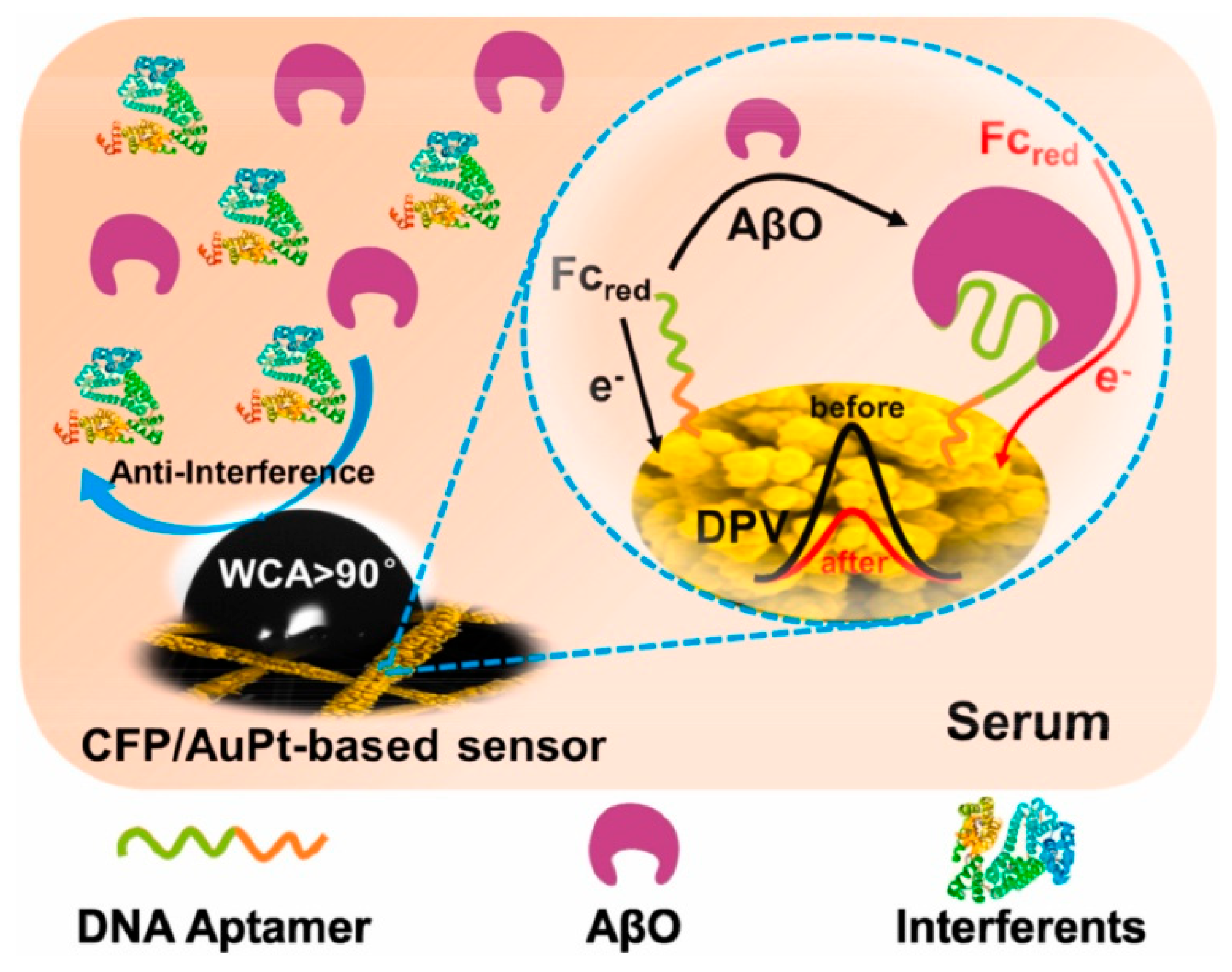
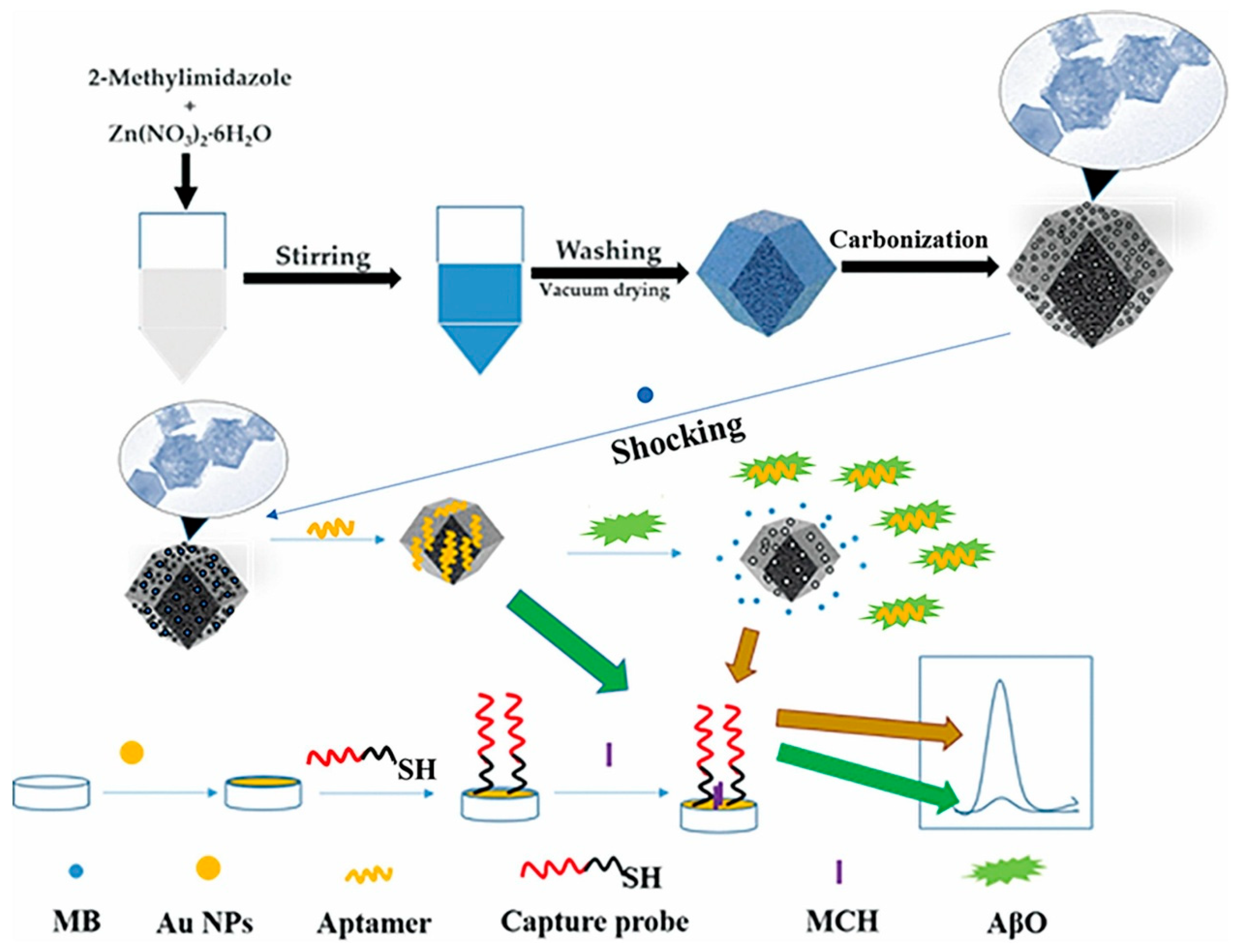
| Method Class | Technique (Examples) | Benefits | Drawbacks | References |
|---|---|---|---|---|
| Clinical/Cognitive | Clinical evaluation; neuropsychological testing | Widely used; accessible | Low accuracy and specificity in the early stages | [6,7] |
| Neuroimaging | MRI (structural changes); PET (amyloid, tau) | In vivo visualization of brain structure and pathology; accurate | Expensive; time-consuming; requires skilled personnel; limited accessibility | [5,6,7] |
| Fluid-based (invasive) | CSF analysis (Aβ, tau) | High accuracy; widely used biomarker detection method | Invasive lumbar puncture; costly; time-consuming; limited accessibility | [5,6,7] |
| Fluid-based (blood-based) | Plasma/serum assays (e.g., pTau217/Aβ1–42 ratio, CLEIA, Lumipulse G1200) | Less invasive; promising for early detection; FDA-authorized test; high sensitivity (~92%) and specificity (~97%) | Currently restricted to specialized labs; biomarker levels 10–100× lower than in CSF; interference from other plasma proteins | [6,8,9] |
| Spectroscopic/Analytical | Spectroscopy, chromatography, Raman spectroscopy | Multiplexed analysis; rapid; non-destructive | Low selectivity in complex matrices; high operational cost; requires specialized equipment and expertise | [5,10,11] |
| Reference | Detection Method | Detection Principle/Platform | LOD | Linear Range | Target/Sample Type | Real Samples |
|---|---|---|---|---|---|---|
| Sethi et al. (2020) [119] | DPV | Antibody (H31L21); external redox probe; signal-off (inhibition): Ab–Aβ1–42 binding hinders [Fe(CN)6]3−/4− electron transfer (current decrease observed in CV and DPV upon Aβ1–42 binding); Platform: dual-layer graphene/rGO SPE with Pyr–NHS linker (π–π to rGO; NHS–amide to Ab); BSA blocking; CV used only for assembly/characterization | 2.398 pM | 11 pM–55 nM | Aβ1–42/PBS; Human plasma (spiked); mouse plasma | Yes—Human plasma (spiked), mouse plasma (no pretreatment); selectivity vs. Aβ1–40, ApoE ε4 (interferents 500 nM vs. 50 pM Aβ1–42, negligible). Incubation 60 min; IHC/MRI concordance (mouse) |
| Li et al. (2020) [120] | Amperometry (i–t) | Antibody; no external redox couple; signal-on (catalytic) via electrocatalytic H2O2 reduction at G/Co9S8–Pd/graphene; Ab–Aβ binding increases catalytic current (i–t readout); EIS (Rct increase) confirms electrode modification and antigen binding; Platform: G/Co9S8–Pd nanocomposite drop-cast on GCE; antibody immobilized via Pd–N coordination; BSA blocking; CV/EIS used only for assembly/characterization | 41.4 fM | 0.1 pg/mL–50 ng/mL | Aβ/PBS; Artificial CSF (spiked) | No—Artificial CSF (standard addition); recovery 96.3–109.5%; RSD 3.9–4.9%; selectivity vs. glucose, HIg, Gln, PSA; reproducibility RSD < 5% (n = 5); stability: ~4.1% signal loss after 3 weeks at 4 °C |
| Karaboga & Sezgintürk (2020) [121] | EIS, CV | Antibody (anti-Tau, monoclonal); external redox probe; signal-off (inhibition): Ab–Tau-441 binding hinders [Fe(CN)6]3−/4− electron transfer (ΔRct increase, current decrease in CV); Platform: disposable ITO electrode coated with rGO–AuNP nanocomposite; anti-Tau immobilized via 11-MUA SAM + EDC/NHS coupling; BSA blocking; CV/EIS used for assembly/characterization | 0.091 pg/mL | 1–500 pg/mL | Tau-441/PBS; Human serum, CSF | Yes—Human serum (n = 5, standard addition) and human CSF (n = 4, standard addition); recovery 96.1–108.6%; repeatability RSD 6.38% (n = 20 electrodes); reproducibility RSD 3.02–3.41% (n = 6 batches); selectivity vs. HSP-70, α-Syn, RACK-1 (negligible); stability 8 weeks at 4 °C |
| Zhou et al. (2021) [122] | DPV | Aptamer-polyT-CuNP conjugates serve as signal tags, binding to AβO previously captured by PrPC (95–110 residues); signal-on via poly(thymine)-templated CuNP tags (DPV peak from Cu0/Cu redox); double amplification by CuNP tags and Au-VG/CC substrate; Platform: Au-nanoparticle-modified vertical graphene on carbon cloth (Au-VG/CC); HS-PrPC immobilized by Au–S; hexanethiol blocking; EIS/CV used only for assembly/characterization | 3.5 pM | 10–2200 pM | Aβ oligomers/buffer; Human serum | Yes—Human serum (n = 9; AD + controls); standard-addition recovery 93–116%, RSD < 10%; repeatability RSD 4.8% (n = 7); reproducibility RSD 6.7% (n = 6); ELISA correlation; selectivity vs. Aβ monomers, Aβ fibrils, insulin (1 μg/mL), TNF-α (500 pg/mL), CRP (100 μg/mL) (negligible); stability: 89.9% signal retained after 4 weeks at 4 °C |
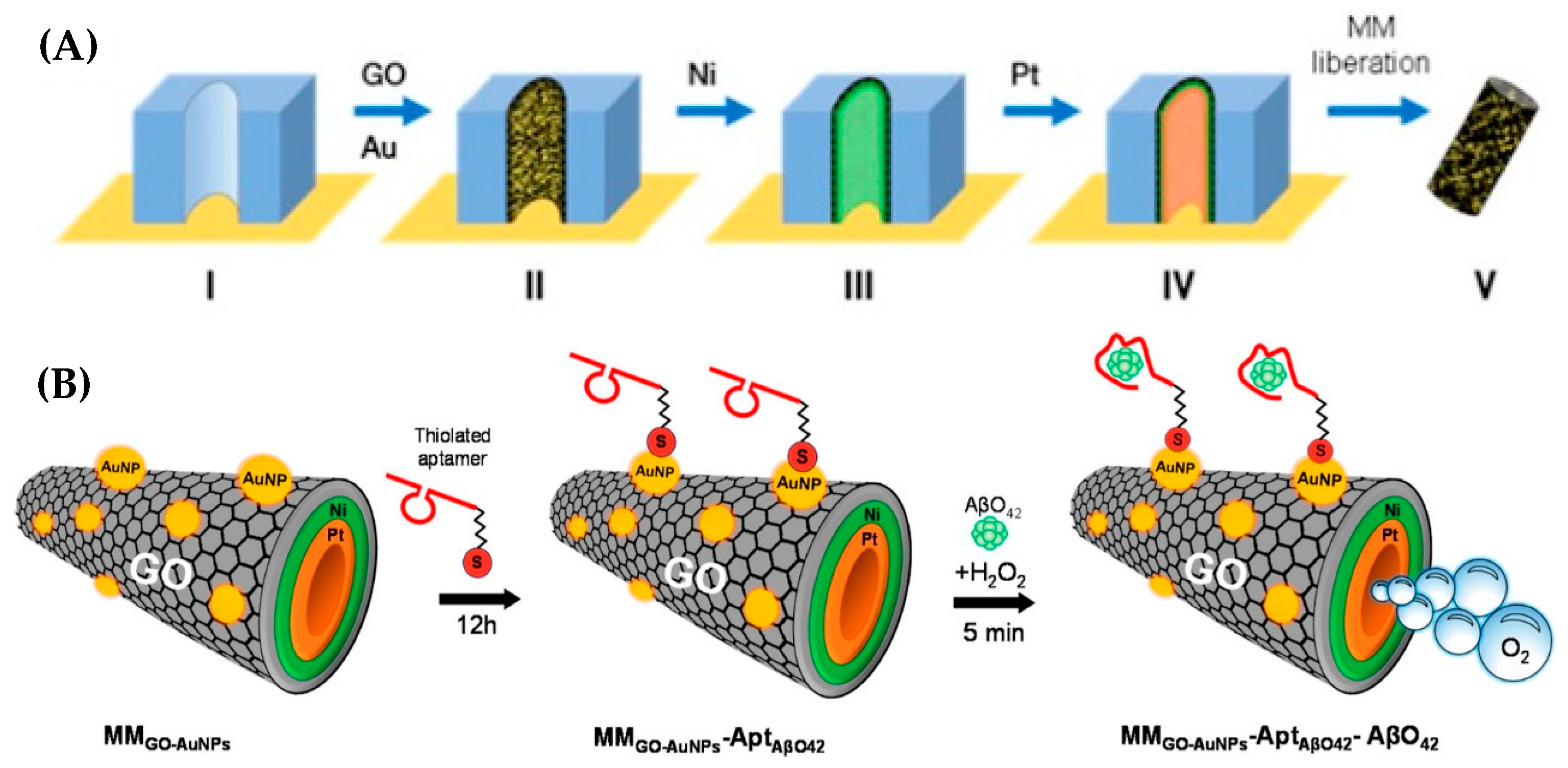
| Gallo-Orive et al. (2024) [123] | SWV | Aptamer (thiolated, AβO42-specific); label-free signal-off via inhibition of [Fe(CN)6]3−/4− redox probe (SWV cathodic current decreases upon AβO42 binding); Platform: catalytic micromotors (GO–AuNPs/Ni/PtNPs tubular MM) providing self-propulsion and enhanced mixing; aptamer immobilized via Au–S, BSA blocking; EIS with Ru(NH3)63+/2+ used only for assembly/binding confirmation (Rct increase); CV used only for characterization | 0.10 pg/mL | 0.5–500 pg/mL | AβO42/Brain tissue, CSF, Human plasma | Yes—Brain tissue, CSF, human plasma (AD patients); 5 µL undiluted; recovery 94–102%; precision RSD < 8% (intra-/inter-assay); selectivity vs. Aβ42 monomer (negligible); validated against dot blot (MM faster, 5 min vs. >14 h); stability of MM–aptamer complexes 15 days at 4 °C |
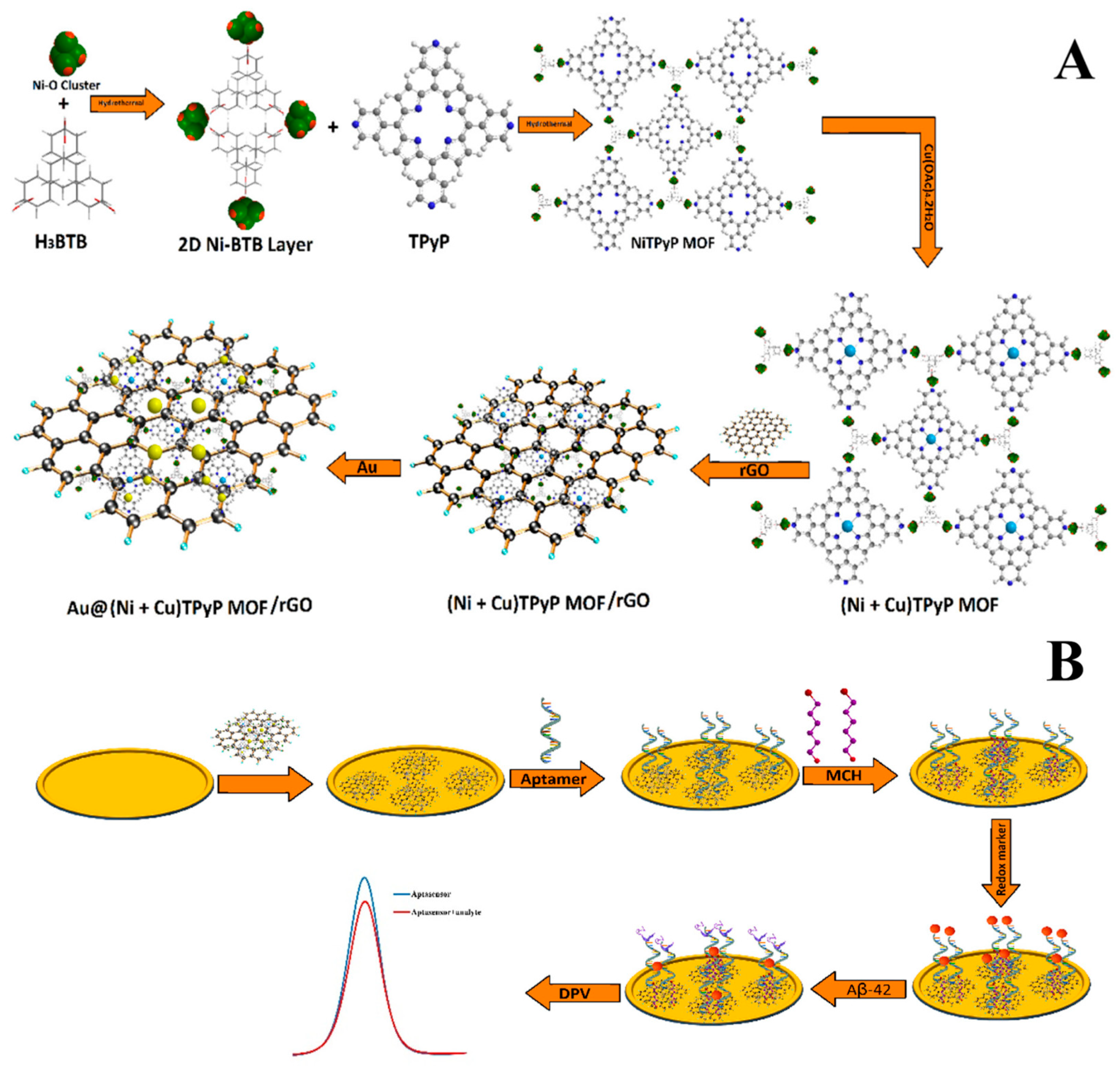
| Vajedi et al. (2024) [124] | DPV | Aptamer (thiolated, Aβ42-specific); label-free inhibition of [Fe(CN)6]3−/4− redox probe (DPV peak current decreases upon binding); Platform: Au@(Ni + Cu)TPyP MOF/rGO nanostructure on GE; aptamer immobilized via Au–S; MCH blocking; CV/EIS used only to confirm assembly and Aβ42 binding (Rct increase) | 48.6 fg/mL | 0.05 pg/mL–5.00 ng/mL | Aβ42/Human serum (spiked) | Yes—Human serum (10% diluted); recovery 95–104%; RSD 4.3% (spiked serum); reproducibility RSD 5.6% (n = 5 sensors); regeneration RSD 3.96% (bound), 2.27% (regenerated), 5 cycles; selectivity vs. Hb, HSA, BSA, HEP (≤1000×, negligible); stability 95.6% after 10 days at 4 °C. |
| Fan et al. (2025) [125] | DPV | Aptamer (Aβ1–40 oligomers, thiolated); signal-off via inhibition of [Fe(CN)6]3−/4− redox probe (ΔI decrease upon binding); ternary PPy–rGO–Fe2O3 nanocomposite enhances conductivity and surface area; AuNPs provide Au–S anchoring for aptamer; BSA blocking; Platform: PPy/rGO–Fe2O3/AuNPs/GCE; CV/EIS used only for assembly confirmation | 40 fM | 0.1 pM–200 nM | AβO (Aβ1–40)/PBS; Artificial serum (spiked) | No—artificial serum (spiked); recovery 96.3–105%; reproducibility RSD < 4.4% (n = 5 electrodes); selectivity not studied; stability ≈ 90.2% signal retained after 14 days |
| Reference | Detection Method | Detection Principle/Platform | LOD | Linear Range | Target/Sample Type | Real Samples |
|---|---|---|---|---|---|---|
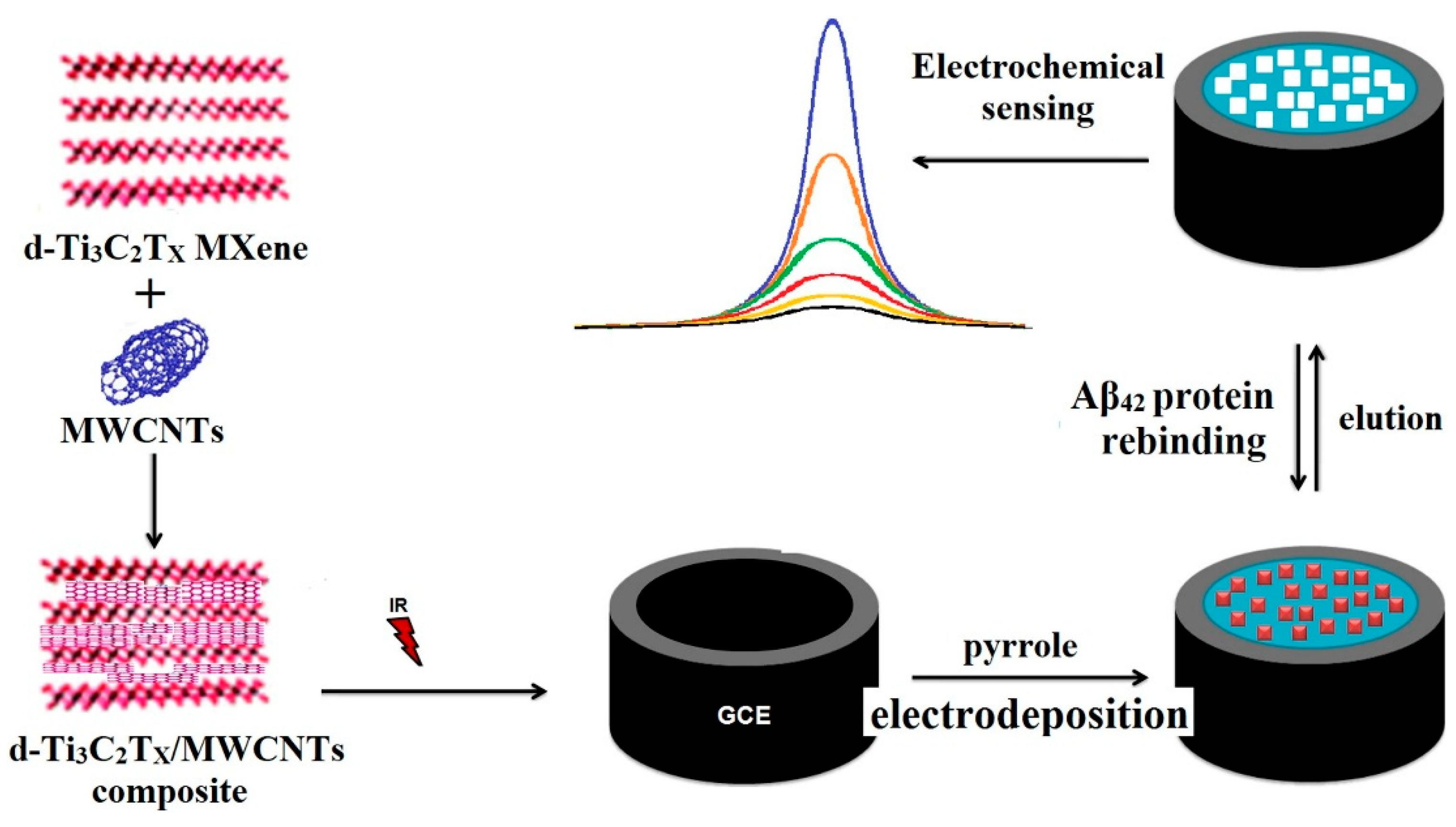
| Özcan et al. (2020) [129] | DPV | MIP (electropolymerized polypyrrole; template Aβ1–42); label-free signal-off via inhibition of [Fe(CN)6]3−/4− redox probe (DPV peak decreases upon rebinding); template eluted with NaCl (1.0 M); Platform: GCE modified with d-Ti3C2Tx MXene/MWCNTs (3:1 (m/m)) composite on GCE, OPDA electropolymerized with Aβ1–42 template; CV/EIS used only for assembly/characterization (ΔEp, Rct changes) | 0.3 fg/mL | 1.0–100.0 fg/mL | Aβ42/PBS; Human plasma (spiked) | Yes—Spiked human plasma; recovery 99.99–100.04% (n = 6); repeatability RSD 0.11% (60 runs); reproducibility RSD 0.15% (20 electrodes); selectivity vs. Hb, HEP, BIL (low cross-response; k up to 33.33); agreement with LC–MS/MS (Wilcoxon, p > 0.05); reusability ≥ 30 cycles; stability 60 days (inter-day RSD 0.13%) |
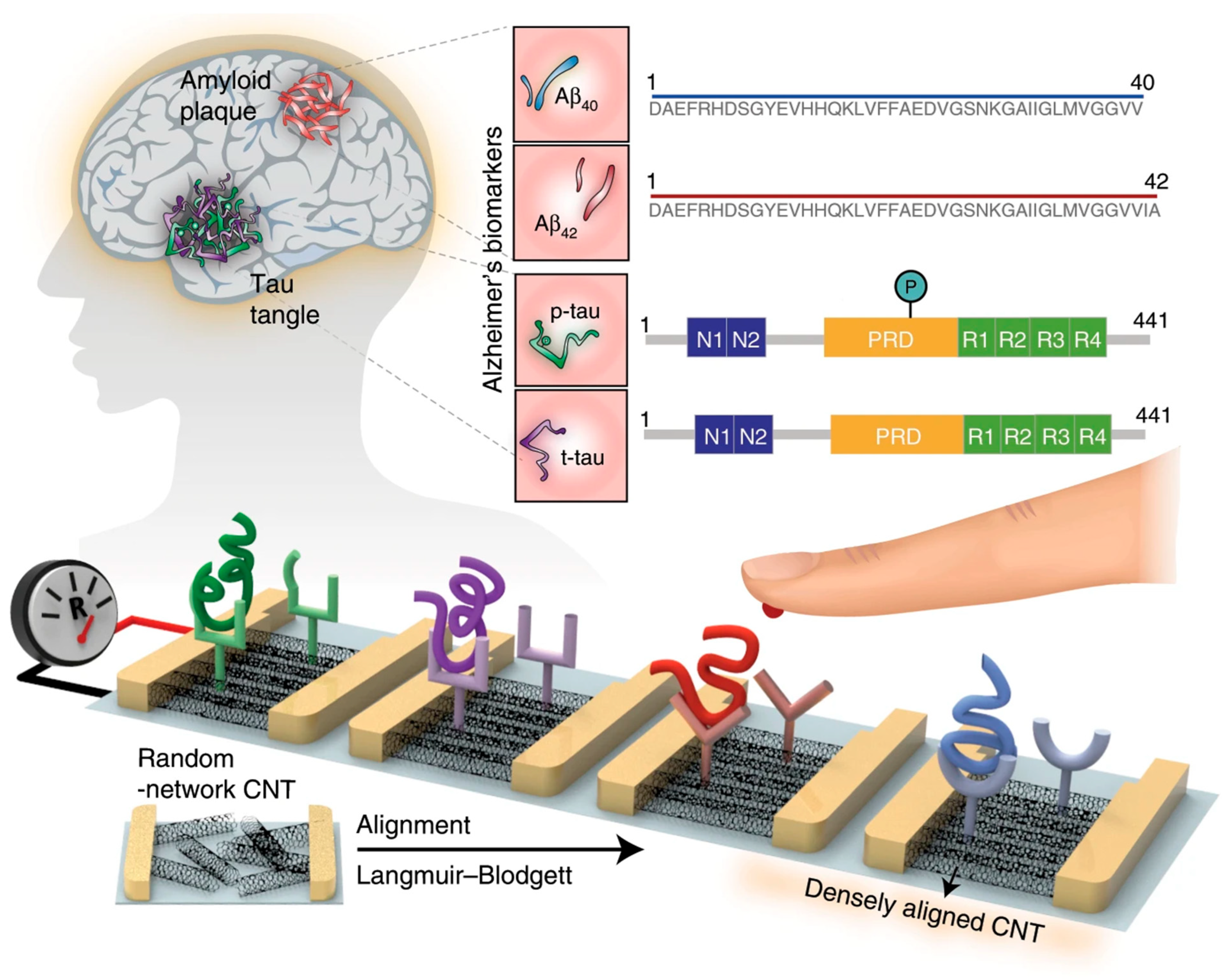
| Kim et al. (2020) [6] | Chemiresistive (ΔR, CNT-FET) | Antibodies (anti-Aβ42, anti-Aβ40, anti-t-tau, anti-p-tau181) immobilized via carbodiimide coupling (EDC/sulfo-NHS) after UV–ozone carboxylation of CNTs; binding of antigens increases resistance (scattering centers in p-type SWCNTs; ΔR readout); multiplexed detection of 4 biomarkers; Platform: densely aligned SWCNT monolayer by Langmuir–Blodgett on Si/SiO2 with Cr/Au contacts; BSA/Tween blocking; CV used only for assembly/characterization | 2.13 fM (Aβ42), 2.20 fM (Aβ40), 2.45 fM (t-tau), 2.72 fM (p-tau181) | ~100–106 fM | Aβ42, Aβ40, t-tau, p-tau181/Human plasma | Yes—Human plasma; spiked (recovery 93.0–97.6%); clinical plasma from AD patients and controls (n = 20 each); composite biomarkers (t-tau/Aβ42, p-tau181/Aβ42, Aβ42/Aβ40) discriminated AD vs. controls with 90.0% sensitivity, 90.0% selectivity, and 88.6% accuracy (AUC ≥ 0.93) |
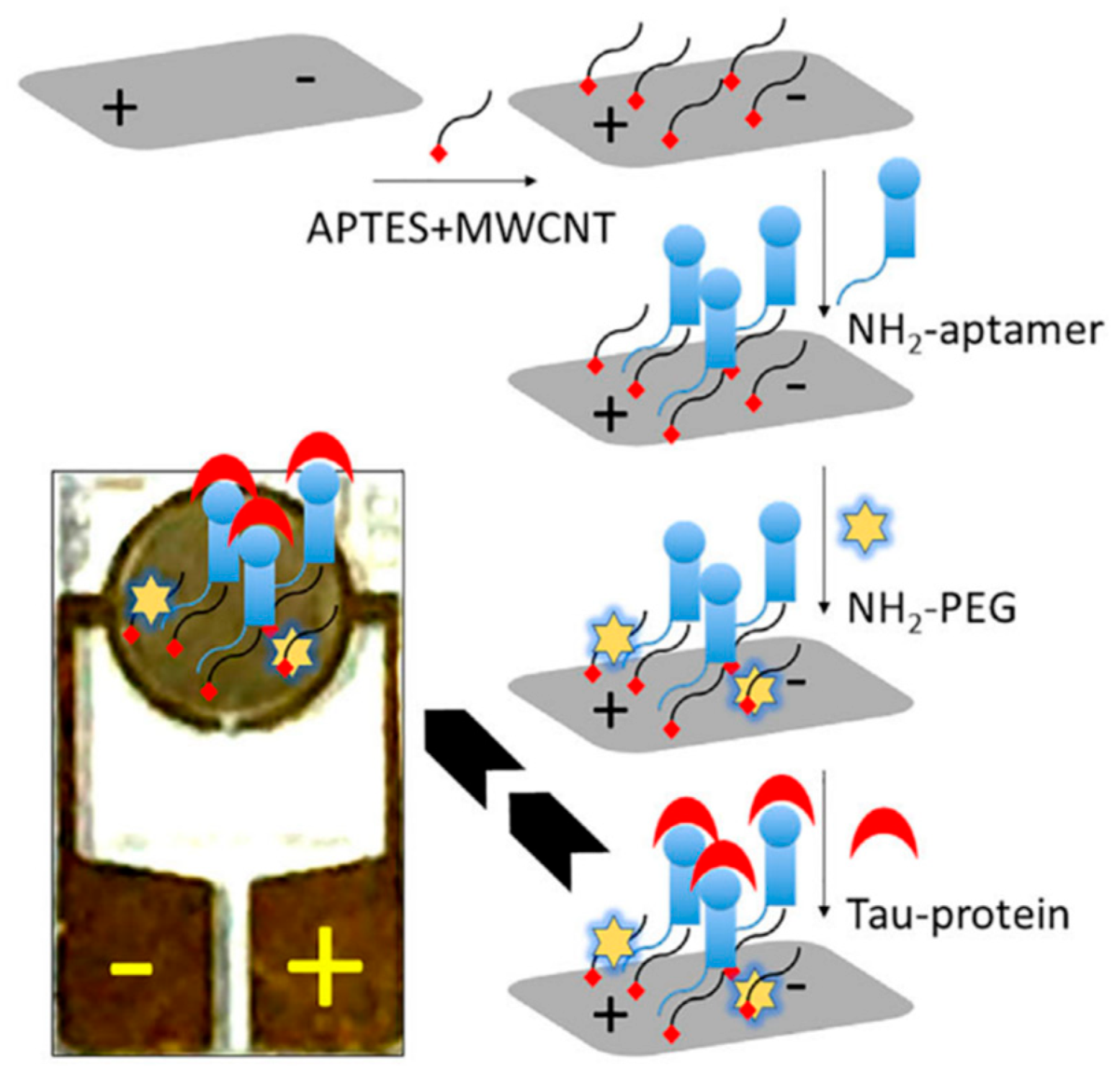
| Yin et al. (2022) [130] | EIS | Aptamer (NH2-ended, tau-specific) immobilized on MWCNTs-modified electrode via APTES linker; NH2-PEG blocking; tau binding decreases charge-transfer resistance (ΔRct, Nyquist plots); Platform: MWCNT-modified electrode with APTES linker and aptamer immobilization; CV/EIS used only for assembly/characterization | 1 fM | 1 fM–1 nM | Tau protein/Human serum (spiked; 1:100 dilution) | Yes—Spiked human serum (1:100 dilution); selectivity confirmed vs. complementary aptamer, CFH, albumin (low-cross response) |
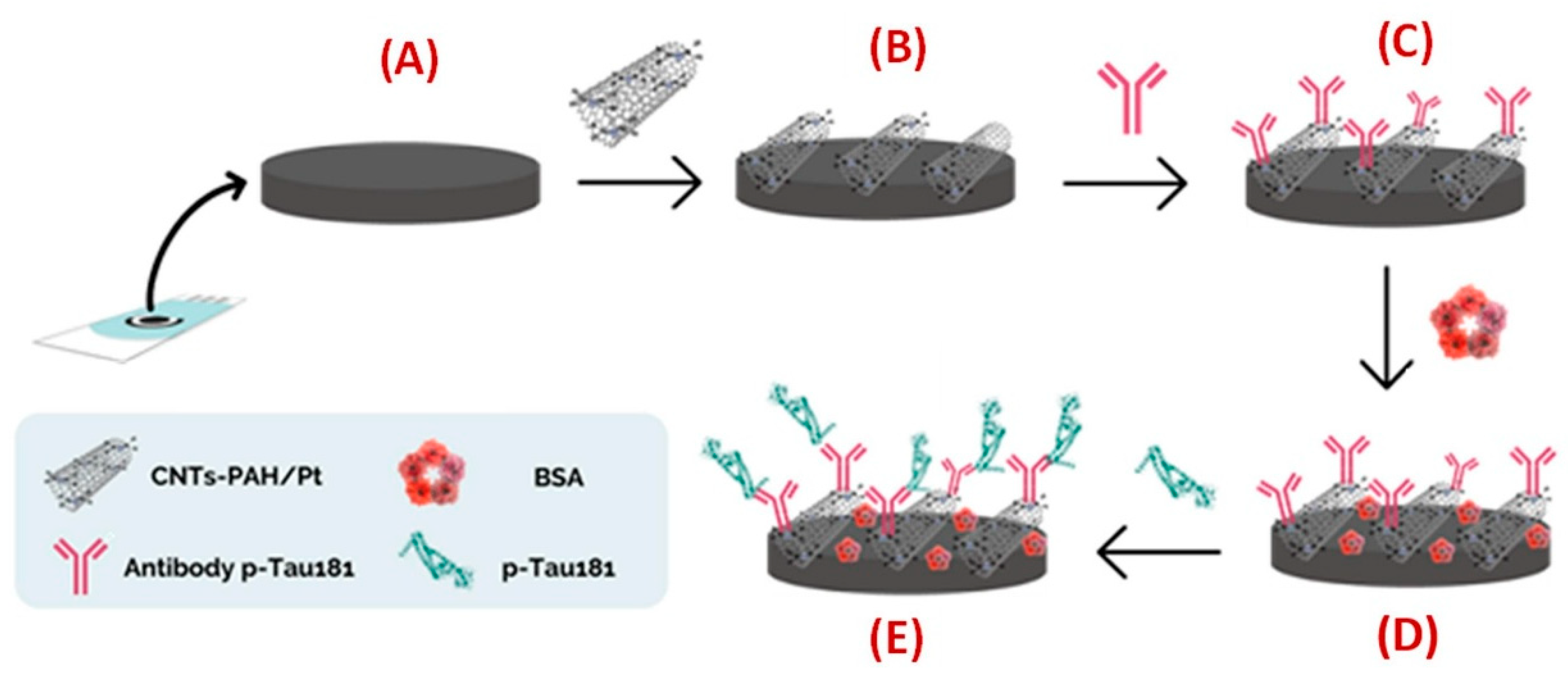
| Schneider et al. (2022) [131] | SWV | Antibody (anti-p-Tau181, polyclonal) randomly adsorbed (“flat-on”) on MWCNTs–PAH/Pt nanocomposite; label-free signal-off via inhibition of [Fe(CN)6]3−/4− redox probe (SWV peak current decreases upon p-Tau181 binding); BSA blocking; Platform: pretreated carbon SPE (C-SPE) modified with MWCNTs–PAH/Pt nanocomposite for Ab anchoring; CV used only for assembly/characterization | 0.24 pg/mL | 8.6 pg/mL–1100 pg/mL | p-Tau181/FBS (1:100, 1:10) | No—FBS (spiked); recovery 87–95%; selectivity vs. IgG, Hb, uric acid, BSA (interference ≤ 8%) |
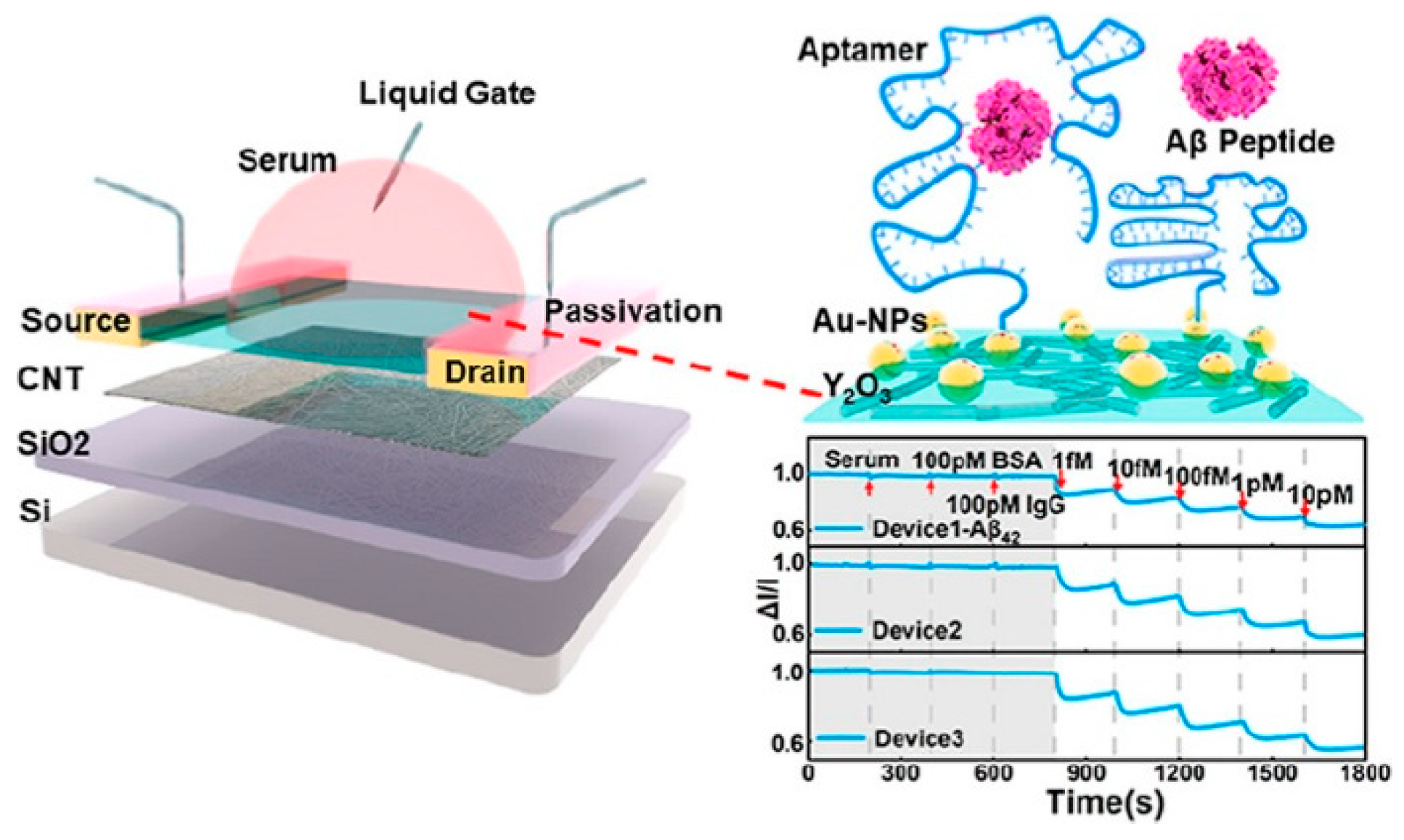
| Chen et al. (2022) [132] | FET | Aptamers (thiolated DNA; Aβ42, Aβ40) immobilized on AuNPs via Au–S; signal-off: aptamer conformational change upon binding causes Vth shift and IDS decrease; Platform: high-purity semiconducting CNT network channels on Si/SiO2 with 6 nm Y2O3 gate dielectric, AuNPs as floating-gate linkers; multi-blocking (MCH, Tween-20, BSA) to suppress nonspecific adsorption; wafer-scale device fabrication; Raman/SEM/fluorescence and electrical transfer curves used only for assembly/characterization | 45 aM (Aβ42), 55 aM (Aβ40) | 1 fM–10 pM | Aβ42, Aβ40/Human serum | Yes—Undiluted human serum (spiked); recovery 88–108%; selectivity vs. BSA, IgG, non-target Aβ peptide; selectivity ratios up to 800% (Aβ42), 730% (Aβ40); CV < 10%; stability 30 min in serum; response ~40 s |
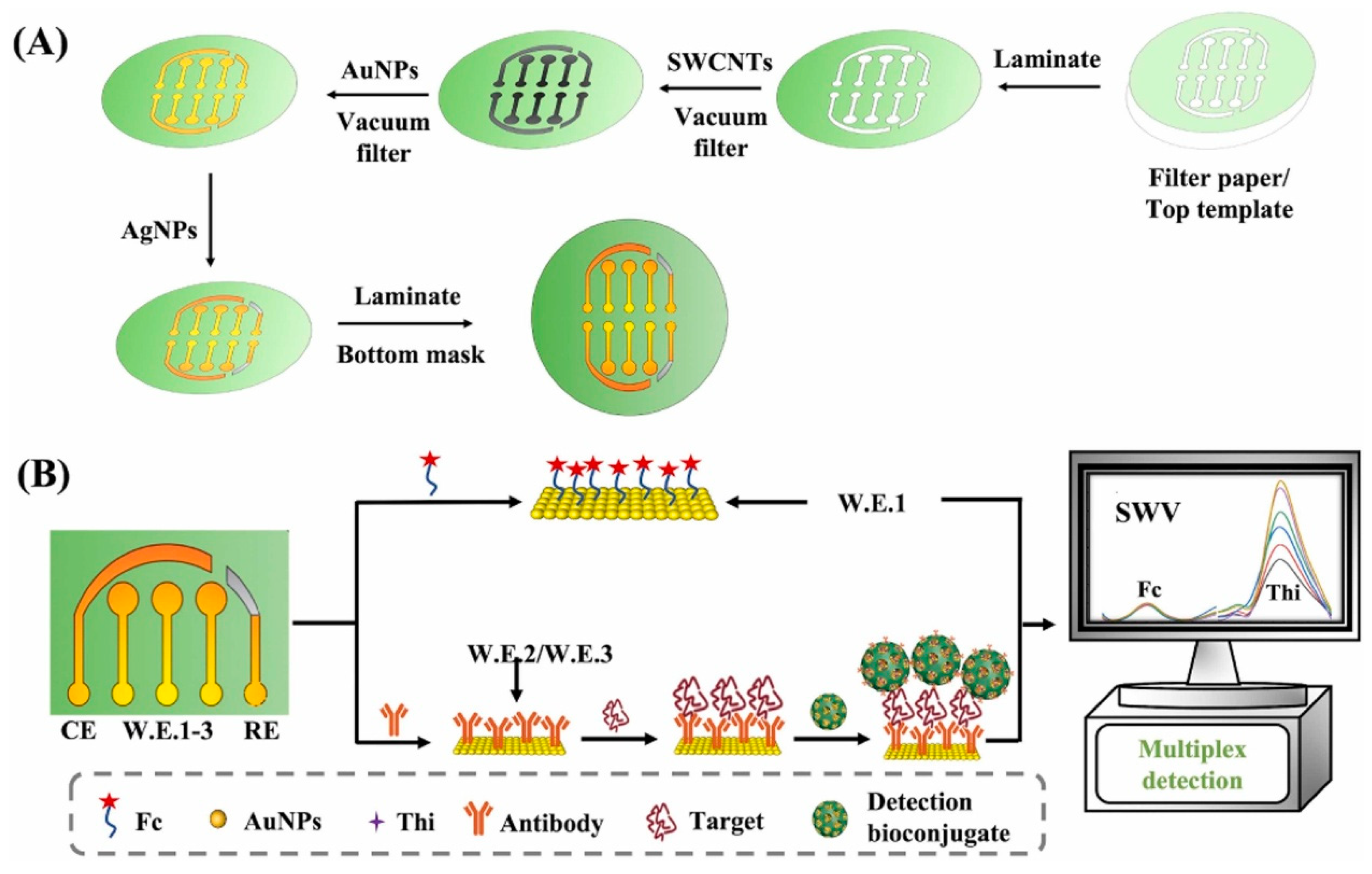
| Gu et al. (2024) [133] | SWV | Antibodies (A11 for Aβ oligomers; anti-Fetuin B); in sandwich format with dmSiO2–Au–Thionine-Ab nanoprobes; ratiometric readout vs. ferrocene (IThi/IFc); Platform: multiplex paper-based electrode made by vacuum-filtered SWCNT underlayer + AuNPs (3 WEs + Au CE + Ag pseudo-RE); BSA blocking; CV used only for assembly confirmation | 0.005 ng/mL (AβO), 0.02 ng/mL (Fetuin B) | 0.01–40 ng/mL (AβO), 0.05–80 ng/mL (Fetuin B) | AβO (hippocampus, cortex, serum) and Fetuin B (serum)/APP/PS1 transgenic mice | Yes—APP/PS1 transgenic mouse hippocampus, cortex, and serum (AβO) and serum (Fetuin B); recovery 98.0–100.9% (AβO hippocampus), 98.9–109.8% (AβO serum), 97.7–106.7% (Fetuin B serum); selectivity vs. dopamine, ascorbic acid, amino acids (Val, Cys, Ser, Glu, Thr), ions (Na+, Fe2+, Ca2+, Cu2+); note: oligomeric Aβ40 gives similar signal to Aβ42 (A11 oligomer-specific); no cross-talk between channels; repeatability RSD 2.51% (AβO), 2.96% (Fetuin B); reproducibility RSD 4.97% (AβO), 4.28% (Fetuin B); stability: ~89% response retained at 28 days (AβO only) |
| Liu et al. (2024) [134] | SWV | Aptamer (amine-terminated Aβ42 ssDNA) covalently immobilized via EDC/NHS onto COOH–CNTs; label-free binding decreases interfacial resistance, measured as increased SWV current; BSA blocking; Platform: freestanding electrospun PA/PANI–CNTs nanofiber membrane electrode; CV/EIS with [Fe(CN)6]3−/4− used only for electrode assembly confirmation | 30 fg/mL | 0.1 pg/mL–500 pg/mL and 500 pg/mL–110 ng/mL | Aβ42/Human serum | Yes—Human serum (spiked; protein-depleted); recovery 97.65–111.50%; RSD 0.09–7.00%; selectivity vs. alpha-fetoprotein, cTnI, CA125, hCG (100 ng/mL each); reproducibility RSD 0.96% (n = 6); electrochemical stability after 30 CV scans (−8.35%, −7.23% peak changes); 30-day storage stability; response time 4 min (72.6% signal at 2 min) |
| Reference | Detection Method | Detection Principle/Platform | LOD | Linear Range | Target/Sample Type | Real Samples |
|---|---|---|---|---|---|---|
| Li et al. (2020) [136] | DPV | Antibody (anti-Tau-441); antigen–antibody complex blocks [Fe(CN)6]3−/4− redox probe (DPV peak current decrease, ΔI); Platform: GE coated with MWCNTs–rGO–CS film; antibody immobilized via GLA; Tau-441–AuNPs conjugate for additional signal amplification; CV/EIS used for assembly confirmation | 0.46 fM | 0.5–80 fM | Tau-441 protein/Human serum | Yes—Human serum (clinical cohorts: 14 healthy, 14 MCI, 14 dementia); recovery 90.67–102.33%, repeatability RSD < 5%; reproducibility RSD 4.74% (n = 3); selectivity vs. Glu, AA, L-cys, α-Syn, HSA (<5% interference); stability 11 days at 4 °C (signal retained 92.86%) |
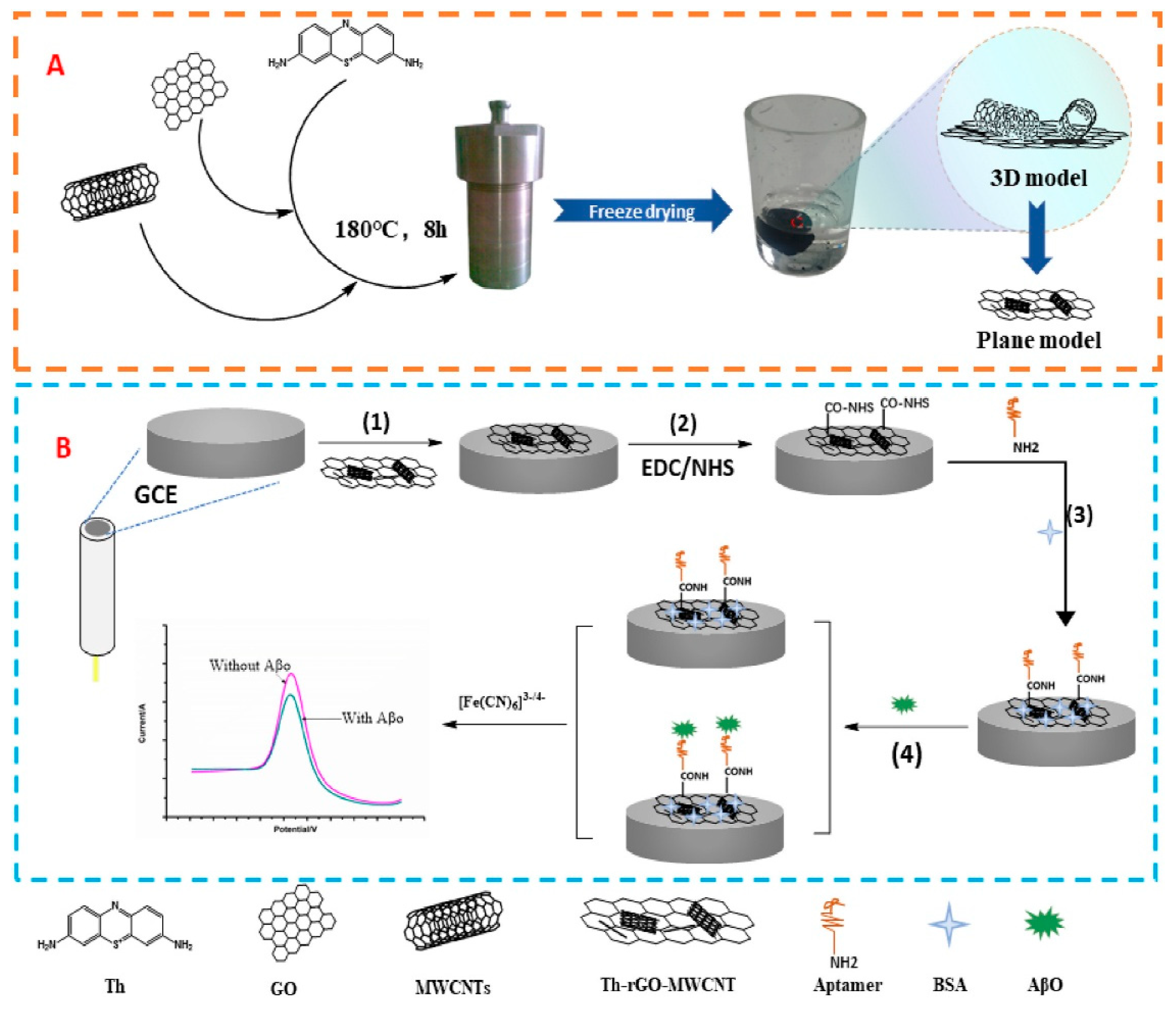
| Tao et al. (2021) [137] | DPV | Aptamer (AβO-specific); AβO–aptamer binding blocks [Fe(CN)6]3−/4− redox probe (DPV ΔI decrease); Platform: GCE modified with Th-rGO-MWCNTs (3D nanocomposite); aptamer immobilized via EDC/NHS; BSA blocking; CV/EIS used only for assembly/characterization | 10 fM | 0.0443–443.00 pM | Aβ oligomers/Human serum (diluted) | Yes—Human serum (diluted); recovery 99.71–103.84%, repeatability RSD < 1% (0.79–0.98%); reproducibility RSD < 2% (n = 3 electrodes, 4.43 pM); selectivity vs. Aβ monomers, Aβ fibrils, α-syn oligomers, tau protein; stability 15 days at 4 °C (signal ≥ 90%), <90% on day 16; incubation time 20 min (optimized) |
| Negahdary et al. (2023) [138] | DPV | Aptamer (NH2-modified, AβO-specific); AβO–aptamer binding blocks [Fe(CN)6]3−/4− redox probe (DPV peak decrease, ΔI); Platform: GCE with electrodeposited jagged Au (JG) nanostructures over-coated with GO-c-MWCNTs; aptamer immobilized via EDC/NHS; CV/EIS used only for assembly/characterization | 0.088 pg/mL | 0.1 pg/mL–1 ng/mL | Aβ oligomers/Human serum | Yes—Human serum (spiked; n = 10; diluted 50% in PBS): recovery 93–110% (overall RSD 5.43%); selectivity vs. AβMs, AβFs, and mixtures (max DPV decrement ≈ 38%); reproducibility RSD 1.28% (n = 5 re-fabrications); reversibility 3 cycles; stability 11 days under refrigerated storage (tracked every other day) |
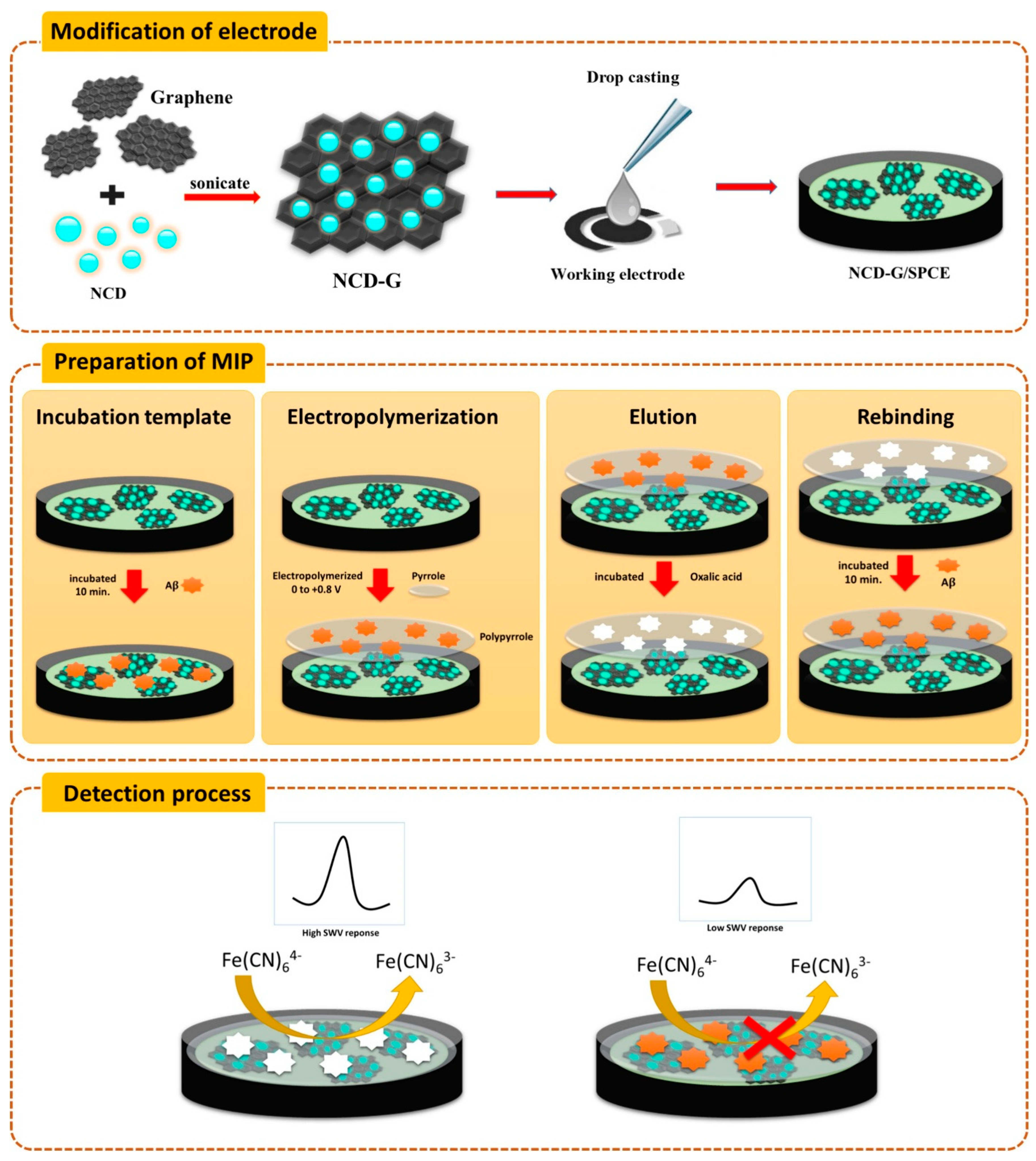
| Pakapongpan et al. (2024) [139] | SWV | MIP (PPY, Aβ42 template); Aβ42 rebinding blocks [Fe(CN)6]3−/4− redox probe (SWV peak decrease, ΔI); Platform: NCD–G nanohybrid-modified SPCE; PPY electropolymerized and film formation/template removal confirmed by CV; template removed with oxalic acid | 1 pg/mL | 5–70 pg/mL | Aβ42/artificial serum | No—Artificial serum (spiked): recovery 92.31–119.25%; repeatability RSD ≤ 5.44% (n = 3); reproducibility RSD 2.08% (n = 15 electrodes); selectivity vs. BNP, IgG, HSA; rebinding time 10 min (optimized) |
| Reference | Detection Method | Detection Principle/Platform | LOD | Linear Range | Target/Sample Type | Real Samples |
|---|---|---|---|---|---|---|
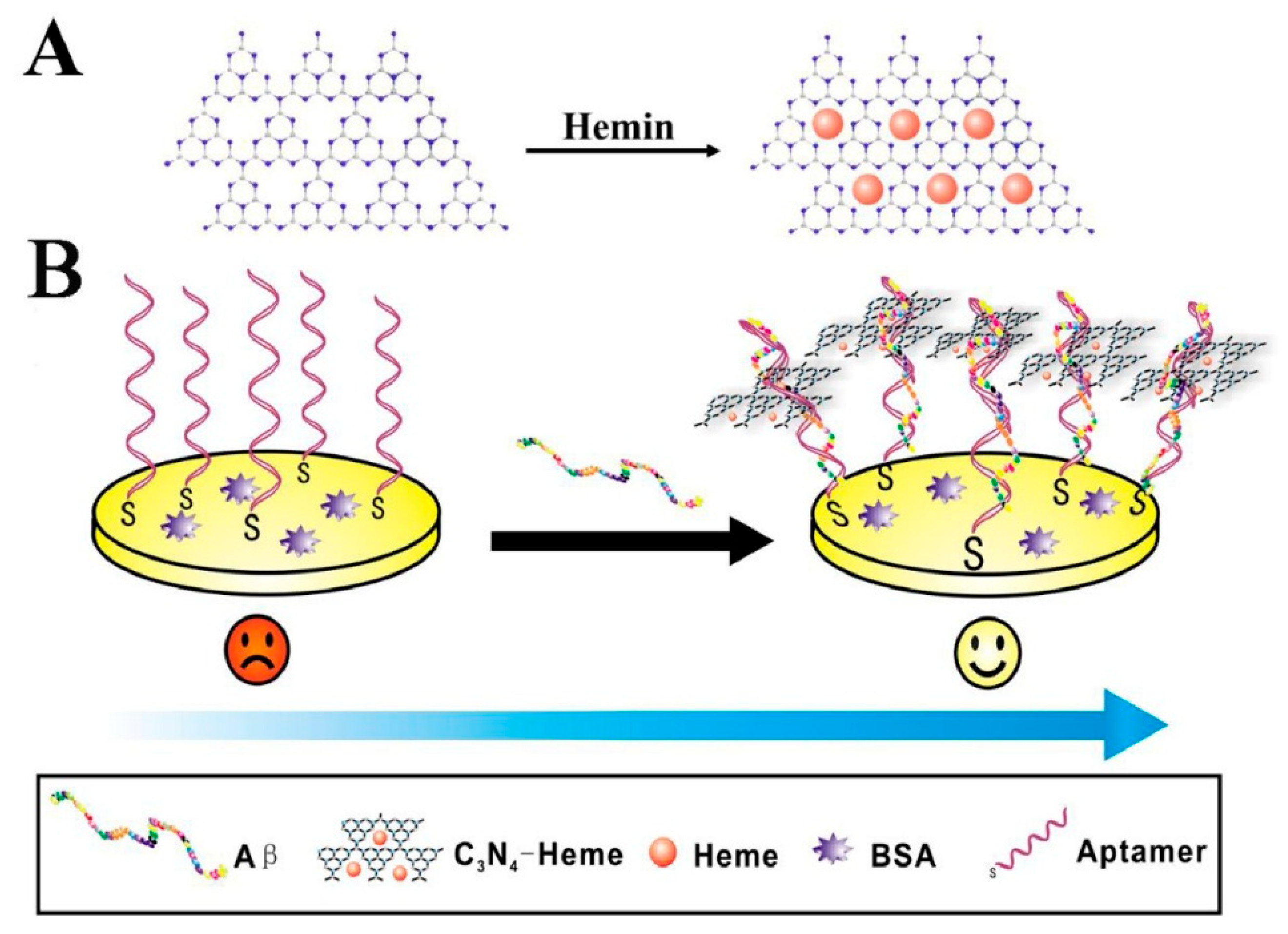
| Zhang et al. (2020) [140] | ECL | Aptamer (Aβ-targeting, thiolated); label-free signal-ON aptasensor; g-C3N4–heme nanocomposite as ECL luminophore; heme catalyzes in situ H2O2 production from Aβ–O2 interaction; K2S2O8 as co-reactant (dual catalytic ECL amplification); readout ΔIECL; Platform: GE with aptamer immobilized via Au–S; BSA blocking; followed by incubation with Aβ and g-C3N4–heme; CV/EIS used only for assembly confirmation | 3.25 fM | 10 fM–0.1 μM | Aβ40 monomer/Human serum (spiked) | Yes—human serum (healthy donors); standard-addition recovery 95.3–104.1%; reproducibility RSD 4.65% at 10 nM (n = 5 electrodes); selectivity vs. Aβ40 oligomers, Aβ40 fibrils, BSA, CEA, thrombin; stability: no significant loss over 200 s consecutive scans; incubation 12 h at 37 °C |
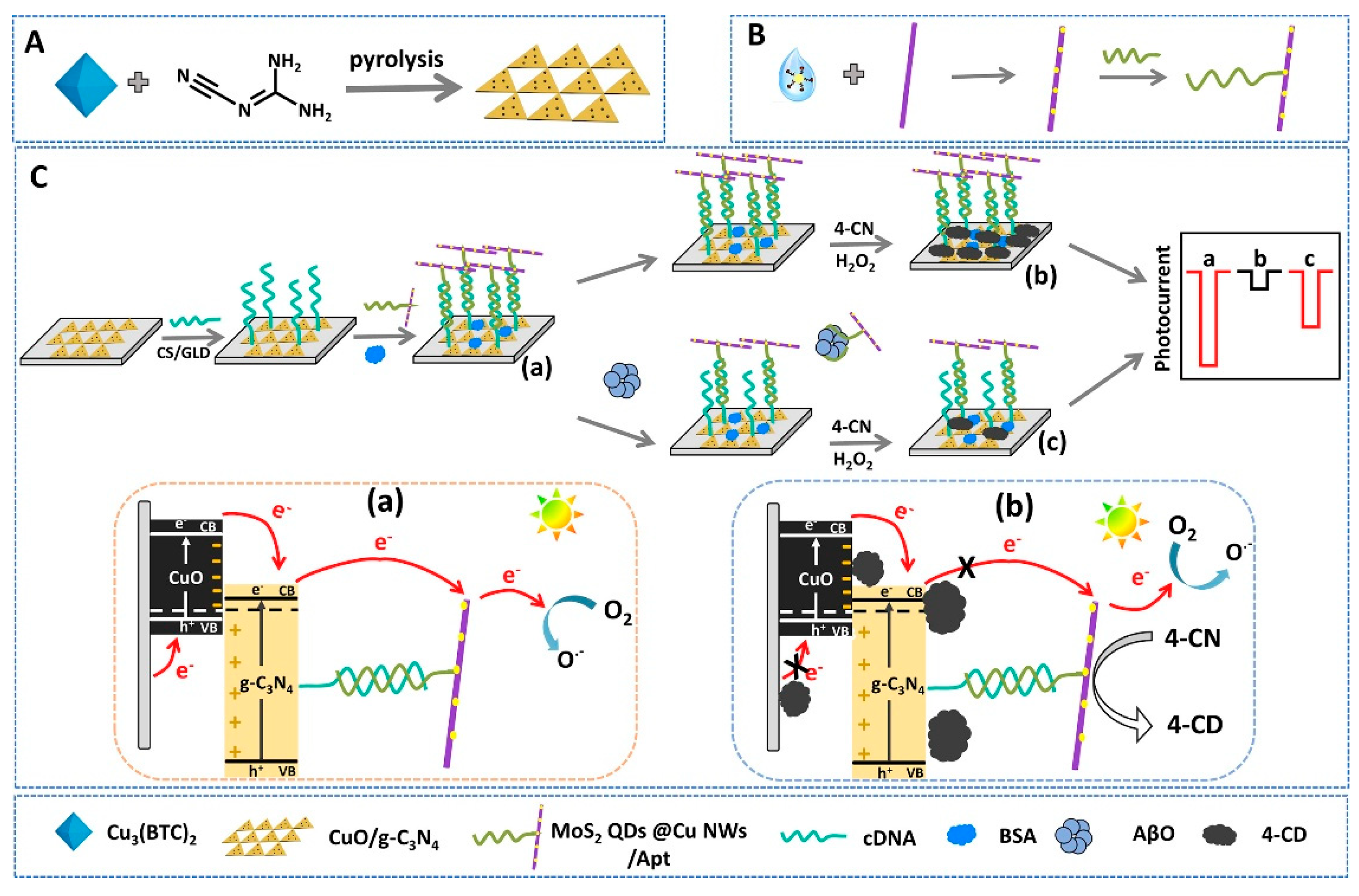
| Zhang et al. (2021) [141] | PEC (cathodic) | Aptamer; on–off–on cathodic PEC aptasensor; p–n heterojunction CuO/g-C3N4 photocathode with aptamer-labelled MoS2 QDs@Cu NWs as dual amplifier (PEC enhancer + nanozyme for 4-CN/H2O2 precipitation); photocurrent decrease via insulating 4-CD and recovery upon AβO binding; readout ΔIPEC; Platform: ITO with CuO/g-C3N4, cDNA, BSA, MoS2 QDs@Cu NWs–aptamer; EIS used only for assembly confirmation | 5.79 fM | 10 fM–0.5 μM | Aβ oligomers/Human serum (spiked, 50-fold dilution) | Yes—Human serum (two samples; standard addition); recovery 98.20–103.12%, RSD 3.31–3.49% (two serum samples); reproducibility RSD 3.35% (n = 6 sensors); selectivity vs. AβM, AβF, TNF-α, Lys, Ins (10×, negligible response); stability: 10 on/off cycles ≈101.13% of initial; long-term storage 14 days at 4 °C ≈ 91.69% signal retained |
| Li et al. (2025) [90] | PEC | Aptamer; signal-on PEC aptasensor; TiO2/Au-g-C3N4 heterojunction on FTO (TiO2 by EPD + annealing, Au–C3N4 drop-coated); AuNPs enhance conductivity, LSPR, and provide Au–S sites for aptamer immobilization; thiolated Aβ40 aptamer immobilized via Au–S, MCH blocking; photocurrent increase upon Aβ40 binding; readout ΔIPEC; no external redox probe used | 0.33 fg/mL | 10−15–10−11 g/mL | Aβ40/PBS, CSF, plasma, artificial saliva | Yes—Clinical CSF (n = 3) and plasma (n = 6) vs. SiMoA; reproducibility RSD 2.69% (n = 6 sensors); RSD ≤ 5.9% in patient samples; selectivity vs. tau, Aβ42, AA, glucose, urea, chitosan (1000×); stability: 9 days at RT, ≈ 6.9% signal loss |
| Reference | Detection Method | Detection Principle/Platform | LOD | Linear Range | Target/Sample Type | Real Samples |
|---|---|---|---|---|---|---|
| Pereira et al. (2020) [142] | SWV | MIP (template Aβ42; polymer OPDA); signal-off rebinding blocks [Fe(CN)6]3−/4−; readout ΔI; Platform: paper-based carbon-ink electrode (CI-HME) coated with PEDOT and ATP linker; OPDA electropolymerized with Aβ42; template removed by trypsin + oxalic acid; CV/EIS used only for assembly confirmation | 0.067 ng/mL | 0.1 ng/mL–1 μg/mL | Aβ42/PBS; serum (FBS, spiked) | Yes—serum (FBS, Cormay, spiked); recovery not reported; repeatability RSD < 10%; detection time 20 min; selectivity vs. BSA (4 mg/mL), glucose (0.7 mg/mL), creatinine (1 μg/mL); low-cost (~€0.03/sensor) |
| Liu et al. (2021) [143] | DPV | Aptamer; signal-off DPV with Fc redox probe; superhydrophobic CFP/AuPt nanoalloy boosts area and resists fouling; thiolated AβO aptamer self-assembled on AuPt; optional BSA blocking; readout ΔI (Fc); Platform: CFP/AuPt electrode with aptamer and BSA (optional); CV used only for characterization | 0.16 pg/mL | 0.5–10,000 pg/mL | AβO/PBS; Human serum (spiked) | Yes—human serum (spiked 1–1000 pg/mL); LOD 0.21 pg/mL (PBS with BSA), 0.90 pg/mL (serum); recovery 92.5–109% (n = 5), RSD < 10%; selectivity vs. Aβ40, Aβ42, Tau441, NFL, HSA (each 1 μg/mL, 1000× of AβO at 1 ng/mL); antifouling: ≈90% current retained after 168 h in serum; stability: sensor maintained performance for 60 days |
| Ren et al. (2023) [144] | DPV | Aptamer; nanoporous carbon from ZIF-8 (ZC-700) loaded with methylene blue and sealed by AβO aptamers; (π–π stacking, stimuli-responsive signal-ON); AβO binding opens gate, MB released and hybrid-captured on AuNP-modified GCE for amplification; readout ΔI; Platform: AuNP-coated GCE with thiolated capture probe (Au–S) + MCH blocking; ZC-700@MB/aptamer used as solution-phase nanocontainer; CV/EIS used only for assembly confirmation | 1.58 fM | 50 fM–10 nM | AβO/PBS; Human serum (spiked, 10× dilution) | Yes—human serum (standard addition, 10×); recovery 102.35–107.14%, RSD 1.54–3.55%; reproducibility RSD 3.42% (n = 5); selectivity vs. AβM, AβF, α-Syn, tau (10 nM interferents vs. 1 nM AβO, negligible); stability 8 days at 4 °C, signal retention ≈98.6–104.4% |
| Recognition Element | Advantages | Limitations | Biomarkers |
|---|---|---|---|
| Aptamers [90,122,123,124,125,130,132,134,137,138,140,141,143,144] | High specificity and selectivity; chemical stability; easy functionalization; effective in complex matrices | Multi-step immobilization required to preserve binding; limited long-term stability data in some cases | Aβ1–42 [124,132,134]; Aβ oligomers [122,123,125,137,138,141,143,144]; Aβ1–40 [90,125,132,140]; tau protein [130] |
| Antibodies [6,119,120,121,131,133,136] | Clinically validated specificity; high affinity; robust selectivity in serum/plasma | Orientation effects can reduce capture efficiency; stability under extended storage not always assessed | Aβ1–42 [6,119]; Aβ1–40 [6]; Aβ (unspecified) [120]; tau-441 [121,136]; t-tau [6]; p-tau181 [6,131]; Fetuin B [133] |
| MIPs [129,139,142] | Low cost; physical/chemical robustness; potential for reuse | Possible lower selectivity for closely related isoforms; insulating layers may hinder electron transfer | Aβ1–42 [139,142]; Aβ oligomers [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şak, B.; Sousa, H.B.A.; Prior, J.A.V. Carbon Nanomaterial-Based Electrochemical Biosensors for Alzheimer’s Disease Biomarkers: Progress, Challenges, and Future Perspectives. Biosensors 2025, 15, 684. https://doi.org/10.3390/bios15100684
Şak B, Sousa HBA, Prior JAV. Carbon Nanomaterial-Based Electrochemical Biosensors for Alzheimer’s Disease Biomarkers: Progress, Challenges, and Future Perspectives. Biosensors. 2025; 15(10):684. https://doi.org/10.3390/bios15100684
Chicago/Turabian StyleŞak, Berfin, Helena B. A. Sousa, and João A. V. Prior. 2025. "Carbon Nanomaterial-Based Electrochemical Biosensors for Alzheimer’s Disease Biomarkers: Progress, Challenges, and Future Perspectives" Biosensors 15, no. 10: 684. https://doi.org/10.3390/bios15100684
APA StyleŞak, B., Sousa, H. B. A., & Prior, J. A. V. (2025). Carbon Nanomaterial-Based Electrochemical Biosensors for Alzheimer’s Disease Biomarkers: Progress, Challenges, and Future Perspectives. Biosensors, 15(10), 684. https://doi.org/10.3390/bios15100684





