A Biofuel Cell for Electricity Generation from Biomass-Derived Cellobiose
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Enzymes and Solutions
2.2. ThCel6A Production and Purification
2.3. Enzymatic Characterization of ThCel6A
2.4. Enzymatic Hydrolysis of Insoluble Biomass
2.5. Bioanode and Biocathode Preparation
2.6. Electrochemical Characterization
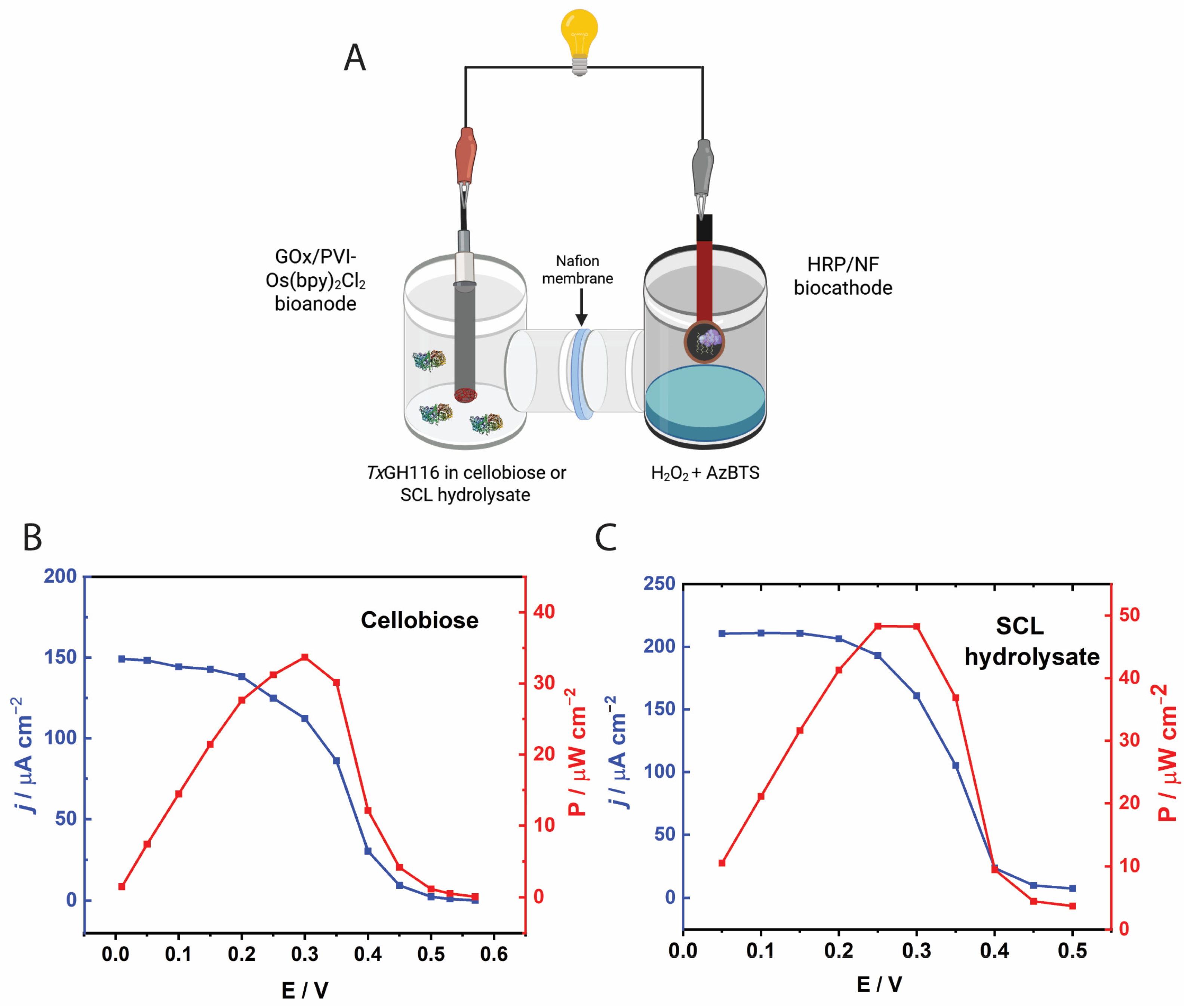
2.7. Biofuel Cell Performance
3. Results and Discussion
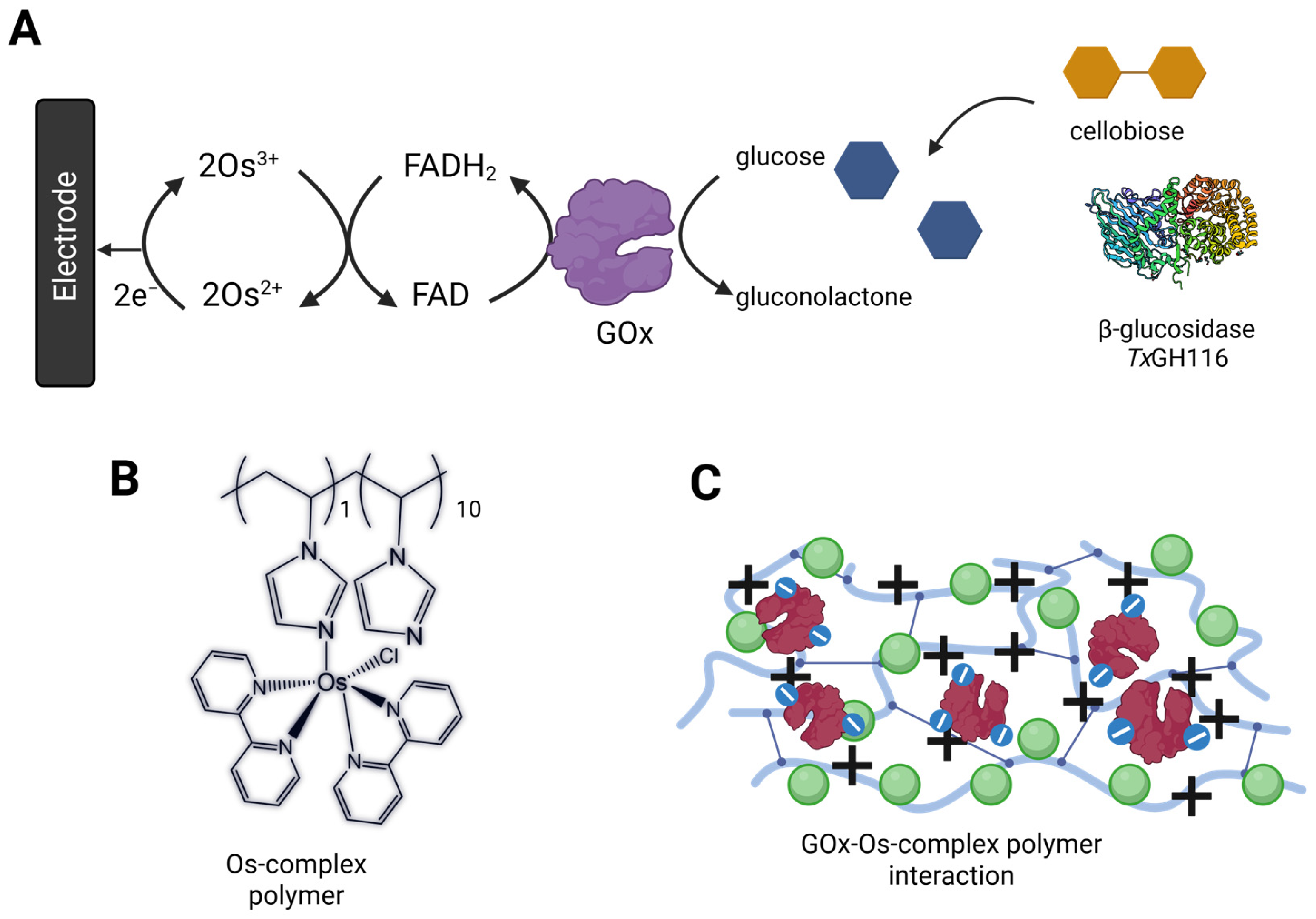
3.1. GOx-Modified Anode Characterization and Optimization
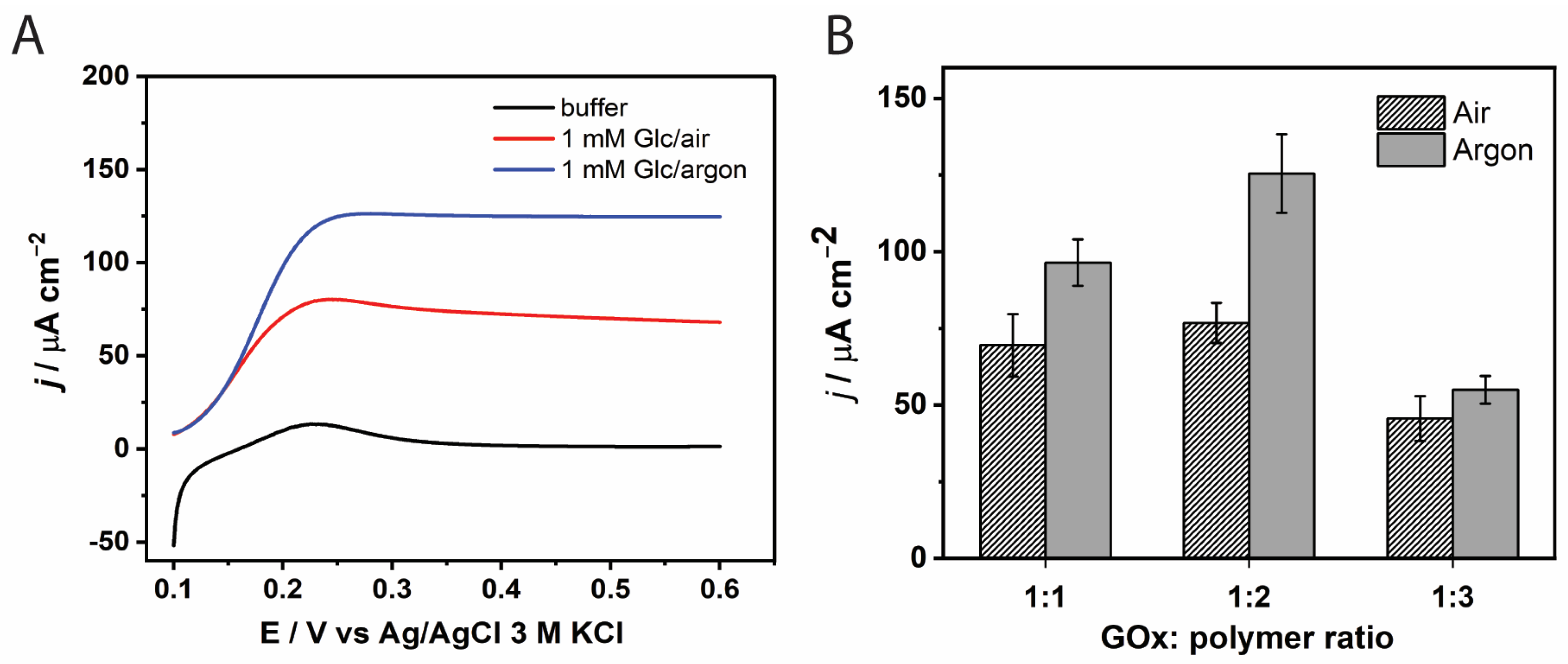
3.1.1. Optimization of GOx to Redox Polymer Mass Ratio
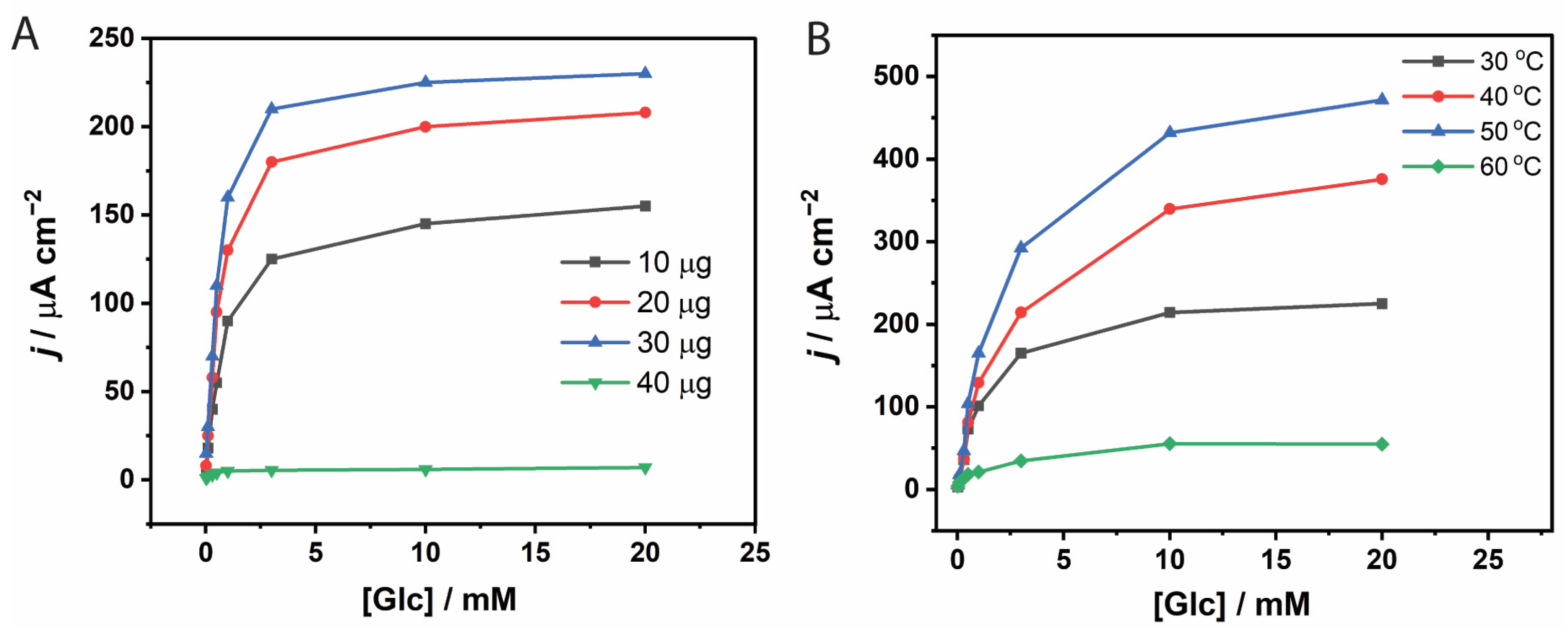
3.1.2. Effect of GOx Loading Amount
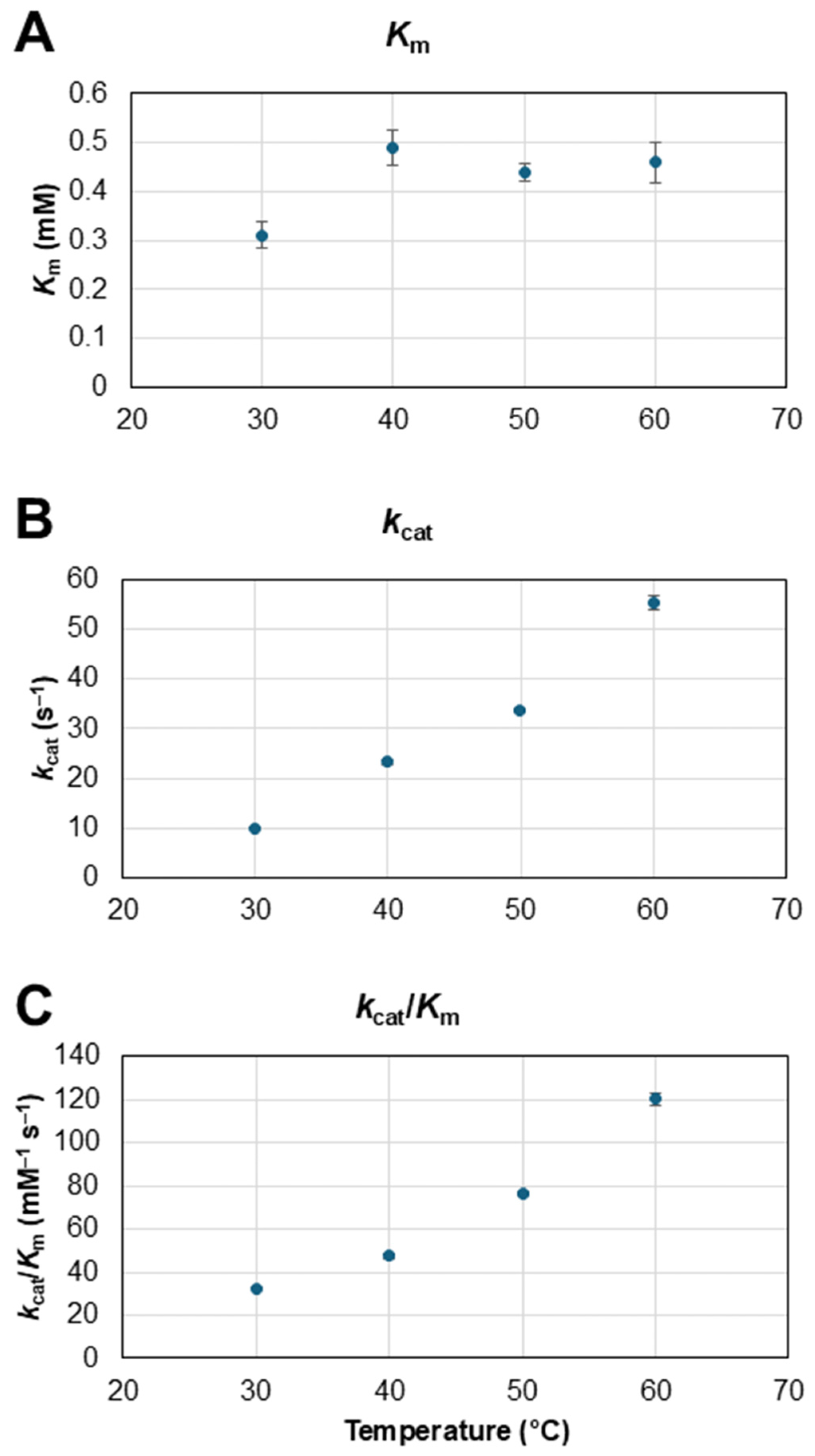
3.1.3. Effect of Temperature on Immobilized GOx Kinetics
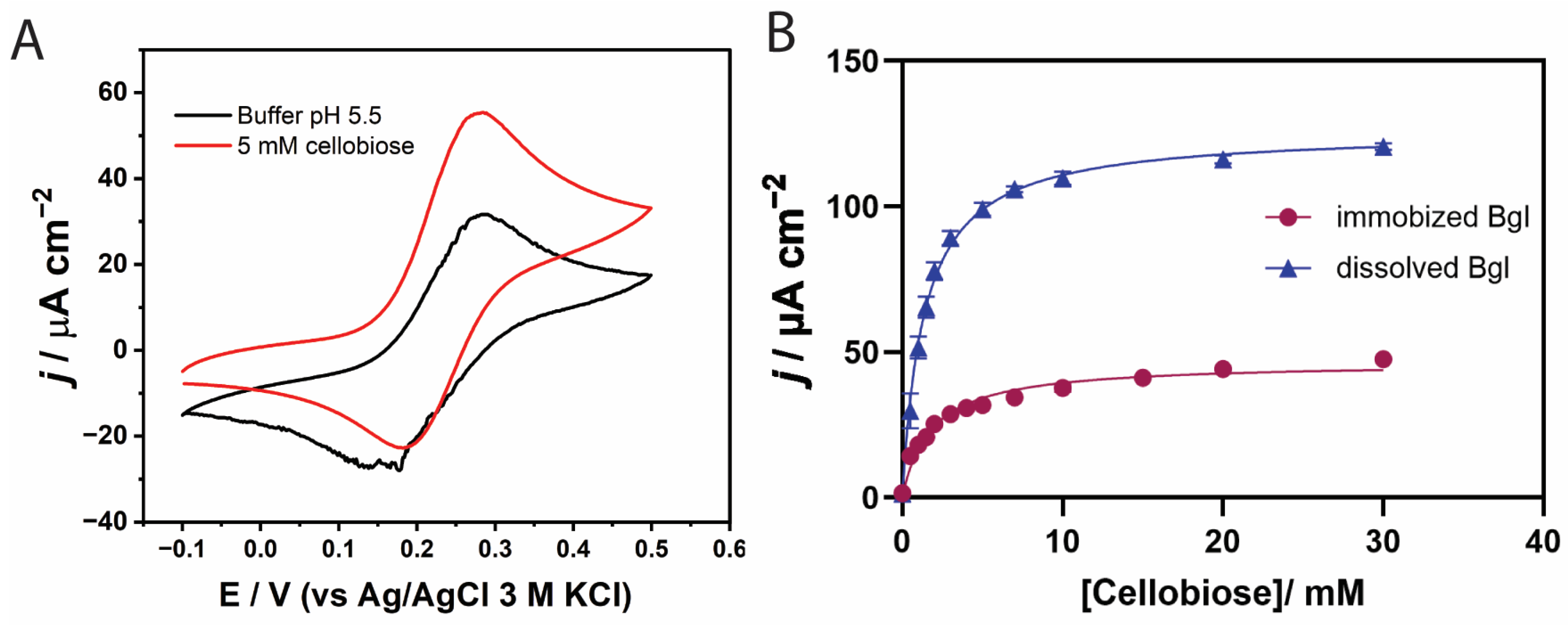
3.2. Effect of Temperature on TxGH116 β-Glucosidase
3.3. Evaluation of Bi-Enzymatic GOx/β-Glucosidase Catalysis
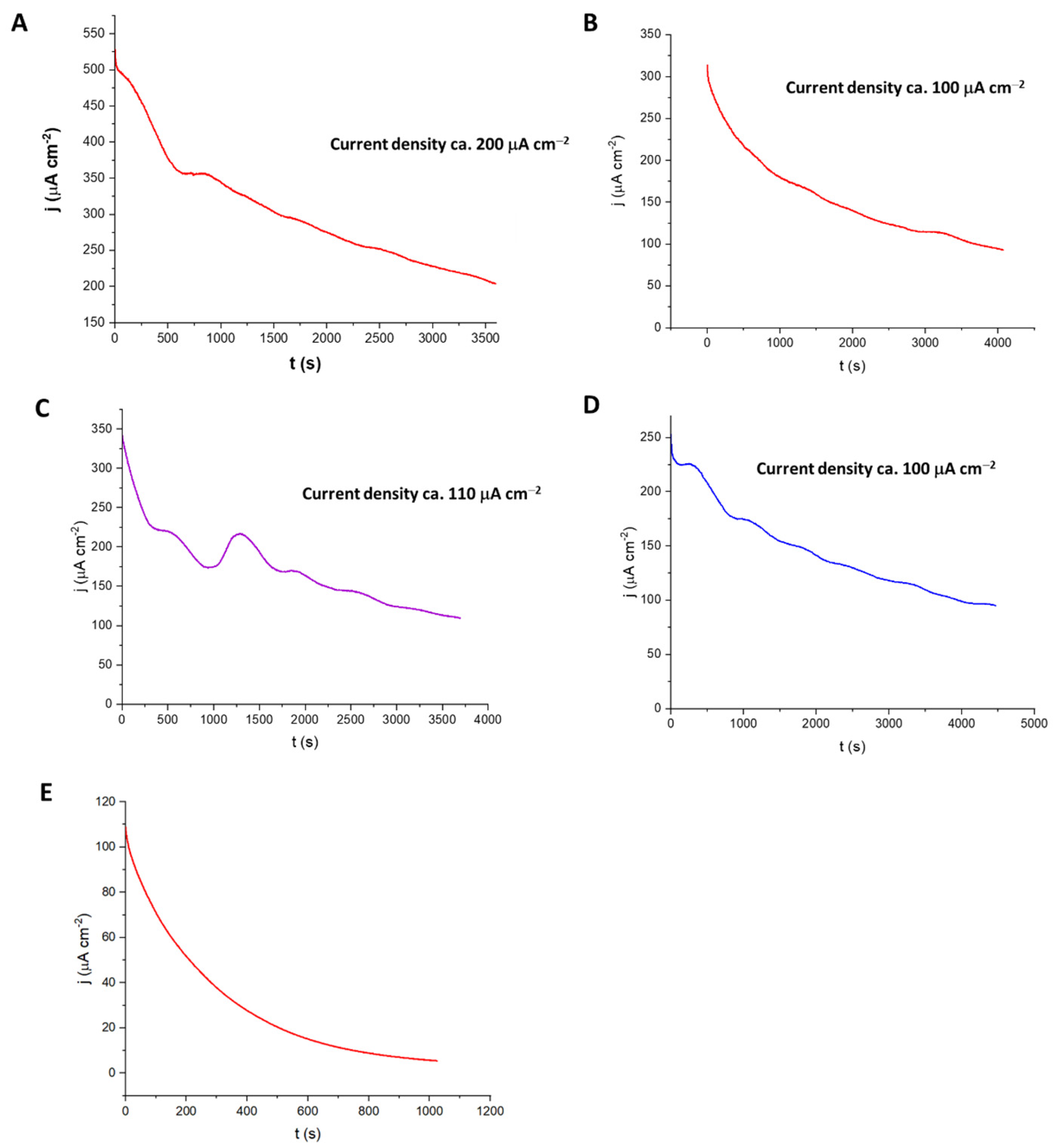
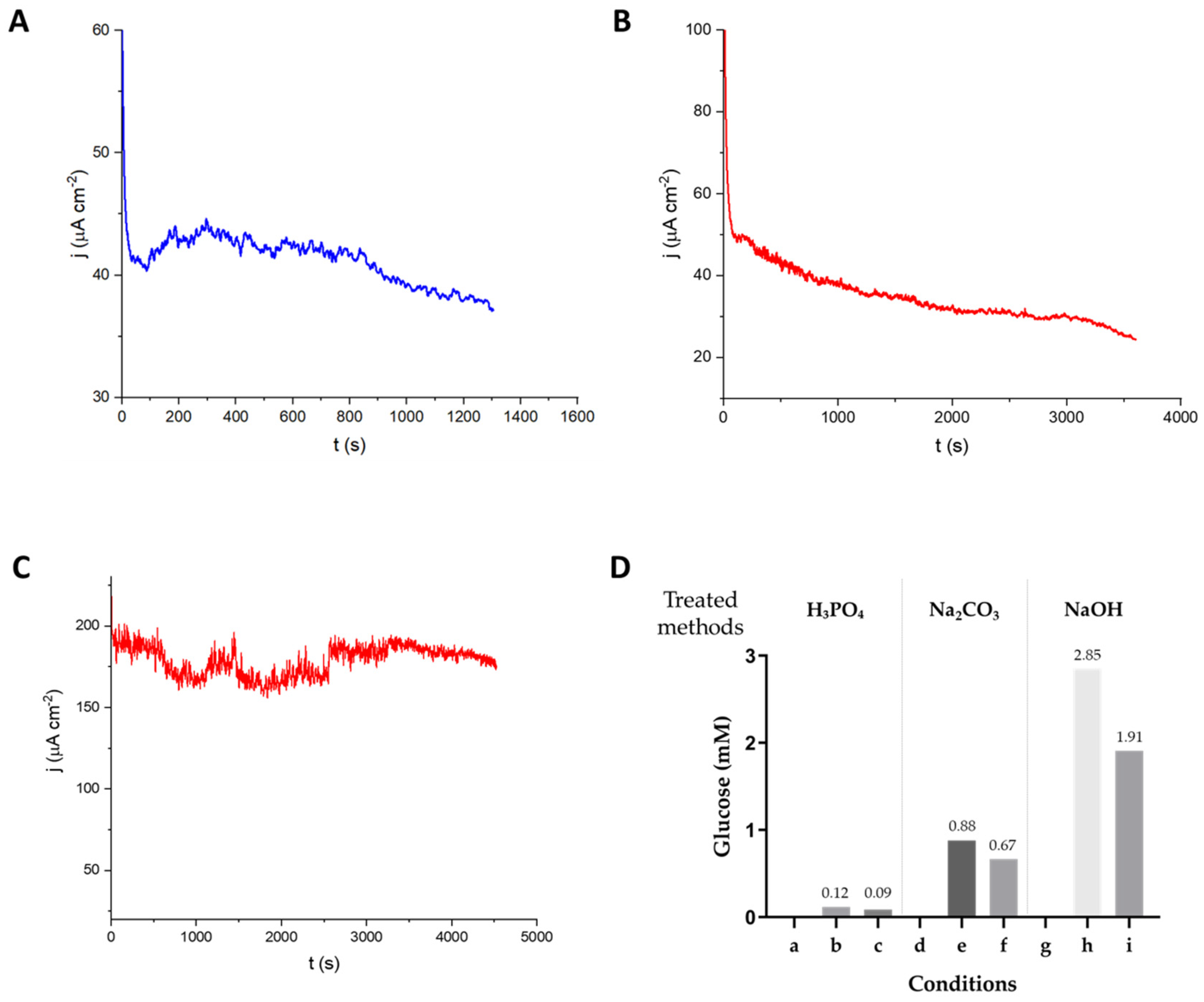
3.4. Current from Sugarcane Leaf Treated with Cellulase
3.5. Current from Sugarcane Leaf Treated with ThCel6A and TxGH116
3.6. BFC Performance with Cellobiose and SCL Hydrolysates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AzBTS | 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) ammonium salt |
| BFCs | Biofuel cells |
| EBFCs | Enzymatic biofuel cells |
| Bgl | β-glucosidase |
| CMC | Carboxymethyl cellulose |
| DNS | 3,5-dinitrosalicylic acid |
| GOx | Glucose oxidase |
| HRP | Horseradish peroxidase |
| MET | Mediated electron transfer |
| NF | Nafion |
| OCV | Open circuit voltage |
| PASC | Phosphoric Acid Swollen Cellulose |
| PGO | Peroxidase-Glucose Oxidase |
| SCL | Sugarcane leaf |
| SDS-PAGE | Sodium dodecyl sulfate-polyacryamide gel electrophoresis |
| TLC | Thin-layer chromatography |
References
- Xiao, X.; Xia, H.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the Challenges of Enzymatic (Bio)Fuel Cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.V.; Giroud, F.; Minteer, S.D. Improved Bioelectrocatalytic Oxidation of Sucrose in a Biofuel Cell with an Enzyme Cascade Assembled on a DNA Scaffold. J. Electrochem. Soc. 2014, 161, H930. [Google Scholar] [CrossRef]
- Tan, Z.; Cheng, H.; Chen, G.; Ju, F.; Fernández-Lucas, J.; Zdarta, J.; Jesionowski, T.; Bilal, M. Designing Multifunctional Biocatalytic Cascade System by Multi-Enzyme Co-Immobilization on Biopolymers and Nanostructured Materials. Int. J. Biol. Macromol. 2023, 227, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Megarity, C.F.; Weald, T.R.I.; Heath, R.S.; Turner, N.J.; Armstrong, F.A. A Nanoconfined Four-Enzyme Cascade Simultaneously Driven by Electrical and Chemical Energy, with Built-in Rapid, Confocal Recycling of NADP(H) and ATP. ACS Catal. 2022, 12, 8811–8821. [Google Scholar] [CrossRef]
- Bai, J.; Li, M.; Xing, F.; Wei, X.; Liu, J. Electrically Driven Biocatalysis for Sustainable CO2-to-Chemicals Transformation. ChemSusChem 2025, 18, e202500334. [Google Scholar] [CrossRef]
- Macazo, F.C.; Minteer, S.D. Enzyme Cascades in Biofuel Cells. Curr. Opin. Electrochem. 2017, 5, 114–120. [Google Scholar] [CrossRef]
- Huang, W.; Zulkifli, M.Y.B.; Chai, M.; Lin, R.; Wang, J.; Chen, Y.; Chen, V.; Hou, J. Recent Advances in Enzymatic Biofuel Cells Enabled by Innovative Materials and Techniques. Exploration 2023, 3, 20220145. [Google Scholar] [CrossRef]
- Shi, P.; Wu, R.; Wang, J.; Ma, C.; Li, Z.; Zhu, Z. Biomass Sugar-Powered Enzymatic Fuel Cells Based on a Synthetic Enzymatic Pathway. Bioelectrochemistry 2022, 144, 108008. [Google Scholar] [CrossRef]
- Yamamoto, K.; Matsumoto, T.; Shimada, S.; Tanaka, T.; Kondo, A. Starchy Biomass-Powered Enzymatic Biofuel Cell Based on Amylases and Glucose Oxidase Multi-Immobilized Bioanode. New Biotechnol. 2013, 30, 531–535. [Google Scholar] [CrossRef]
- Herzallh, N.S.; Cohen, Y.; Cohen, R.; Chmelnik, O.; Shoham, Y.; Yehezkeli, O. Cellulose to Electricity Conversion by an Enzymatic Biofuel Cell. Sustain. Energy Fuels 2021, 5, 4580–4586. [Google Scholar] [CrossRef]
- Rafighi, P.; Nordberg Karlsson, E.; Zubaida Gulshan Ara, K.; Pankratova, G.; Bollella, P.; Peterbauer, C.K.; Gorton, L. A Novel Membraneless β-Glucan/O2 Enzymatic Fuel Cell Based on β-Glucosidase (RmBgl3B)/Pyranose Dehydrogenase (AmPDH) Co-Immobilized onto Buckypaper Electrode. Bioelectrochemistry 2022, 148, 108254. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Nakagawa, W.; Mori, Y.; Azhari, S.; Méhes, G.; Nishina, Y.; Kawano, T.; Miyake, T. A Plant-Insertable Multi-Enzyme Biosensor for the Real-Time Monitoring of Stomatal Sucrose Uptake. Biosens. Bioelectron. 2025, 287, 117674. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Patil, R.; Dey, T. Electron Transfer-Driven Single and Multi-Enzyme Biofuel Cells for Self-Powering and Energy Bioscience. Nano Energy 2022, 96, 107074. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A.K.; Kumar, V. Catalytic Properties, Functional Attributes and Industrial Applications of β-Glucosidases. 3 Biotech 2015, 6, 3. [Google Scholar] [CrossRef]
- Zang, X.; Liu, M.; Fan, Y.; Xu, J.; Xu, X.; Li, H. The Structural and Functional Contributions of β-Glucosidase-Producing Microbial Communities to Cellulose Degradation in Composting. Biotechnol. Biofuels 2018, 11, 51. [Google Scholar] [CrossRef]
- Ketudat Cairns, J.R.; Esen, A. β-Glucosidases. Cell. Mol. Life Sci. 2010, 67, 3389–3405. [Google Scholar] [CrossRef]
- Withers, S.G.; Warren, R.A.J.; Street, I.P.; Rupitz, K.; Kempton, J.B.; Aebersold, R. Unequivocal Demonstration of the Involvement of a Glutamate Residue as a Nucleophile in the Mechanism of a Retaining Glycosidase. J. Am. Chem. Soc. 1990, 112, 5887–5889. [Google Scholar] [CrossRef]
- Teugjas, H.; Väljamäe, P. Selecting β-Glucosidases to Support Cellulases in Cellulose Saccharification. Biotechnol. Biofuels 2013, 6, 105. [Google Scholar] [CrossRef]
- Alves, L.d.F.; Meleiro, L.P.; Silva, R.N.; Westmann, C.A.; Guazzaroni, M.-E. Novel Ethanol- and 5-Hydroxymethyl Furfural-Stimulated β-Glucosidase Retrieved from a Brazilian Secondary Atlantic Forest Soil Metagenome. Front. Microbiol. 2018, 9, 2556. [Google Scholar] [CrossRef]
- Sørensen, A.; Lübeck, M.; Lübeck, P.S.; Ahring, B.K. Fungal Beta-Glucosidases: A Bottleneck in Industrial Use of Lignocellulosic Materials. Biomolecules 2013, 3, 612–631. [Google Scholar] [CrossRef]
- Kannan, P.; Shafreen, M.M.; Achudhan, A.B.; Gupta, A.; Saleena, L.M. A Review on Applications of β-Glucosidase in Food, Brewery, Pharmaceutical and Cosmetic Industries. Carbohydr. Res. 2023, 530, 108855. [Google Scholar] [CrossRef] [PubMed]
- Cota, J.; Corrêa, T.L.R.; Damásio, A.R.L.; Diogo, J.A.; Hoffmam, Z.B.; Garcia, W.; Oliveira, L.C.; Prade, R.A.; Squina, F.M. Comparative Analysis of Three Hyperthermophilic GH1 and GH3 Family Members with Industrial Potential. New Biotechnol. 2015, 32, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Rathour, R.; Jha, S.; Pandey, K.; Srivastava, M.; Thakur, V.K.; Sengar, R.S.; Gupta, V.K.; Mazumder, P.B.; Khan, A.F.; et al. Microbial Beta Glucosidase Enzymes: Recent Advances in Biomass Conversation for Biofuels Application. Biomolecules 2019, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.R.K.; Karunambigai, A.; Jeon, J.-S.; Svasti, J. Functions of Rice Beta-Glucosidases and Transglucosidases. ScienceAsia 2023, 49, 635–645. [Google Scholar] [CrossRef]
- Matsuzaki, C.; Hidaka, M.; Nakashima, Y.; Honda, Y.; Koyanagi, T.; Ishikawa, K.; Katoh, T.; Katayama, T.; Kumagai, H. A Thermostable and Highly Active Fungal GH3 β-Glucosidase Generated by Random and Saturation Mutagenesis. Proc. Jpn. Acad. Ser. B 2025, 101, 177–195. [Google Scholar] [CrossRef]
- Xia, W.; Bai, Y.; Shi, P. Improving the Substrate Affinity and Catalytic Efficiency of β-Glucosidase Bgl3A from Talaromyces Leycettanus JCM12802 by Rational Design. Biomolecules 2021, 11, 1882. [Google Scholar] [CrossRef]
- Goswami, S.; Das, S.; Datta, S. Understanding the Role of Residues around the Active Site Tunnel towards Generating a Glucose-Tolerant β-Glucosidase from Agrobacterium Tumefaciens 5A. Protein Eng. Des. Sel. 2017, 30, 523–530. [Google Scholar] [CrossRef]
- Charoenwattanasatien, R.; Pengthaisong, S.; Breen, I.; Mutoh, R.; Sansenya, S.; Hua, Y.; Tankrathok, A.; Wu, L.; Songsiriritthigul, C.; Tanaka, H.; et al. Bacterial β-Glucosidase Reveals the Structural and Functional Basis of Genetic Defects in Human Glucocerebrosidase 2 (GBA2). ACS Chem. Biol. 2016, 11, 1891–1900. [Google Scholar] [CrossRef]
- Huang, M.; Pengthaisong, S.; Charoenwattanasatien, R.; Thinkumrob, N.; Jitonnom, J.; Ketudat Cairns, J.R. Systematic Functional and Computational Analysis of Glucose-Binding Residues in Glycoside Hydrolase Family GH116. Catalysts 2022, 12, 343. [Google Scholar] [CrossRef]
- Pengthaisong, S.; Piniello, B.; Davies, G.J.; Rovira, C.; Ketudat Cairns, J.R. Reaction Mechanism of Glycoside Hydrolase Family 116 Utilizes Perpendicular Protonation. ACS Catal. 2023, 13, 5850–5863. [Google Scholar] [CrossRef]
- Gorantla, J.N.; Pengthaisong, S.; Choknud, S.; Kaewpuang, T.; Manyum, T.; Promarak, V.; Cairns, J.R.K. Gram Scale Production of 1-Azido-β-D-glucose via Enzyme Catalysis for the Synthesis of 1,2,3-Triazole-glucosides. RSC Adv. 2019, 9, 6211–6220. [Google Scholar] [CrossRef]
- Gorantla, J.N.; Maniganda, S.; Pengthaisong, S.; Ngiwsara, L.; Sawangareetrakul, P.; Chokchaisiri, S.; Kittakoop, P.; Svasti, J.; Ketudat Cairns, J.R. Chemoenzymatic and Protecting-Group-Free Synthesis of 1,4-Substituted 1,2,3-Triazole-α-d-glucosides with Potent Inhibitory Activity toward Lysosomal α-Glucosidase. ACS Omega 2021, 6, 25710–25719. [Google Scholar] [CrossRef]
- VandeZande, G.R.; Olvany, J.M.; Rutherford, J.L.; Rasmussen, M. Enzyme Immobilization and Mediation with Osmium Redox Polymers. In Enzyme Stabilization and Immobilization: Methods and Protocols; Minteer, S.D., Ed.; Springer: New York, NY, USA, 2017; pp. 165–179. ISBN 978-1-4939-6499-4. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Wood, T.M. Preparation of Crystalline, Amorphous, and Dyed Cellulase Substrates. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1988; Volume 160, pp. 19–25. ISBN 0076-6879. [Google Scholar]
- Zhang, J.; Zhang, J.; Lin, L.; Chen, T.; Zhang, J.; Liu, S.; Li, Z.; Ouyang, P. Dissolution of Microcrystalline Cellulose in Phosphoric Acid—Molecular Changes and Kinetics. Molecules 2009, 14, 5027–5041. [Google Scholar] [CrossRef] [PubMed]
- Gregg, B.A.; Heller, A. Redox Polymer Films Containing Enzymes. 2. Glucose Oxidase Containing Enzyme Electrodes. J. Phys. Chem. 1991, 95, 5976–5980. [Google Scholar] [CrossRef]
- Reiter, S.; Habermüller, K.; Schuhmann, W. A Reagentless Glucose Biosensor Based on Glucose Oxidase Entrapped into Osmium-Complex Modified Polypyrrole Films. Sens. Actuators B Chem. 2001, 79, 150–156. [Google Scholar] [CrossRef]
- Flexer, V.; Brun, N. Fundamentals of Enzymatic Electrochemical Systems. In Functional Electrodes for Enzymatic and Microbial Electrochemical Systems; World Scientific (Europe): London, UK, 2017; pp. 3–50. ISBN 978-1-78634-353-6. [Google Scholar]
- Rusling, J.F.; Wang, B.; Yun, S. Electrochemistry of Redox Enzymes. In Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 39–85. ISBN 978-0-470-75384-2. [Google Scholar]
- Bambhania, H.M.; Chakraborty, D.; Wen, H.; Barton, S.C. Impact of Oxygen on Glucose Oxidation Kinetics in a Redox Polymer Mediated Glucose Oxidase Electrode. J. Electrochem. Soc. 2017, 164, H232. [Google Scholar] [CrossRef]
- Osadebe, I.; Conghaile, P.Ó.; Kavanagh, P.; Leech, D. Glucose Oxidation by Osmium Redox Polymer Mediated Enzyme Electrodes Operating at Low Potential and in Oxygen, for Application to Enzymatic Fuel Cells. Electrochim. Acta 2015, 182, 320–326. [Google Scholar] [CrossRef]
- Contin, A.; Frasca, S.; Vivekananthan, J.; Leimkühler, S.; Wollenberger, U.; Plumeré, N.; Schuhmann, W. A pH Responsive Redox Hydrogel for Electrochemical Detection of Redox Silent Biocatalytic Processes. Control of Hydrogel Solvation. Electroanalysis 2015, 27, 938–944. [Google Scholar] [CrossRef]
- Mao, F.; Mano, N.; Heller, A. Long Tethers Binding Redox Centers to Polymer Backbones Enhance Electron Transport in Enzyme “Wiring” Hydrogels. J. Am. Chem. Soc. 2003, 125, 4951–4957. [Google Scholar] [CrossRef]
- Timur, S.; Yigzaw, Y.; Gorton, L. Electrical Wiring of Pyranose Oxidase with Osmium Redox Polymers. Sens. Actuators B Chem. 2006, 113, 684–691. [Google Scholar] [CrossRef]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose Oxidase—An Overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Figueiredo, C.; García-Ortega, A.; Mandal, T.; Lielpetere, A.; Cervantes, F.; Demurtas, D.; Magner, E.; Plou, F.J.; Schuhmann, W.; Leech, D.; et al. An Oxygen-Insensitive Amperometric Galactose Biosensor Based on Galactose Oxidase Co-Immobilized with an Os-Complex Modified Redox Polymer. Electrochim. Acta 2023, 472, 143438. [Google Scholar] [CrossRef]
- Yin, Y.-R.; Zhang, F.; Hu, Q.-W.; Xian, W.-D.; Hozzein, W.N.; Zhou, E.-M.; Ming, H.; Nie, G.-X.; Li, W.-J. Heterologous Expression and Characterization of a Novel Halotolerant, Thermostable, and Alkali-Stable GH6 Endoglucanase from Thermobifida Halotolerans. Biotechnol. Lett. 2015, 37, 857–862. [Google Scholar] [CrossRef]
- da Silva, A.S.; Espinheira, R.P.; Teixeira, R.S.S.; de Souza, M.F.; Ferreira-Leitão, V.; Bon, E.P.S. Constraints and Advances in High-Solids Enzymatic Hydrolysis of Lignocellulosic Biomass: A Critical Review. Biotechnol. Biofuels 2020, 13, 58. [Google Scholar] [CrossRef]
- Martin, S.A.; Akin, D.E. Effect of Phenolic Monomers on the Growth and Beta-Glucosidase Activity of Bacteroides Ruminicola and on the Carboxymethylcellulase, Beta-Glucosidase, and Xylanase Activities of Bacteroides Succinogenes. Appl. Environ. Microbiol. 1988, 54, 3019–3022. [Google Scholar] [CrossRef]
- Ashande, C.M.; Masunda, A.T.; Ngbolua, K.-N.; Kilembe, J.T.; Matondo, A.; Clément, I.L.; Benjamin, G.Z.; Emmanuel, L.M.; Tshibangu, D.S.T.; Tshilanda, D.D.; et al. Glucose Oxidase as a Model Enzyme for Antidiabetic Activity Evaluation of Medicinal Plants: In Vitro and in Silico Evidence. NRFHH 2022, 2, 265–273. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinyou, P.; Jatooratthawichot, P.; Sribrahma, L.; Pengthaisong, S.; Beagbandee, C.; Chansaenpak, K.; Blay, V.; Ketudat Cairns, J.R. A Biofuel Cell for Electricity Generation from Biomass-Derived Cellobiose. Biosensors 2025, 15, 674. https://doi.org/10.3390/bios15100674
Pinyou P, Jatooratthawichot P, Sribrahma L, Pengthaisong S, Beagbandee C, Chansaenpak K, Blay V, Ketudat Cairns JR. A Biofuel Cell for Electricity Generation from Biomass-Derived Cellobiose. Biosensors. 2025; 15(10):674. https://doi.org/10.3390/bios15100674
Chicago/Turabian StylePinyou, Piyanut, Peeranat Jatooratthawichot, Luciranon Sribrahma, Salila Pengthaisong, Chamaipon Beagbandee, Kantapat Chansaenpak, Vincent Blay, and James R. Ketudat Cairns. 2025. "A Biofuel Cell for Electricity Generation from Biomass-Derived Cellobiose" Biosensors 15, no. 10: 674. https://doi.org/10.3390/bios15100674
APA StylePinyou, P., Jatooratthawichot, P., Sribrahma, L., Pengthaisong, S., Beagbandee, C., Chansaenpak, K., Blay, V., & Ketudat Cairns, J. R. (2025). A Biofuel Cell for Electricity Generation from Biomass-Derived Cellobiose. Biosensors, 15(10), 674. https://doi.org/10.3390/bios15100674








