Rationally Designed Molecularly Imprinted Polymer Electrochemical Biosensor with Graphene Oxide Interface for Selective Detection of Matrix Metalloproteinase-8 (MMP-8)
Abstract
1. Introduction
2. Results and Discussion
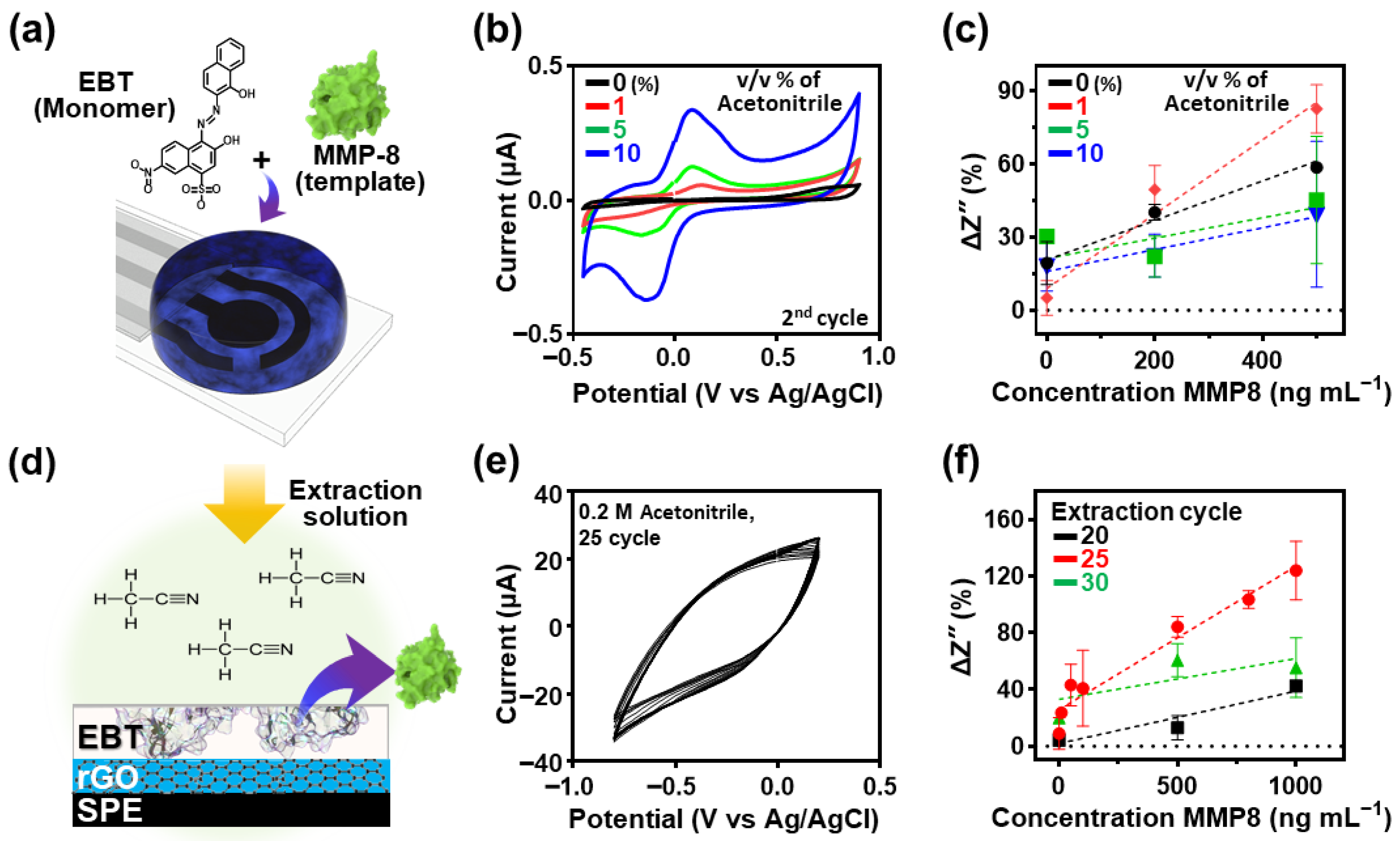
2.1. Fabrication of EBT/GO-Based MIP Electrodes Using Electropolymerization
2.2. Surface and Electrochemical Characteristics of rGO/SPCE and MIP/rGO/SPCE
2.3. Electrochemical Properties of PEBT/rGO/SPCE-Based MIP Electrodes
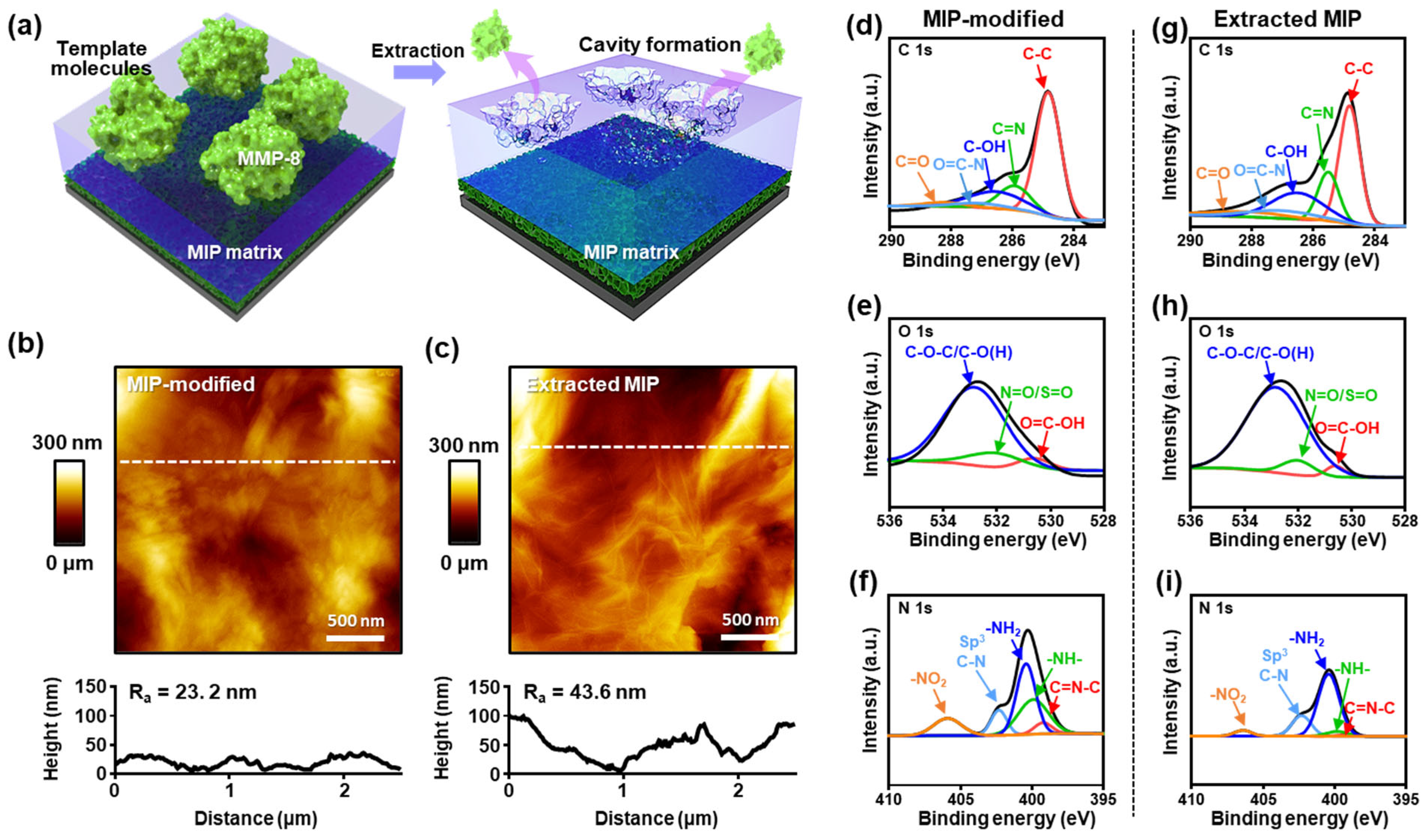
2.4. Surface Characterization of MIP and Extracted MIP Electrodes
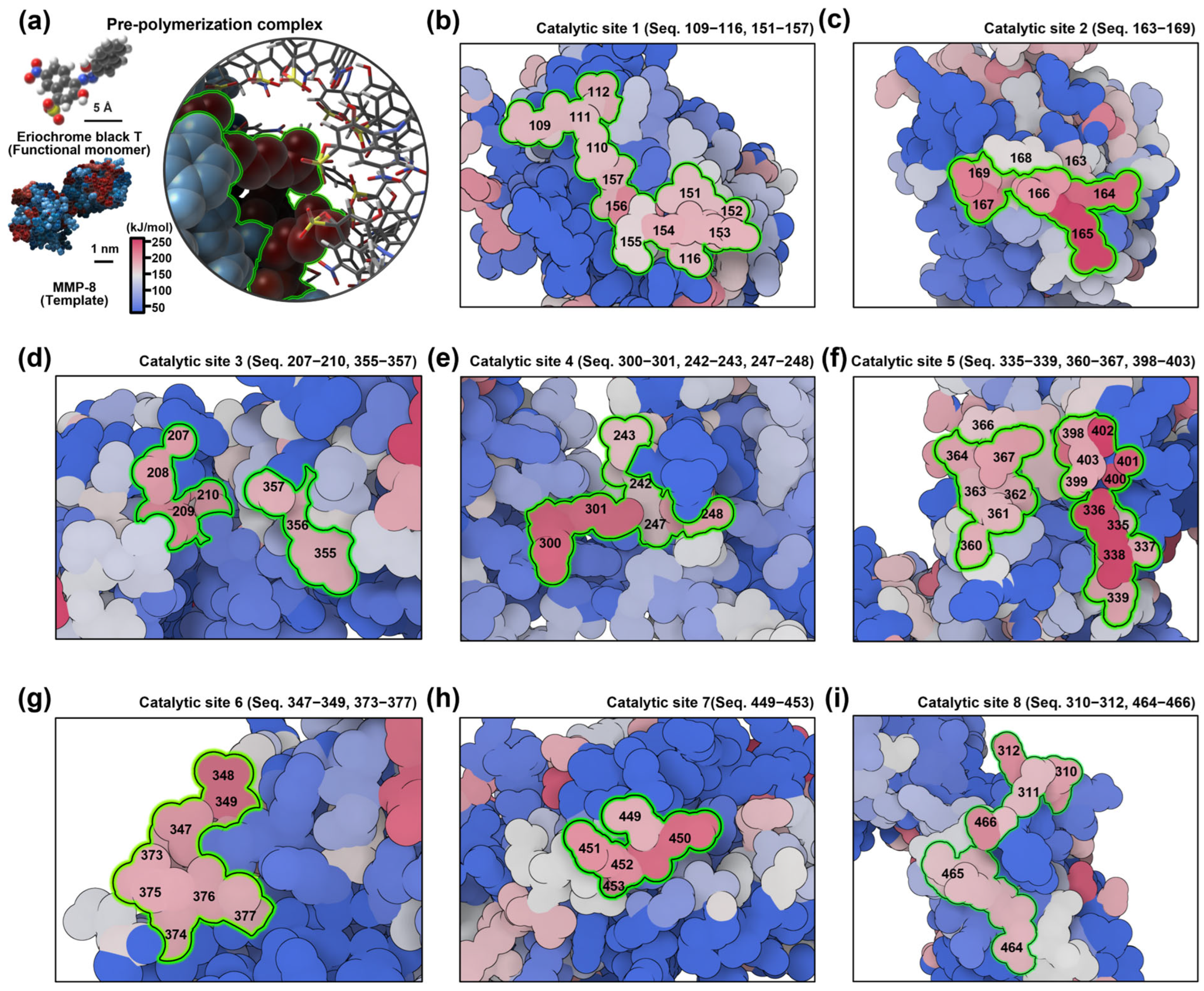
2.5. Computational Characterization of Binding Specificity Between EBT and MMP-8
2.6. Optimization of Fabrication Parameters for Enhanced Sensing
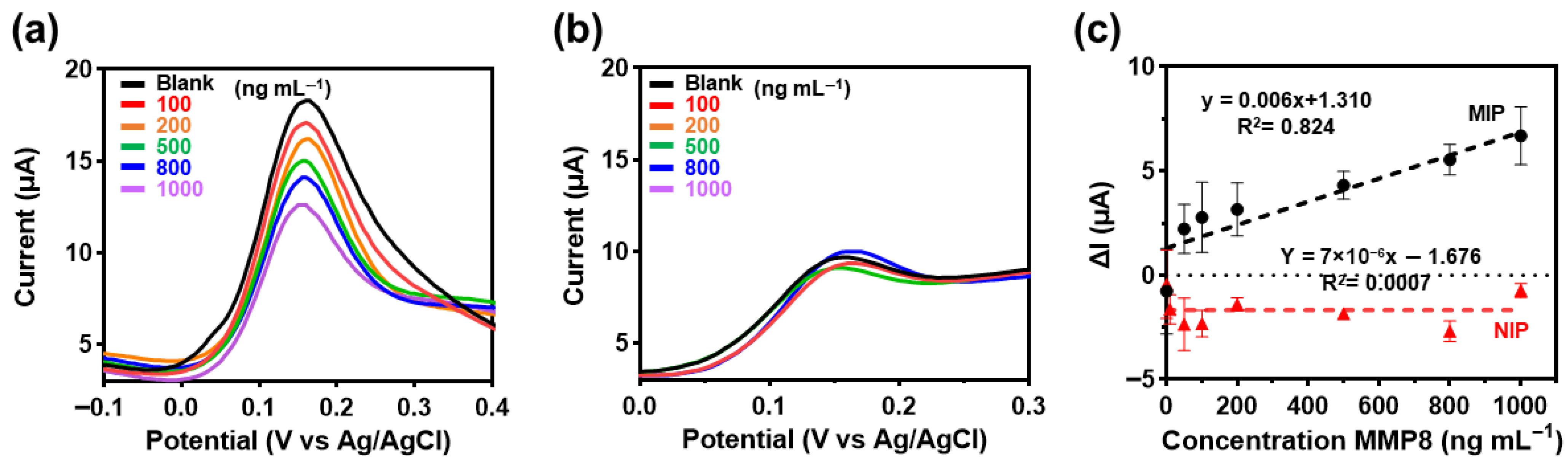
2.7. Electrochemical Sensing Performance Based on SWV Analytical Characteristics
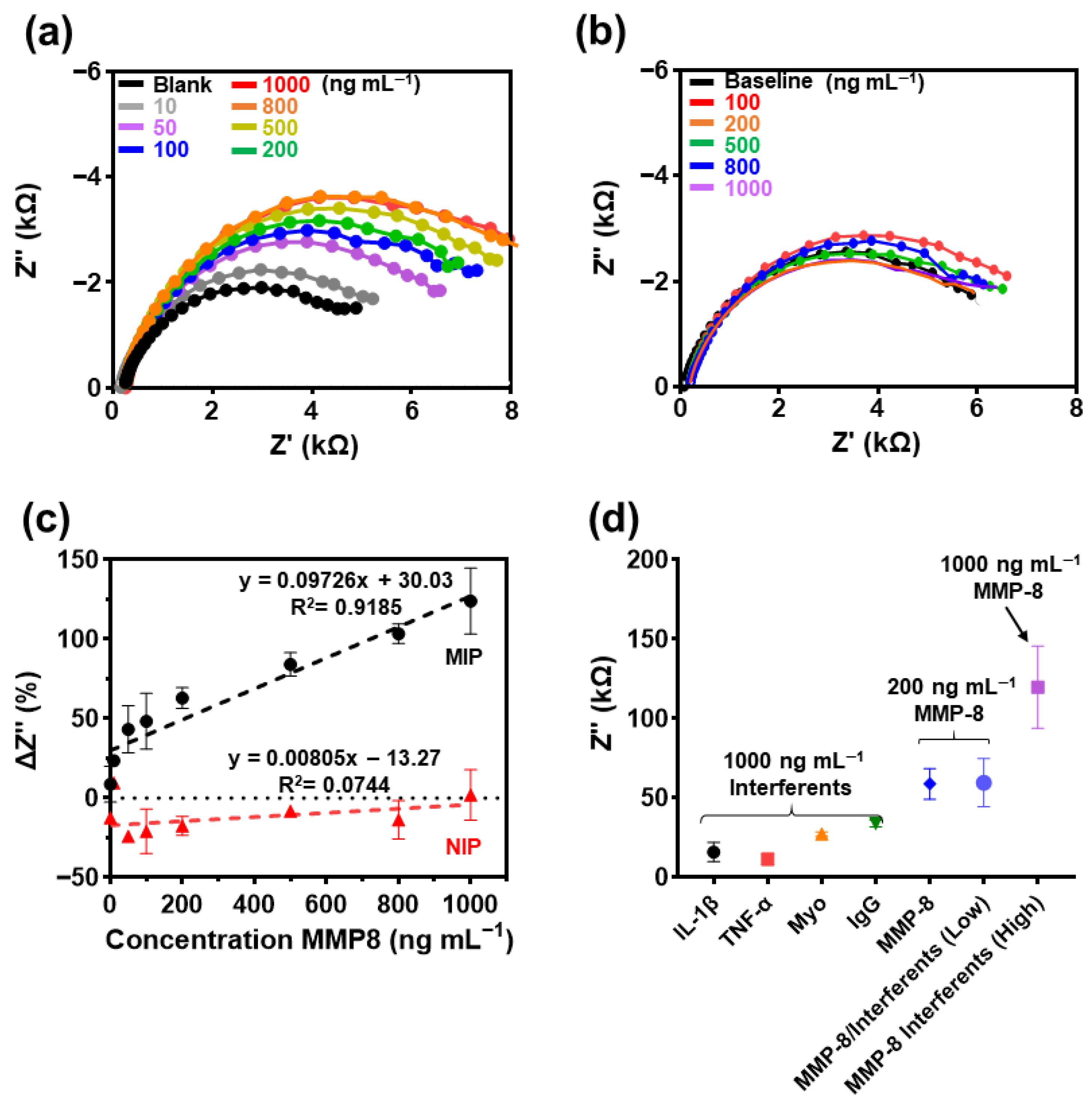
2.8. Impedimetric Analysis of MIP-Based Biosensor for MMP-8 Determination
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. MIP-Based Biosensor Fabrication
4.3. Surface Characterization
4.4. Characterization of the MIP Sensor Performance
4.5. Data Analysis and Statistics
4.6. Simulation of Molecular Electrostatic Potential
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Oral Disorders Collaborators; Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: A systematic analysis for the global burden of disease 2017 study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Hobbs, F.D.R.; Huck, O.; Hummers, E.; et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: Consensus report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European arm of the World Organization of Family Doctors (WONCA Europe). J. Clin. Periodontol. 2023, 50, 819–841. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S149–S161. [Google Scholar] [CrossRef]
- Griffith, A.; Chande, C.; Kulkarni, S.; Morel, J.; Cheng, Y.-H.; Shimizu, E.; Cugini, C.; Basuray, S.; Kumar, V. Point-of-Care Diagnostic Devices for Periodontitis—Current Trends and Urgent Need. Sens. Diagn. 2024, 3, 1003–1033. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Dong, T.; Pires, N.M.M.; Yang, Z.; Jiang, Z. Advances in Electrochemical Biosensors Based on Saliva for Disease-Signaling Protein Biomarkers. Biosensors 2022, 12, 93. [Google Scholar] [CrossRef]
- Domokos, Z.; Simon, F.; Uhrin, E.; Duda, S.; Fenyvesi, F.; Balogh, Z.; Németh, I.; Czumbel, L.M.; Varga, G.; Németh, O. Evaluating Salivary MMP-8 as a Biomarker for Periodontal Diseases: A Systematic Review and Meta-Analysis. Heliyon 2024, 10, e40402. [Google Scholar] [CrossRef]
- Umeizudike, K.A.; Lähteenmäki, H.; Räisänen, I.T.; Taylor, J.J.; Preshaw, P.M.; Bissett, S.M.; Tervahartiala, T.; Nwhator, S.O.; Pärnänen, P.; Sorsa, T. Ability of Matrix Metalloproteinase-8 Biosensor, IFMA, and ELISA to Differentiate between Periodontal Health, Gingivitis, and Periodontitis. J. Periodontal Res. 2022, 57, 558–567. [Google Scholar] [CrossRef]
- Rathnayake, N.; Gieselmann, D.-R.; Heikkinen, A.M.; Tervahartiala, T.; Sorsa, T. Salivary Diagnostics—Point-of-Care Diagnostics of MMP-8 in Dentistry and Medicine. Diagnostics 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Korgaonkar, J.; Tarman, A.Y.; Koydemir, H.C.; Chukkapalli, S.S. Periodontal Disease and Emerging Point-of-Care Technologies for Its Diagnosis. Lab Chip 2024, 24, 3326–3346. [Google Scholar] [CrossRef]
- Ramavath, C.; Katam, S.K.; Vardhelli, V.; Deshabhotla, S.; Oleti, T.P. Examining the Utility of Rapid Salivary C-Reactive Protein as a Predictor for Neonatal Sepsis: An Analytical Cross-Sectional Pilot Study. Diagnostics 2023, 13, 867. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Koyun, O.; Dilgin, Y. Molecularly imprinted polymer-based sensors for protein detection. Polymers 2023, 15, 629. [Google Scholar] [CrossRef]
- Park, R.; Jeon, S.; Jeong, J.; Park, S.Y.; Han, D.W.; Hong, S.W. Recent Advances of Point-of-Care Devices Integrated with Molecularly Imprinted Polymers-Based Biosensors: From Biomolecule Sensing Design to Intraoral Fluid Testing. Biosensors 2022, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Choi, D.Y.; Yang, J.C.; Hong, S.W.; Park, J. Molecularly imprinted polymer-based electrochemical impedimetric sensors on screen-printed carbon electrodes for the detection of trace cytokine IL-1β. Biosens. Bioelectron. 2022, 204, 114073. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, L.; Kong, Y.; Li, Y.; Wang, Q.; Wang, M.; Li, Y.; Davenport, A.; Li, B. Recent advances in molecularly imprinted polymer-based electrochemical sensors. Biosens. Bioelectron. 2024, 249, 116018. [Google Scholar] [CrossRef]
- Yarman, A.; Scheller, F.W. How Reliable Is the Electrochemical Readout of MIP Sensors? Sensors 2020, 20, 2677. [Google Scholar] [CrossRef] [PubMed]
- Erdőssy, J.; Horváth, V.; Yarman, A.; Scheller, F.W.; Gyurcsányi, R.E. Electrosynthesized Molecularly Imprinted Polymers for Protein Recognition. TrAC Trends Anal. Chem. 2016, 79, 179–190. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Suni, I.I. Substrate materials for biomolecular immobilization within electrochemical biosensors. Biosensors 2021, 11, 239. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Lai, G.; Yu, A.; Zhang, H. Disease diagnosis and application analysis of molecularly imprinted polymer biosensors based on saliva biomarkers. Talanta 2024, 269, 125394. [Google Scholar] [CrossRef]
- Ahmad, K.; Kim, H. Fabrication of Nitrogen-Doped Reduced Graphene Oxide Modified Screen-Printed Carbon Electrode (N-rGO/SPCE) as Hydrogen Peroxide Sensor. Nanomaterials 2022, 12, 2443. [Google Scholar] [CrossRef]
- Cardoso, A.R.; de Sá, M.H.; Sales, M.G.F. An Impedimetric Molecularly-Imprinted Biosensor for Interleukin-1β Determination, Prepared by In-Situ Electropolymerization on Carbon Screen-printed Electrodes. Bioelectrochemistry 2019, 130, 107287. [Google Scholar] [CrossRef] [PubMed]
- Ainla, A.; Mousavi, M.P.S.; Tsaloglou, M.N.; Redston, J.; Bell, J.G.; Fernandez-Abedul, M.T.; Whitesides, G.M. Open-Source Potentiostat for Wireless Electrochemical Detection with Smartphones. Anal. Chem. 2018, 90, 6240–6246. [Google Scholar] [CrossRef]
- Shen, F.; Ju, F.; Li, G.; Ma, L. Flexible and Highly Sensitive Electrochemical Sensor Based on PEDOT:PSS for Dopamine Detection. Sensors 2020, 20, 2781. [Google Scholar] [CrossRef]
- Su, W.-Y.; Wang, S.-M.; Cheng, S.-H. Electrochemically Pretreated Screen-Printed Carbon Electrodes for the Simultaneous Determination of Aminophenol Isomers. J. Electroanal. Chem. 2011, 651, 166–172. [Google Scholar] [CrossRef]
- Wei, W.; Guo, H.; Ye, Y.; Guo, X.; Guo, W.; Wang, E. Enhanced Electrochemical Performance at Screen-Printed Carbon Electrodes Following a Simple Pretreating Procedure. Anal. Chim. Acta 2007, 588, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of Graphene and Related Materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef]
- Guo, H.-L.; Wang, X.-F.; Qian, Q.-Y.; Wang, F.-B.; Xia, X.-H. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Marques, A.C.; Santos, L.; Carvalho, A.F.; Costa, F.M.; Martins, R.; Sales, M.G.F.; Fortunato, E. Molecularly-imprinted chloramphenicol sensor with laser-induced graphene electrodes. Biosens. Bioelectron. 2019, 124, 167–175. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, S.H.; Heo, G.; Heo, H.J.; Chae, S.Y.; Kwon, Y.W.; Lee, S.-K.; Han, D.-W.; Kim, H.-J.; Kim, Y.H.; et al. A Wearable Electrochemical Biosensor for Salivary Detection of Periodontal Inflammation Biomarkers: Molecularly Imprinted Polymer Sensor with Deep Learning Integration. Adv. Sci. 2025, e09658. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. Precise Tuning of Surface Composition and Electron-Transfer Properties of Graphene Oxide Films through Electroreduction. Chemistry 2013, 19, 4748–4753. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.A.; Bai, J.; Hong, W.; Bai, H. Electrochemically reduced graphene oxide: Preparation, composites, and applications. Carbon 2022, 191, 301–332. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Wilson, D.; Gonçalves, D.; Oliveira, O.N., Jr. Low-cost screen-printed electrodes based on electrochemically reduced graphene oxide–carbon black nanocomposites for dopamine, epinephrine and paracetamol detection. J. Colloid Interface Sci. 2018, 515, 101–108. [Google Scholar] [CrossRef]
- Lorenzetti, A.S.; Sierra, T.; Domini, C.E.; Lista, A.G.; Crevillén, A.G.; Escarpa, A. Electrochemically reduced graphene oxide-based screen-printed electrodes for total tetracycline determination by adsorptive transfer stripping differential pulse voltammetry. Sensors 2020, 20, 76. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Banks, C.E. Electroanalytical overview: Screen-printed electrochemical sensing platforms. Anal. Chim. Acta 2007, 597, 98–104. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Morgan, D.J. Comments on the XPS Analysis of Carbon Materials. C 2021, 7, 51. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene Oxide and Reduced Graphene Oxide Studied by the XRD, TEM and Electron Spectroscopy Methods. J. Electron. Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Vesel, A.; Junkar, I.; Mozetič, M. A Review of Strategies for the Synthesis of N-Doped Graphene-Like Materials. Nanomaterials 2020, 10, 2286. [Google Scholar] [CrossRef]
- Sandoval, S.; Tobias, G. Tuning the Nature of N-Based Groups From N-Containing Reduced Graphene Oxide: Enhanced Thermal Stability Using Post-Synthesis Treatments. Nanomaterials 2020, 10, 1451. [Google Scholar] [CrossRef]
- Yamada, Y.; Tanaka, H.; Kubo, S.; Sato, S. Unveiling bonding states and roles of edges in nitrogen-doped graphene nanoribbon by X-ray photoelectron spectroscopy. Carbon 2021, 185, 342–367. [Google Scholar] [CrossRef]
- Genorio, B.; Harrison, K.L.; Connell, J.G.; Dražić, G.; Zavadil, K.R.; Markovic, N.M.; Strmcnik, D. Tuning the Selectivity and Activity of Electrochemical Interfaces with Defective Graphene Oxide and Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2019, 11, 34517–34525. [Google Scholar] [CrossRef]
- Yao, H.; Sun, Y.; Lin, X.; Tang, Y.; Huang, L. Electrochemical Characterization of Poly(Eriochrome Black T) Modified Glassy-Carbon Electrode and Its Application to Simultaneous Determination of Dopamine, Ascorbic Acid and Uric Acid. Electrochim. Acta 2007, 52, 6165–6171. [Google Scholar] [CrossRef]
- Ondráčková, A.; Stiborová, M.; Dračínská, H.; Havran, L.; Schwarzová-Pecková, K.; Fojta, M. A Study on Redox Reactions of the Azo Dye Sudan I and Its Hydroxylated Metabolites on Pyrolytic Graphite and Boron-Doped Diamond Electrodes to Support Electrochemical Studies of Metabolic Transformations. Electrochim. Acta 2023, 468, 143162. [Google Scholar] [CrossRef]
- Hezarkhani, M.; Ghadari, R. Exploration of the Binding Properties of the Azo Dye Pollutants with Nitrogen-Doped Graphene Oxide by Computational Modeling for Wastewater Treatment Improvement. ChemistrySelect 2019, 4, 5968–5978. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.-X.; Chen, Y.-G.; Yang, L.-J.; Yang, X.-D.; Ye, J.-Y.; Xia, H.-P.; Zhou, Z.-Y.; Sun, S.-G. Identifying the Active Site of N-Doped Graphene for Oxygen Reduction by Selective Chemical Modification. ACS Energy Lett. 2018, 3, 986–991. [Google Scholar] [CrossRef]
- Silva, R.M.; da Silva, A.D.; Camargo, J.R.; de Castro, B.S.; Meireles, L.M.; Silva, P.S.; Janegitz, B.C.; Silva, T.A. Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications. Biosensors 2023, 13, 453. [Google Scholar] [CrossRef]
- Lasia, A. Impedance of the Faradaic Reactions in the Presence of Mass Transfer. In Electrochemical Impedance Spectroscopy and Its Applications; Lasia, A., Ed.; Springer: New York, NY, USA, 2014; pp. 85–125. [Google Scholar]
- Verheyen, E.; Schillemans, J.P.; van Wijk, M.; Demeniex, M.-A.; Hennink, W.E.; van Nostrum, C.F. Challenges for the effective molecular imprinting of proteins. Biomaterials 2011, 32, 3008–3020. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Chianella, I.; Larcombe, L.; Piletsky, S.A.; Noble, J.; Porter, R.; Horgan, A. The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem. Soc. Rev. 2011, 40, 1547–1571. [Google Scholar] [CrossRef]
- Bossi, A.; Bonini, F.; Turner, A.P.F.; Piletsky, S.A. Molecularly imprinted polymers for the recognition of proteins: The state of the art. Biosens. Bioelectron. 2007, 22, 1131–1137. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Tricase, A.; Marchianò, V.; Macchia, E.; Ditaranto, N.; Torsi, L.; Bollella, P. Ultrasensitive and Highly Selective o-Phenylenediamine Molecularly Imprinted Polymer for the Detection of 2,4-Dichlorophenoxyacetic Acid. Electrochim. Acta 2024, 494, 144430. [Google Scholar] [CrossRef]
- Feier, B.; Blidar, A.; Pusta, A.; Carciuc, P.; Cristea, C. Electrochemical sensor based on molecularly imprinted polymer for the detection of cefalexin. Biosensors 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Jeon, S.; Lee, J.W.; Jeong, J.; Kwon, Y.W.; Kim, S.H.; Jang, J.; Han, D.-W.; Hong, S.W. Mobile Point-of-Care Device Using Molecularly Imprinted Polymer-Based Chemosensors Targeting Interleukin-1β Biomarker. Biosensors 2023, 13, 1013. [Google Scholar] [CrossRef]
- Nicholls, I.A.; Chianella, I.; Ekberg, B.; Karlsson, B.C.G.; Olsson, G.D.; Suriyanarayanan, S.; Wiklander, J.G.; Wulff, G.; Ye, L. The use of computational methods for the development of molecularly imprinted materials for the recognition of proteins. Polymers 2021, 13, 2841. [Google Scholar] [CrossRef] [PubMed]
- Suryana, S.; Ibrahim, S.; Baharum, A.; Nasir, N.A.M.; Hashim, U.; Ahmad, S.A.A. An Update on Molecularly Imprinted Polymer Design: A Guide to Efficient Computational Methods. Molecules 2021, 26, 1891. [Google Scholar] [CrossRef]
- Rajpal, S.; Mishra, P.; Mizaikoff, B. Rational In Silico Design of Molecularly Imprinted Polymers: Current Challenges and Future Potential. Int. J. Mol. Sci. 2023, 24, 6785. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing Structure Coverage for over 214 Million Protein Sequences. Nucleic Acids Res. 2024, 52, D368–D375. [Google Scholar] [CrossRef] [PubMed]
- Reinemer, P.; Grams, F.; Huber, R.; Kleine, T.; Schnierer, S.; Pieper, M.; Tschesche, H.; Bode, W. Structural Implications for the Role of the N Terminus in the “Superactivation” of Collagenases: A Crystallographic Study. FEBS Lett. 1994, 338, 227–233. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Gimeno, A.; Beltrán-Debón, R.; Mulero, M.; Pujadas, G.; Garcia-Vallvé, S. Understanding the Variability of the S1’ Pocket to Improve Matrix Metalloproteinase Inhibitor Selectivity Profiles. Drug Discov. Today 2020, 25, 38–57. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Maskos, K.; Bode, W. Structural basis of the matrix metalloproteinases and their physiological inhibitors, the tissue inhibitors of metalloproteinases. Biol. Chem. 2003, 384, 863–872. [Google Scholar] [CrossRef]
- Murdaya, I.W.; Handayani, W.; Djojo, M.T.; Hartati, Y.W. Using Multiple Templates for Molecular Imprinted Polymer—A Review. Polymers 2022, 14, 4441. [Google Scholar] [CrossRef]
- Jadreško, D.; Lovrić, M. A Theory of Square-Wave Voltammetry of Surface-Active, Electroinactive Compounds. Electrochim. Acta 2008, 53, 8045–8050. [Google Scholar] [CrossRef]
- Yarman, A.; Kurbanoglu, S.; Zebger, I.; Scheller, F.W. Simple and robust: The claims of protein sensing by molecularly imprinted polymers. Sens. Actuators B Chem. 2021, 330, 129369. [Google Scholar] [CrossRef]
- Gulaboski, R.; Mirceski, V. Calculating of Square-Wave Voltammograms—A Practical On-Line Simulation Platform. J. Solid State Electrochem. 2024, 28, 1121–1130. [Google Scholar] [CrossRef]
- Kim, K.H.; Ahn, G.H.; Seol, Y.J.; Park, S.Y. Establishing cut-off values for salivary MMP-8 and IL-1β in the diagnosis of active periodontal disease: A preliminary cohort study toward the development of a diagnostic kit. J. Periodontal Implant Sci. 2025. [Google Scholar] [CrossRef]
- Ali, B.J.; Hassan, B.K.; Mahmood, A.A. The assessment of MMP-8 among different stages of periodontitis in the Iraqi population. J. Emerg. Med. Trauma Acute Care 2023, 2023, 5. [Google Scholar] [CrossRef]
- Becskereki, G.; Horvai, G.; Tóth, B. The Selectivity of Molecularly Imprinted Polymers. Polymers 2021, 13, 1781. [Google Scholar] [CrossRef]
- Currie, L.A. Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]
- Tortolini, C.; Gigli, V.; Angeloni, A.; Tasca, F.; Thanh, N.T.K.; Antiochia, R. A Disposable Immunosensor for the Detection of Salivary MMP-8 as Biomarker of Periodontitis. Bioelectrochemistry 2024, 156, 108590. [Google Scholar] [CrossRef] [PubMed]
- Lowpradit, P.; Janmanee, R.; Tansriratanawong, K. Performance Validation of Fabricated Nanomaterial-Based Biosensor for Matrix Metalloproteinase-8 Protein Detection. Eur. J. Dent. 2025. [Google Scholar] [CrossRef]
- Zhang, W.; Du, J.; Wang, K.; Li, Y.; Chen, C.; Yang, L.; Kan, Z.; Dong, B.; Wang, L.; Xu, L. Integrated Dual-Channel Electrochemical Immunosensor for Early Diagnosis and Monitoring of Periodontitis by Detecting Multiple Biomarkers in Saliva. Anal. Chim. Acta 2023, 1247, 340878. [Google Scholar] [CrossRef] [PubMed]
- Guida, L.; Bencivenga, D.; Annunziata, M.; Arcadio, F.; Borriello, A.; Della Ragione, F.; Formisano, A.; Piccirillo, A.; Zeni, L.; Cennamo, N. An Optical Fiber-Based Point-of-Care Test for Periodontal MMP-8 Detection: A Proof of Concept. J. Dent. 2023, 134, 104553. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.W.; Park, R.; Jeon, S.; Kim, S.H.; Kwon, Y.W.; Han, D.-W.; Hong, S.W. Rationally Designed Molecularly Imprinted Polymer Electrochemical Biosensor with Graphene Oxide Interface for Selective Detection of Matrix Metalloproteinase-8 (MMP-8). Biosensors 2025, 15, 671. https://doi.org/10.3390/bios15100671
Lee JW, Park R, Jeon S, Kim SH, Kwon YW, Han D-W, Hong SW. Rationally Designed Molecularly Imprinted Polymer Electrochemical Biosensor with Graphene Oxide Interface for Selective Detection of Matrix Metalloproteinase-8 (MMP-8). Biosensors. 2025; 15(10):671. https://doi.org/10.3390/bios15100671
Chicago/Turabian StyleLee, Jae Won, Rowoon Park, Sangheon Jeon, Sung Hyun Kim, Young Woo Kwon, Dong-Wook Han, and Suck Won Hong. 2025. "Rationally Designed Molecularly Imprinted Polymer Electrochemical Biosensor with Graphene Oxide Interface for Selective Detection of Matrix Metalloproteinase-8 (MMP-8)" Biosensors 15, no. 10: 671. https://doi.org/10.3390/bios15100671
APA StyleLee, J. W., Park, R., Jeon, S., Kim, S. H., Kwon, Y. W., Han, D.-W., & Hong, S. W. (2025). Rationally Designed Molecularly Imprinted Polymer Electrochemical Biosensor with Graphene Oxide Interface for Selective Detection of Matrix Metalloproteinase-8 (MMP-8). Biosensors, 15(10), 671. https://doi.org/10.3390/bios15100671






