Core–Shell PEDOT-PVDF Nanofiber-Based Ammonia Gas Sensor with Robust Humidity Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of CSNF Sensors
2.3. Characterization and Testing of CSNF Sensors
3. Results and Discussion
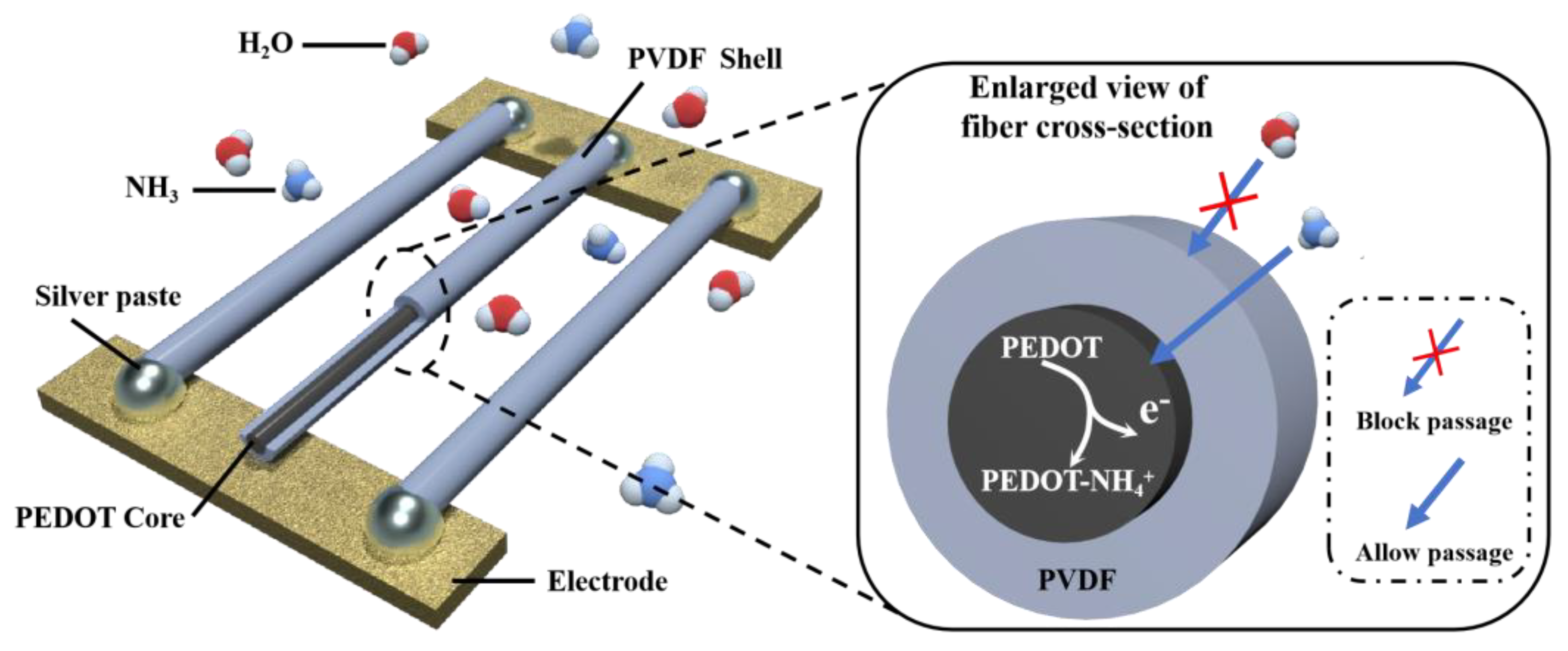
3.1. Design Concept
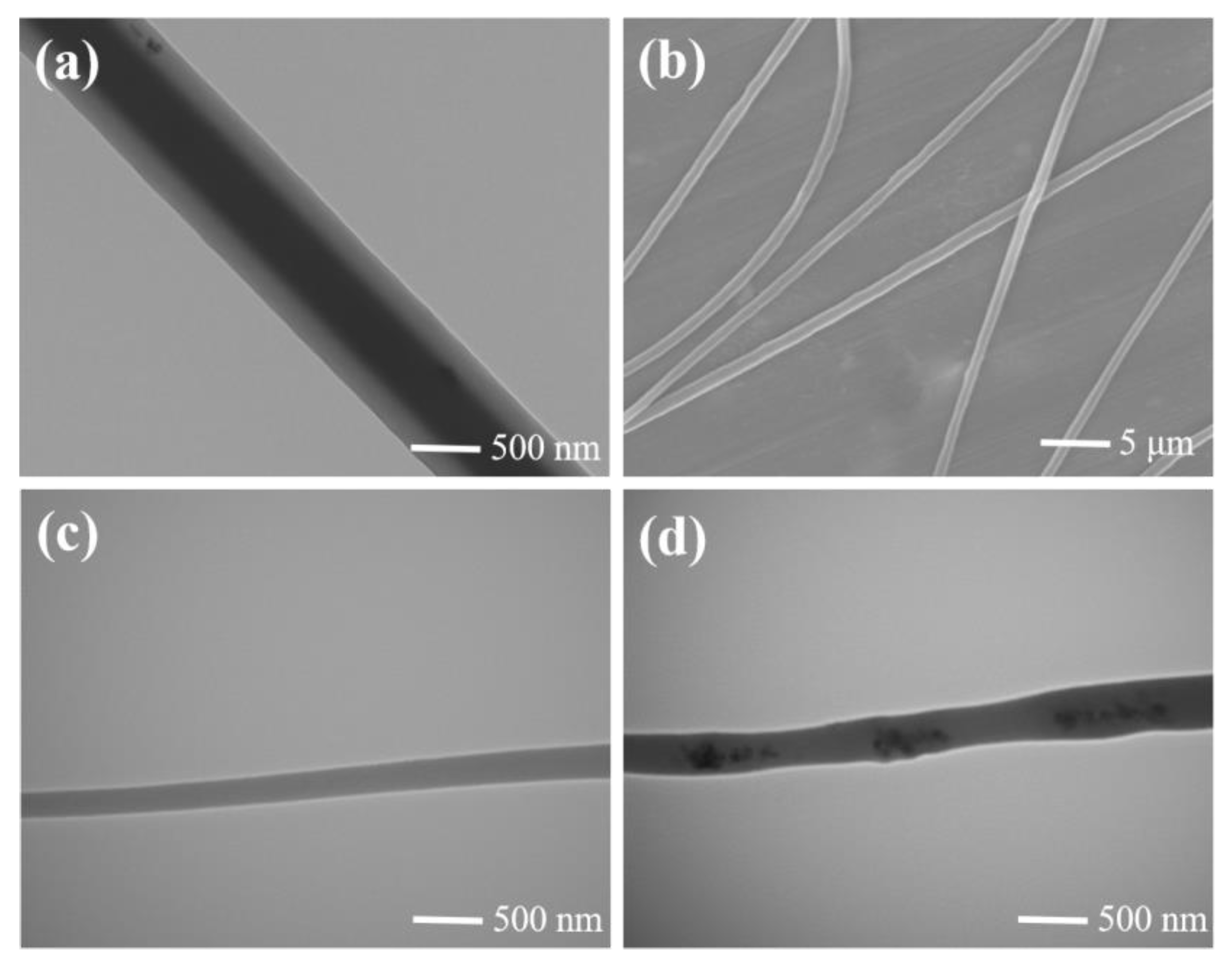
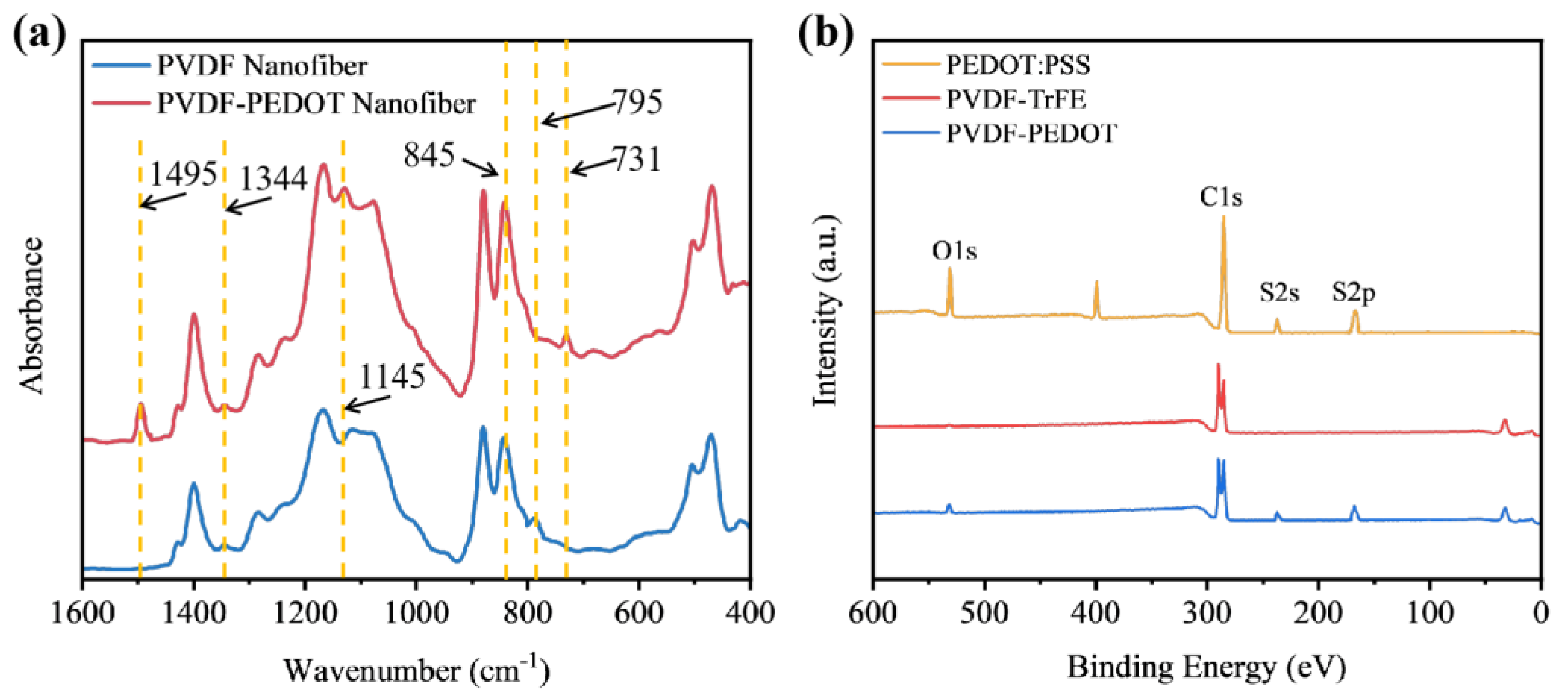
3.2. Structural and Spectral Characterization of Nanofiber
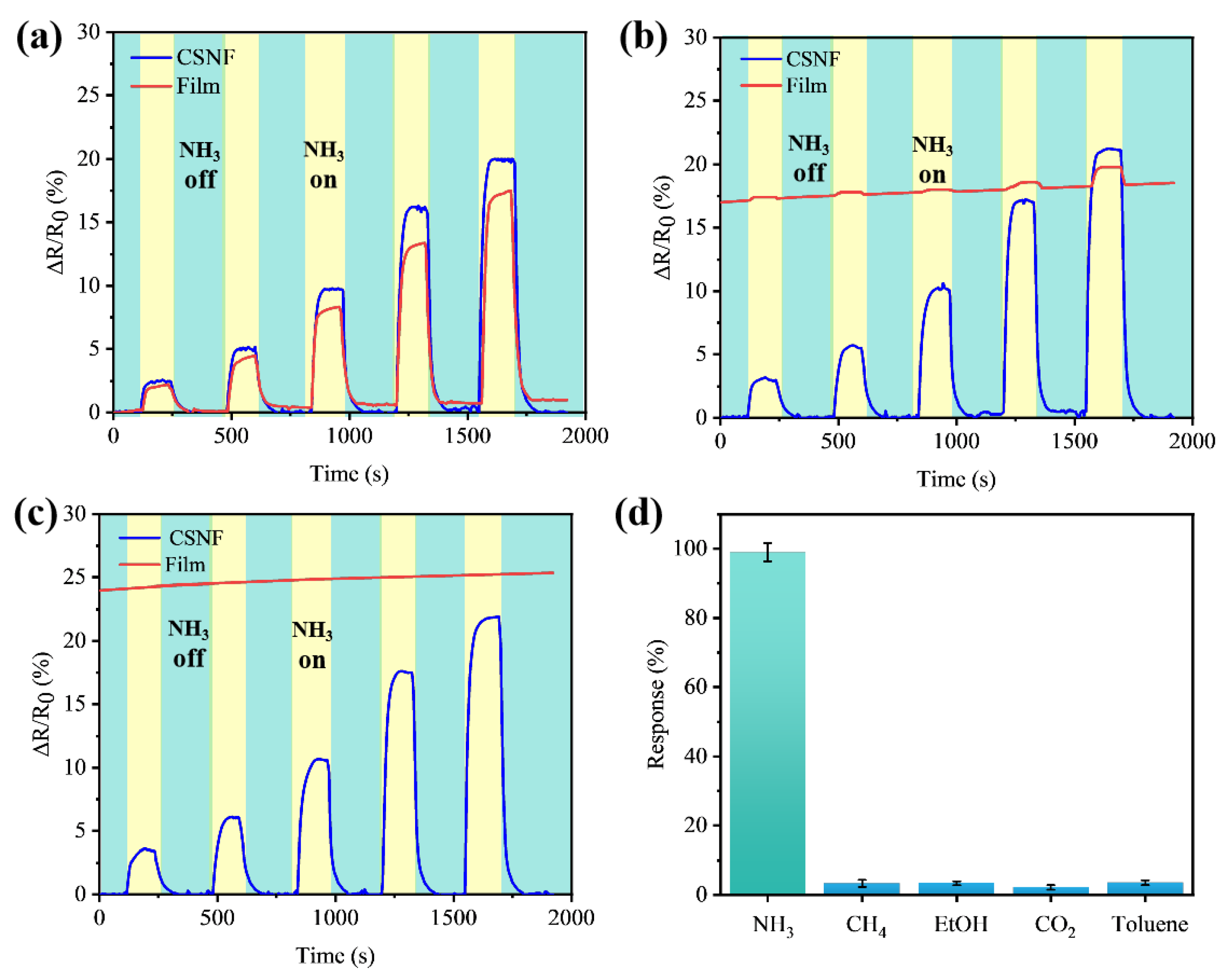
3.3. Sensing Performance and Selectivity of Gas Sensors
3.4. Resistance to Humidity Interference
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Q.; Solomon, P.; Österlund, L.; Zhang, Z. Nanotransistor-based gas sensing with record-high sensitivity enabled by electron trapping effect in nanoparticles. Nat. Commun. 2024, 15, 5259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, H.; Ni, Z.; Li, M.; Chen, Y.; Xu, P.; Li, X. 1ppm-detectable hydrogen gas sensors by using highly sensitive P+/N+ single-crystalline silicon thermopiles. Microsyst. Nanoeng. 2023, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Ma, Y.; Luo, H.; Hou, J.; Hou, C.; Huo, D. Ultra-sensitive electrochemical sensors through self-assembled MOF composites for the simultaneous detection of multiple heavy metal ions in food samples. Anal. Chim. Act 2024, 1289, 342155. [Google Scholar] [CrossRef]
- Qu, G.; Liu, G.; Zhao, C.; Yuan, Z.; Yang, Y.; Xiang, K. Detection and treatment of mono and polycyclic aromatic hydrocarbon pollutants in aqueous environments based on electrochemical technology: Recent advances. Environ. Sci. Pollut. Res. 2024, 31, 23334–23362. [Google Scholar] [CrossRef] [PubMed]
- Esteves, H.A.; Gonçalves, W.B.; Teixeira, W.S.R.; da Silva Pádua, A.C.C.; Gruber, J. Conductive Polymer-Based Sensors. In Organic and Inorganic Materials Based Sensors; Wiley-VCH: Weinheim, Germany, 2024; pp. 559–590. [Google Scholar]
- Liu, Y.; Li, J.; Xiao, S.; Liu, Y.; Bai, M.; Gong, L.; Zhao, J.; Chen, D. Revolutionizing Precision Medicine: Exploring Wearable Sensors for Therapeutic Drug Monitoring and Personalized Therapy. Biosensors 2023, 13, 726. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhu, L.; Bai, M.; Liu, Y.; Zhu, Q.; Zhao, J.; Chen, D. Continuous glucose metabolism monitoring platform for long-term analysis of tumor cell proliferation and drug response. J. Electroanal. Chem. 2023, 948, 117808. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Lin, X.; Dai, J.; Liu, S.; Fei, T.; Zhang, T. Proton-Conductive Gas Sensor: A New Way to Realize Highly Selective Ammonia Detection for Analysis of Exhaled Human Breath. ACS Sens. 2020, 5, 346–352. [Google Scholar] [CrossRef]
- Hong, S.-Z.; Huang, Q.-Y.; Wu, T.-M. Facile Synthesis of Polyaniline/Carbon-Coated Hollow Indium Oxide Nanofiber Composite with Highly Sensitive Ammonia Gas Sensor at the Room Temperature. Sensors 2022, 22, 1570. [Google Scholar] [CrossRef]
- Korotcenkov, G. Factors Controlling Stability of Polymers Acceptable for Gas Sensor Application. In Handbook of Gas Sensor Materials: Properties, Advantages and Shortcomings for Applications Volume 2: New Trends and Technologies; Korotcenkov, G., Ed.; Springer: New York, NY, USA, 2014; pp. 249–263. [Google Scholar]
- Van Duy, L.; Nguyet, T.T.; Le, D.T.T.; van Duy, N.; Nguyen, H.; Biasioli, F.; Tonezzer, M.; Di Natale, C.; Hoa, N.D. Room Temperature Ammonia Gas Sensor Based on p-Type-like V2O5 Nanosheets towards Food Spoilage Monitoring. Nanomaterials 2023, 13, 146. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Nakamae, T.; Fujisada, R.; Shiba, S. A Highly Sensitive Ammonia Gas Sensor Using Micrometer-Sized Core–Shell-Type Spherical Polyaniline Particles. Sensors 2021, 21, 7522. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Wang, N.; Huang, B.; Li, X. SnTe/SnSe Heterojunction Based Ammonia Sensors with Excellent Withstand to Ambient Humidities. Small 2024, 20, 2309831. [Google Scholar] [CrossRef] [PubMed]
- Lesego, M.; Ndinteh, D.T.; Ndungu, P.; Mamo, M.A. Zeolitic imidazolate framework as humidity-resistant solid state-chemiresistive gas sensors: A review. Heliyon 2023, 9, e22329. [Google Scholar] [CrossRef]
- Vasiliev, A.A.; Varfolomeev, A.E.; Volkov, I.A.; Simonenko, N.P.; Arsenov, P.V.; Vlasov, I.S.; Ivanov, V.V.; Pislyakov, A.V.; Lagutin, A.S.; Jahatspanian, I.E.; et al. Reducing Humidity Response of Gas Sensors for Medical Applications: Use of Spark Discharge Synthesis of Metal Oxide Nanoparticles. Sensors 2018, 18, 2600. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Rupom, R.H.; Adhikari, P.R.; Demchuk, Z.; Popov, I.; Sokolov, A.P.; Wu, H.F.; Advincula, R.C.; Dahotre, N.; Jiang, Y.; et al. Boosting Piezoelectricity by 3D Printing PVDF-MoS2 Composite as a Conformal and High-Sensitivity Piezoelectric Sensor. Adv. Funct. Mater. 2023, 33, 2302946. [Google Scholar] [CrossRef]
- Dunst, K.; Karczewski, J.; Jasiński, P. Nitrogen dioxide sensing properties of PEDOT polymer films. Sens. Actuators B Chem. 2017, 247, 108–113. [Google Scholar] [CrossRef]
- Hakimi, M.; Salehi, A.; Boroumand, F.A. Fabrication and Characterization of an Ammonia Gas Sensor Based on PEDOT-PSS With N-Doped Graphene Quantum Dots Dopant. IEEE Sens. J. 2016, 16, 6149–6154. [Google Scholar] [CrossRef]
- Ahmed, Y.M.; Eldin, M.A.; Galal, A.; Atta, N.F. Electrochemical sensor based on PEDOT/CNTs-graphene oxide for simultaneous determination of hazardous hydroquinone, catechol, and nitrite in real water samples. Sci. Rep. 2024, 14, 5654. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.; Choi, J.; Jung, D.; Shim, S. Electrospun PEDOT: PSS/PVP nanofibers as the chemiresistor in chemical vapour sensing. Synth. Met. 2010, 160, 1415–1421. [Google Scholar] [CrossRef]
- Shiu, B.C.; Liu, Y.L.; Yuan, Q.Y.; Lou, C.W.; Lin, J.H. Preparation and Characterization of PEDOT: PSS/TiO2 Micro/Nanofiber-Based Gas Sensors. Polymers 2022, 14, 1780. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Jaiswal, M.; Satapathy, D.K. Swelling kinetics and electrical charge transport in PEDOT: PSS thin films exposed to water vapor. J. Phys. Condens. Matter 2018, 30, 225101. [Google Scholar] [CrossRef]
- Xiong, Z.; Liu, C. Optimization of inkjet printer PEDOT: PSS thin films through annealing processes. Org. Electron. 2012, 13, 1532–1540. [Google Scholar] [CrossRef]
- Perret, B. Peter Gründler: Chemical sensors. An introduction for scientists and engineers. Anal. Bioanal. Chem. 2008, 392, 21–22. [Google Scholar] [CrossRef]
- Sezen-Edmonds, M.; Yeh, Y.-W.; Yao, N.; Loo, Y.-L. Humidity and Strain Rate Determine the Extent of Phase Shift in the Piezoresistive Response of PEDOT: PSS. ACS Appl. Mater. Interfaces 2019, 11, 16888–16895. [Google Scholar] [CrossRef] [PubMed]
- Sobola, D.; Kaspar, P.; Částková, K.; Dallaev, R.; Papež, N.; Sedlák, P.; Trčka, T.; Orudzhev, F.; Kaštyl, J.; Weiser, A.; et al. PVDF Fibers Modification by Nitrate Salts Doping. Polymers 2021, 13, 2439. [Google Scholar] [CrossRef]
- Greco, G.; Giuri, A.; Bagheri, S.; Seiti, M.; Degryse, O.; Rizzo, A.; Mele, C.; Ferraris, E.; Corcione, C.E. PEDOT: PSS/Graphene Oxide (GO) Ternary Nanocomposites for Electrochemical Applications. Molecules 2023, 28, 2963. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Li, J.; Zhao, H.; Wang, Y.; Zhou, Y. Mesoporous cellulose nanofibers-interlaced PEDOT: PSS hybrids for chemoreceptive ammonia detection. Microchem. Acta 2022, 189, 308. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Hu, M.; Hong, Y.; Hu, M.; Sun, T.; Chen, D. Core–Shell PEDOT-PVDF Nanofiber-Based Ammonia Gas Sensor with Robust Humidity Resistance. Biosensors 2024, 14, 411. https://doi.org/10.3390/bios14090411
Xiao S, Hu M, Hong Y, Hu M, Sun T, Chen D. Core–Shell PEDOT-PVDF Nanofiber-Based Ammonia Gas Sensor with Robust Humidity Resistance. Biosensors. 2024; 14(9):411. https://doi.org/10.3390/bios14090411
Chicago/Turabian StyleXiao, Shenghao, Mengjie Hu, Yinhui Hong, Mengjia Hu, Tongtong Sun, and Dajing Chen. 2024. "Core–Shell PEDOT-PVDF Nanofiber-Based Ammonia Gas Sensor with Robust Humidity Resistance" Biosensors 14, no. 9: 411. https://doi.org/10.3390/bios14090411
APA StyleXiao, S., Hu, M., Hong, Y., Hu, M., Sun, T., & Chen, D. (2024). Core–Shell PEDOT-PVDF Nanofiber-Based Ammonia Gas Sensor with Robust Humidity Resistance. Biosensors, 14(9), 411. https://doi.org/10.3390/bios14090411



