Polarized and Evanescent Guided Wave Surface-Enhanced Raman Spectroscopy of Ligand Interactions on a Plasmonic Nanoparticle Optical Chemical Bench
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Au@Ag Nanoparticles (NPs)
2.2. Preparation of the Optical Chemical Bench (OCB)
2.3. Binding of 4MPy and 4ABA Molecules
2.4. Fe2+ Binding to 4MPy on the OCB
2.5. Binding of 4ABA-Functionalized Magnetite Nanoparticles (MNPs)
2.6. Characterization Techniques
- X-ray Photoelectron Spectroscopy (XPS): XPS was performed using a Thermo Scientific K-Alpha spectrometer with an Al-Kα X-ray source, operating under a vacuum of <2 × 10⁻7 mbar. Spectra for N (1s) and O (1s) were collected for MNPs before and after 4ABA functionalization.
- Integrated Optics (IO) SERS Spectrometry: Polarization-dependent SERS spectra were acquired using a custom-built IO-SERS instrument containing a Triax 550 Raman monochromator (Horiba Scientific, Piscataway, NJ, USA). Details of the custom-built IO-SERS setup is described in SI. A 532 nm CW laser diode was used, and polarized waveguide modes were excited via prism coupling.
- Scanning Electron Microscopy (SEM): High-resolution SEM and EDX were performed using a Helios NanoLab™ 660 instrument (Thermo-Fisher Scientific, St. Laurent, QC, Canada).
- Transmission Electron Microscopy (TEM): Light-field and dark-field TEM, along with EDX, were performed using an FEI Titan Krios 300 kV Cryo-STEM (Thermo-Fisher Scientific, St. Laurent, QC, Canada).
- Simulation of Plasmonic Electric Field: Three-dimensional finite difference time domain (3D FDTD) simulations were conducted using Lumerical Solutions software ((2020 R2), Vancouver, BC, Canada). to model the resonant optical response of the Au@Ag nanoparticles on the waveguide.
2.7. Guided-Wave Raman Spectroscopy and Prism Coupling
3. Results and Discussion
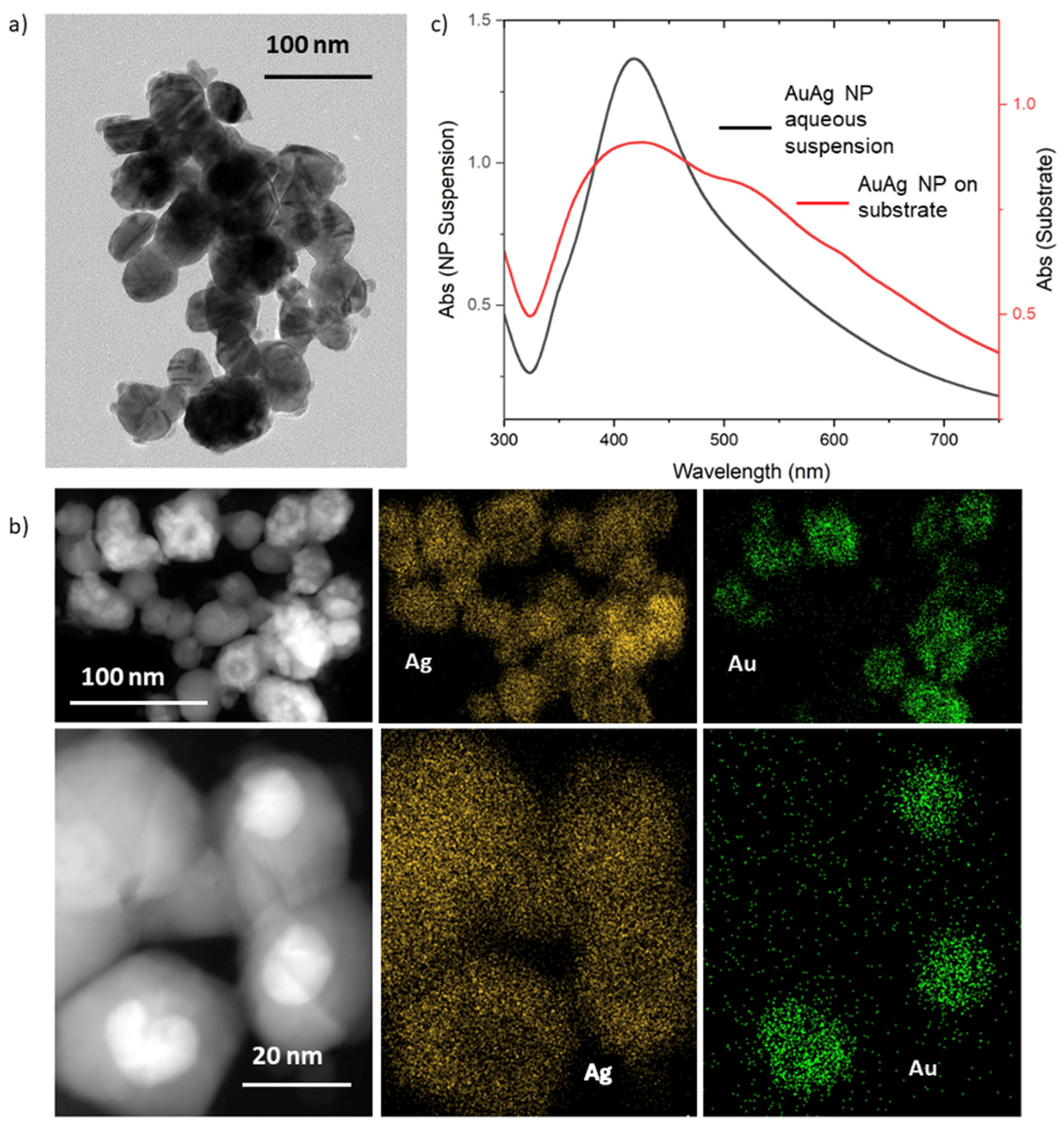
3.1. Characterization of OCB Overlayed with Au@Ag Nanoparticles
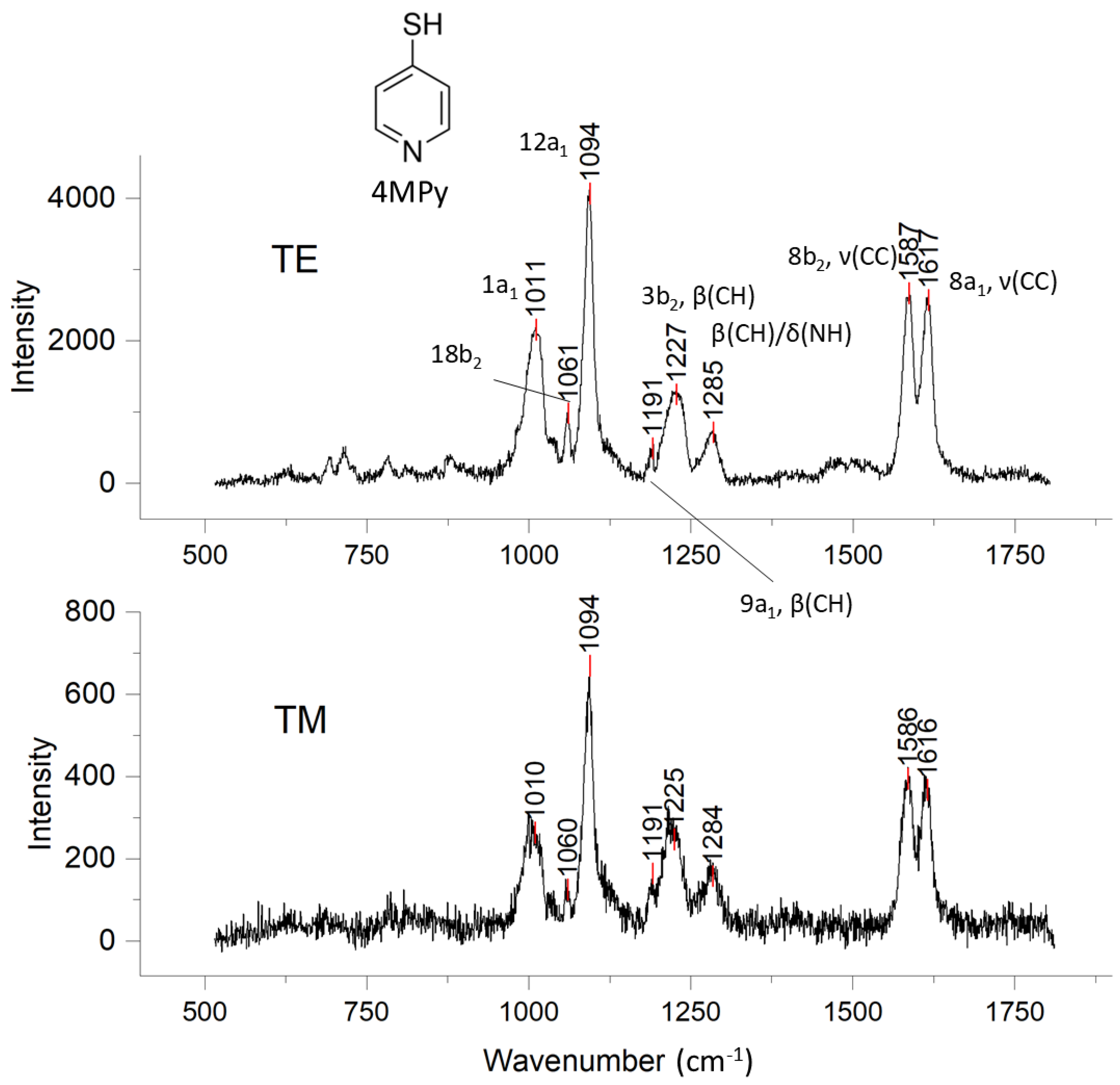
3.2. SER Spectra of 4-Mercaptopyridine (4MPy) Binding on Au@Ag NP Surface
3.3. SER Response of 4MPy to TE and TM Evanescent Wave Polarization
3.4. Orientation of 4MPy on Au@Ag Nanoparticles on the OCB
3.5. Binding of Fe2+ to 4MPy
3.6. SERS of 4-Aminobenzoic Acid (4ABA) Binding on OCB in Comparison to That of 4MPy
3.7. Caveat on Assignment of Molecule Orientation on Plasmonic Au@Ag PNPs on the OCB
3.8. Exploring the Nanoscale Interface: Detection of 4ABA-Functionalized Magnetic Nanoparticles (MNP) Using Evanescent Waveguide SERS on the OCB
3.9. FDTD Simulation of Electric Fields of Au@Ag NPs Excited by TE/TM Polarized Light
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhang, R.; Jiang, S.; Zhang, Y.; Dong, Z.-C. Probing Adsorption Configurations of Small Molecules on Surfaces by Single-Molecule Tip-Enhanced Raman Spectroscopy. Chem. Phys. Chem. 2019, 20, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M.; Cheng, H.; Yan, Y.; Iqbal, H.M.N. Multi-point enzyme immobilization, surface chemistry, and novel platforms: A paradigm shift in biocatalyst design. Crit. Rev. Biotechnol. 2019, 39, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Rusling, J.F.; Dixit, C.K. Site-selective orientated immobilization of antibodies and conjugates for immunodiagnostics development. Methods 2017, 116, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Coluccio, M.L.; Grillo, F.; Onesto, V.; Garo, V.; Scala, C.; Cuzzola, P.; Calfa, M.; Candeloro, P.; Gentile, F.; Piletsky, S.; et al. Enhancing Antibodies’ Binding Capacity through Oriented Functionalization of Plasmonic Surfaces. Nanomaterials 2021, 11, 2620. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, M.; Perotto, S.; Messina, G.C.; Lovato, L.; De Angelis, F. Chemical Functionalization of Plasmonic Surface Biosensors: A Tutorial Review on Issues, Strategies, and Costs. ACS Appl. Mater. Interfaces 2017, 9, 29394–29411. [Google Scholar] [CrossRef]
- Mai, A.; Mai, C.; Steglich, P. From Lab-on-chip to Lab-in-App: Challenges towards silicon photonic biosensors product developments. Results Opt. 2022, 9, 100317. [Google Scholar] [CrossRef]
- Shahbaz, M.; Butt, M.A.; Piramidowicz, R. A Concise Review of the Progress in Photonic Sensing Devices. Photonics 2023, 10, 698. [Google Scholar] [CrossRef]
- Levy, Y.; Imbert, C.; Cipriani, J.; Racine, S.; Dupeyrat, R. Raman scattering of thin films as a waveguide. Opt. Commun. 1974, 11, 66–69. [Google Scholar] [CrossRef]
- Wang, P.; Miller, B.L. Waveguide-Enhanced Raman Spectroscopy (WERS): An Emerging Chip-Based Tool for Chemical and Biological Sensing. Sensors 2022, 22, 9058. [Google Scholar] [CrossRef]
- Ettabib, M.A.; Marti, A.; Liu, Z.; Bowden, B.M.; Zervas, M.N.; Bartlett, P.N.; Wilkinson, J.S. Waveguide Enhanced Raman Spectroscopy for Biosensing: A Review. ACS Sens. 2021, 6, 2025–2045. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, J.; Xu, S.; Geng, Y. Surface Plasmon Field-Enhanced Raman Scattering Co-Excited by P-Polarized and S-Polarized Light Based on Waveguide-Coupled Surface Plasmon Resonance Configuration. ACS Omega 2023, 8, 41953–41959. [Google Scholar] [CrossRef]
- Wang, J.; Lin, W.; Cao, E.; Xu, X.; Liang, W.; Zhang, X. Surface Plasmon Resonance Sensors on Raman and Fluorescence Spectroscopy. Sensors 2017, 17, 2719. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, Y.; Liu, Y.; Hu, X.; Chen, H. Current strategies of plasmonic nanoparticles assisted surface-enhanced Raman scattering toward biosensor studies. Biosens. Bioelectron. 2023, 228, 115231. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Pai, P.; Shetty, M.G.; Babitha, K.S. Gold nanoparticle based biosensors for rapid pathogen detection: A review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100756. [Google Scholar] [CrossRef]
- Das, G.M.; Managò, S.; Mangini, M.; De Luca, A.C. Biosensing Using SERS Active Gold Nanostructures. Nanomaterials 2021, 11, 2679. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Singh, R.S.; Sarswat, P.K. From fundamentals to applications: The development of magnetoplasmonics for next-generation technologies. Mater. Today Electron. 2023, 4, 100033. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chen, L.-Y.; Huang, Y.-C.; Kumar, P.; Guo, Y.-Z.; Wu, C.-H.; Wang, L.-M.; Chen, K.-L. Improving Sensitivity and Reproducibility of Surface-Enhanced Raman Scattering Biochips Utilizing Magnetoplasmonic Nanoparticles and Statistical Methods. ACS Sens. 2024, 9, 305–314. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface plasmon resonance: A versatile technique for biosensor applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef]
- Vinogradov, A.P.; Dorofeenko, A.V.; Pukhov, A.A.; Lisyansky, A.A. Exciting surface plasmon polaritons in the Kretschmann configuration by a light beam. Phys. Rev. B 2018, 97, 235407. [Google Scholar] [CrossRef]
- David, S.; Polonschii, C.; Luculescu, C.; Gheorghiu, M.; Gáspár, S.; Gheorghiu, E. Magneto-plasmonic biosensor with enhanced analytical response and stability. Biosens. Bioelectron. 2015, 63, 525–532. [Google Scholar] [CrossRef]
- Baldwin, J.; Schühler, N.; Butler, I.S.; Andrews, M.P. Integrated Optics Evanescent Wave Surface Enhanced Raman Scattering (IO-EWSERS) of Mercaptopyridines on a Planar Optical Chemical Bench: Binding of Hydrogen and Copper Ion. Langmuir 1996, 12, 6389–6398. [Google Scholar] [CrossRef]
- Kong, L.; Lee, C.; Earhart, C.M.; Cordovez, B.; Chan, J.W. A nanotweezer system for evanescent wave excited surface enhanced Raman spectroscopy (SERS) of single nanoparticles. Opt. Express 2015, 23, 6793–6802. [Google Scholar] [CrossRef] [PubMed]
- Ismail, W.Z.W.; Dawes, J.M. Synthesis and Characterization of Silver-Gold Bimetallic Nanoparticles for Random Lasing. Nanomaterials 2022, 12, 607. [Google Scholar] [CrossRef]
- Kiani, M.T.; Wang, Y.; Bertin, N.; Cai, W.; Gu, X.W. Strengthening Mechanism of a Single Precipitate in a Metallic Nanocube. Nano Lett. 2019, 19, 255–260. [Google Scholar] [CrossRef]
- Qi, W.H.; Lee, S.T. Phase Stability, Melting, and Alloy Formation of Au−Ag Bimetallic Nanoparticles. J. Phys. Chem. C 2010, 114, 9580–9587. [Google Scholar] [CrossRef]
- Lim, D.-K.; Kim, I.-J.; Nam, J.-M. DNA-embedded Au/Ag core–shell nanoparticles. Chem. Commun. 2008, 42, 5312–5314. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.-I.; Kiyama, T.; Hirahara, K.; Tanaka, N.; Kuwabata, S.; Torimoto, T. Single-step synthesis of gold–silver alloy nanoparticles in ionic liquids by a sputter deposition technique. Chem. Commun. 2008, 6, 691–693. [Google Scholar] [CrossRef]
- Rivas, L.; Sanchez-Cortes, S.; García-Ramos, J.V.; Morcillo, G. Mixed Silver/Gold Colloids: A Study of Their Formation, Morphology, and Surface-Enhanced Raman Activity. Langmuir 2000, 16, 9722–9728. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, B.; Xu, W.; Li, B.; Fan, Y. Surface-enhanced Raman spectroscopy study on the structure changes of 4-mercaptopyridine adsorbed on silver substrates and silver colloids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. Spectrochim. 2002, 58, 2827–2834. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, Y.; Shang, Z.; Zhang, Y.; Mo, Y. Experimental and theoretical studies of Raman spectroscopy on 4-mercaptopyridine aqueous solution and 4-mercaptopyridine/Ag complex system. J. Raman Spectrosc. 2007, 38, 1106–1111. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, K.; Kim, M.S. Raman spectroscopic investigation of the adsorption of 4-mercaptopyridine on a silver-sol surface. J. Mol. Struct. 1997, 407, 139–147. [Google Scholar] [CrossRef]
- Spinner, E. 717. The vibration spectra of some monosubstituted pyridines and pyridinium ions. J. Chem. Soc. (Resumed) 1963, 3860–3870. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.-Y.; Wang, L.; Lu, D.-F.; Qi, Z.-M. Waveguide-coupled directional Raman radiation for surface analysis. Phys. Chem. Chem. Phys. 2015, 17, 21278–21287. [Google Scholar] [CrossRef]
- Sun, L.; Yip, G.L. Analysis of metal-clad optical waveguide polarizers by the vector beam propagation method. Appl. Opt. 1994, 33, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Pal, S. Pyridine: A Useful Ligand in Transition Metal Complexes. Pyridine 2018, 57, 57–74. [Google Scholar] [CrossRef]
- Ramírez, E.A.; Cortés, E.; Rubert, A.A.; Carro, P.; Benítez, G.; Vela, M.E.; Salvarezza, R.C. Complex Surface Chemistry of 4-Mercaptopyridine Self-Assembled Monolayers on Au(111). Langmuir 2012, 28, 6839–6847. [Google Scholar] [CrossRef]
- Mohamed, H.; Ghith, A.; Bell, S.G. The binding of nitrogen-donor ligands to the ferric and ferrous forms of cytochrome P450 enzymes. J. Inorg. Biochem. 2023, 242, 112168. [Google Scholar] [CrossRef]
- Suh, J.S.; DiLella, D.P.; Moskovits, M. Surface-enhanced Raman spectroscopy of colloidal metal systems: A two-dimensional phase equilibrium in p-aminobenzoic acid adsorbed on silver. J. Phys. Chem. 1983, 87, 1540–1544. [Google Scholar] [CrossRef]
- Liang, E.J.; Engert, C.; Kiefer, W. Surface-enhanced Raman spectroscopy of p-aminobenzoic acid with excitation in the visible and near infrared spectral region. Vib. Spectrosc. 1993, 6, 79–85. [Google Scholar] [CrossRef]
- Świsłocka, R.; Regulska, E.; Samsonowicz, M.; Hrynaszkiewicz, T.; Lewandowski, W. Molecular structure of 3-aminobenzoic acid complexes with alkali metals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. Spectrochim. 2005, 61, 2966–2973. [Google Scholar] [CrossRef]
- Park, H.; Lee, S.B.; Kim, K.; Kim, M.S. Surface-enhanced Raman scattering of p-aminobenzoic acid at silver electrode. J. Phys. Chem. 1990, 94, 7576–7580. [Google Scholar] [CrossRef]
- Uznanski, P.; Zakrzewska, J.; Favier, F.; Kazmierski, S.; Bryszewska, E. Synthesis and characterization of silver nanoparticles from (bis)alkylamine silver carboxylate precursors. J. Nanopart. Res. 2017, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Al-Johani, H.; Abou-Hamad, E.; Jedidi, A.; Widdifield, C.M.; Viger-Gravel, J.; Sangaru, S.S.; Gajan, D.; Anjum, D.H.; Ould-Chikh, S.; Hedhili, M.N.; et al. The structure and binding mode of citrate in the stabilization of gold nanoparticles. Nat. Chem. 2017, 9, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Pakiari, A.H.; Jamshidi, Z. Interaction of Amino Acids with Gold and Silver Clusters. J. Phys. Chem. A 2007, 111, 4391–4396. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Meyer, S.A.; Artur, C.; Etchegoin, P.G.; Grand, J.; Lang, P.; Maurel, F. Experimental demonstration of surface selection rules for SERS on flat metallic surfaces. Chem. Commun. 2011, 47, 3903–3905. [Google Scholar] [CrossRef]
- Buchanan, S.; Le Ru, E.C.; Etchegoin, P.G. Plasmon-dispersion corrections and constraints for surface selection rules of single molecule SERS spectra. Phys. Chem. Chem. Phys. 2009, 11, 7406–7411. [Google Scholar] [CrossRef]
- Maksymov, I.S. Magneto-plasmonic nanoantennas: Basics and applications. Rev. Phys. 2016, 1, 36–51. [Google Scholar] [CrossRef]
- Lai, T.F.; Marsh, R.E. The crystal structure of p-aminobenzoic acid. Acta Crystallogr. 1967, 22, 885–893. [Google Scholar] [CrossRef]
- Johansson, P.; Xu, H.; Käll, M. Surface-enhanced Raman scattering and fluorescence near metal nanoparticles. Phys. Rev. B 2005, 72, 035427. [Google Scholar] [CrossRef]
- Sriram, S.; Bhaskaran, M.; Chen, S.; Jayawardhana, S.; Stoddart, P.R.; Liu, J.Z.; Medhekar, N.V.; Kalantar-Zadeh, K.; Mitchell, A. Influence of Electric Field on SERS: Frequency Effects, Intensity Changes, and Susceptible Bonds. J. Am. Chem. Soc. 2012, 134, 4646–4653. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, Y.; Hakuta, M.; Furuya, K.; Chiba, K.; Hasumi, R. Fundamental transverse electric field (TE0) mode selection for thin-film asymmetric light guides. Appl. Phys. Lett. 1972, 21, 291–293. [Google Scholar] [CrossRef]
- Medrano-Lopez, J.A.; Villalpando, I.; Salazar, M.I.; Torres-Torres, C. Hierarchical Nanobiosensors at the End of the SARS-CoV-2 Pandemic. Biosensors 2024, 14, 108. [Google Scholar] [CrossRef]
- Calagua, A.; Alarcon, H.; Paraguay, F.; Rodriguez, J. Synthesis and Characterization of Bimetallic Gold-Silver Core-Shell Nanoparticles: A Green Approach. Adv. Nanopart. 2015, 4, 116. [Google Scholar] [CrossRef][Green Version]
- Petcharoen, K.; Sirivat, A. Synthesis and Characterization of Magnetite Nanoparticles via the Chemical Coprecipitation Method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- He, R.X.; Liang, R.; Peng, P.; Zhou, Y.N. Effect of the Size of Silver Nanoparticles on SERS Signal Enhancement. J. Nanopart. Res. 2017, 19, 267. [Google Scholar] [CrossRef]
- Wilson, D.; Langell, M.A. XPS Analysis of Oleylamine/Oleic Acid Capped Fe3O4 Nanoparticles as a Function of Temperature. Appl. Surf. Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Wagner, A.J.; Wolfe, G.M.; Fairbrother, D.H. Reactivity of Vapor-Deposited Metal Atoms with Nitrogen-Containing Polymers and Organic Surfaces Studied by In Situ XPS. Appl. Surf. Sci. 2003, 219, 317–328. [Google Scholar] [CrossRef]
- Degaga, G.D.; Trought, M.; Nemsak, S.; Crumlin, E.J.; Seel, M.; Pandey, R.; Perrine, K.A. Investigation of N₂ Adsorption on Fe3O4 (001) Using Ambient Pressure X-ray Photoelectron Spectroscopy and Density Functional Theory. J. Chem. Phys. 2020, 152, 054717. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Andrews, M.P. Polarized and Evanescent Guided Wave Surface-Enhanced Raman Spectroscopy of Ligand Interactions on a Plasmonic Nanoparticle Optical Chemical Bench. Biosensors 2024, 14, 409. https://doi.org/10.3390/bios14090409
Chen X, Andrews MP. Polarized and Evanescent Guided Wave Surface-Enhanced Raman Spectroscopy of Ligand Interactions on a Plasmonic Nanoparticle Optical Chemical Bench. Biosensors. 2024; 14(9):409. https://doi.org/10.3390/bios14090409
Chicago/Turabian StyleChen, Xining, and Mark P. Andrews. 2024. "Polarized and Evanescent Guided Wave Surface-Enhanced Raman Spectroscopy of Ligand Interactions on a Plasmonic Nanoparticle Optical Chemical Bench" Biosensors 14, no. 9: 409. https://doi.org/10.3390/bios14090409
APA StyleChen, X., & Andrews, M. P. (2024). Polarized and Evanescent Guided Wave Surface-Enhanced Raman Spectroscopy of Ligand Interactions on a Plasmonic Nanoparticle Optical Chemical Bench. Biosensors, 14(9), 409. https://doi.org/10.3390/bios14090409




