Enhanced Electrochemical Sensing of Oxalic Acid Based on VS2 Nanoflower-Decorated Glassy Carbon Electrode Prepared by Hydrothermal Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of VS2 Nanoflowers
2.3. Preparation of VS2 = −Modified GCE (VS2/GCE)
2.4. Electrochemical Measurements
2.5. Characterization
2.6. Electrochemical Sensing of OA
2.7. Interference Study and Real Sample Analysis
3. Results and Discussion
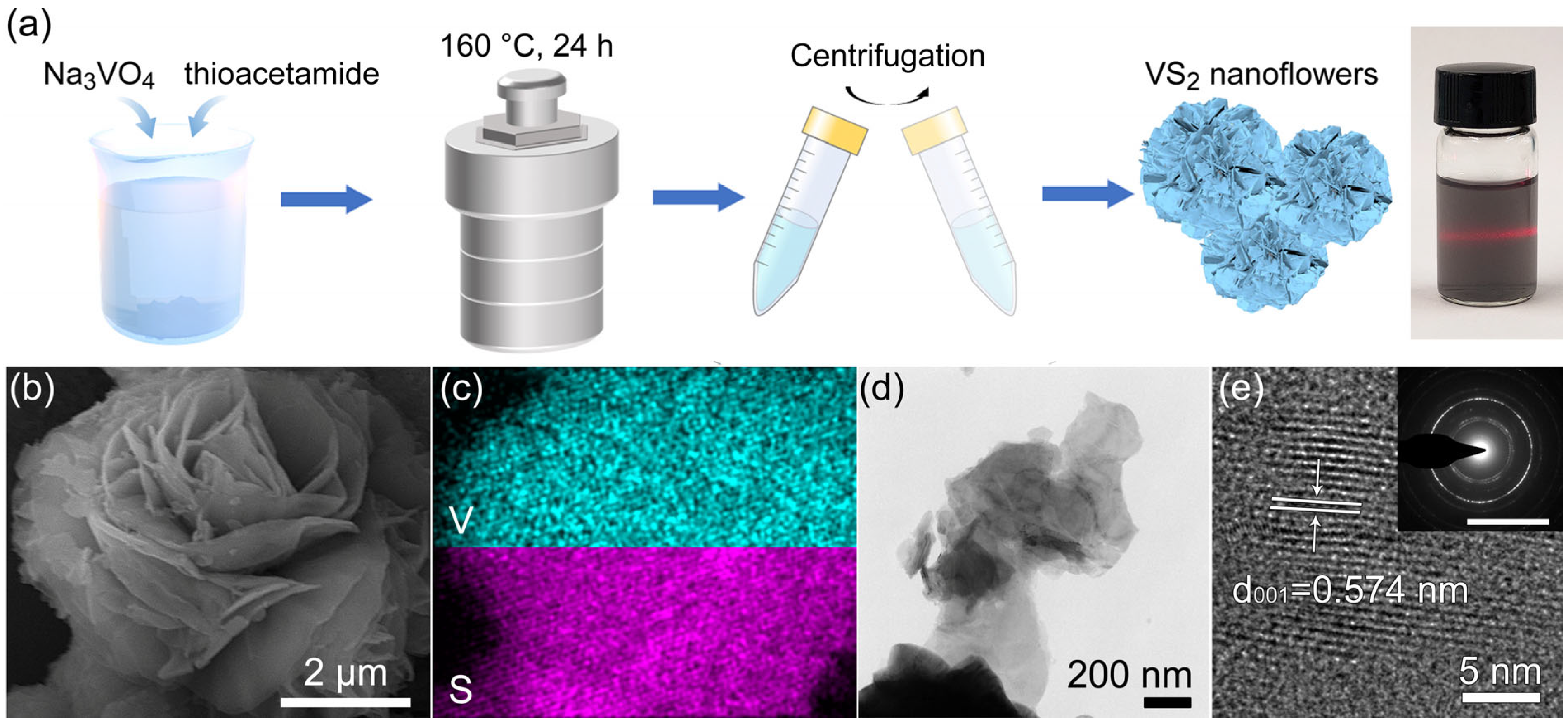
3.1. Morphology and Structure Characterization of VS2 Nanoflowers
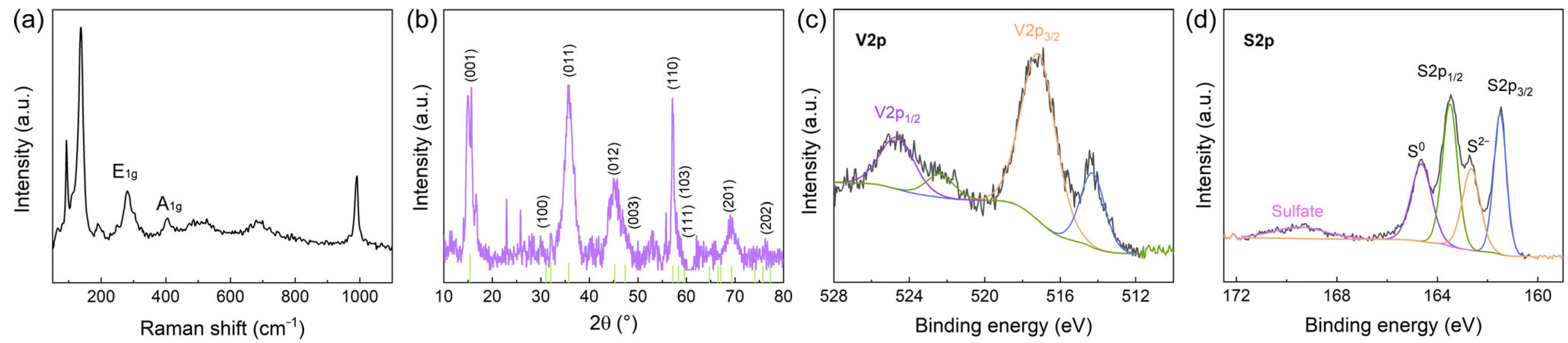
3.2. Chemical Composition and Crystal Structure Analysis of VS2 Nanoflowers
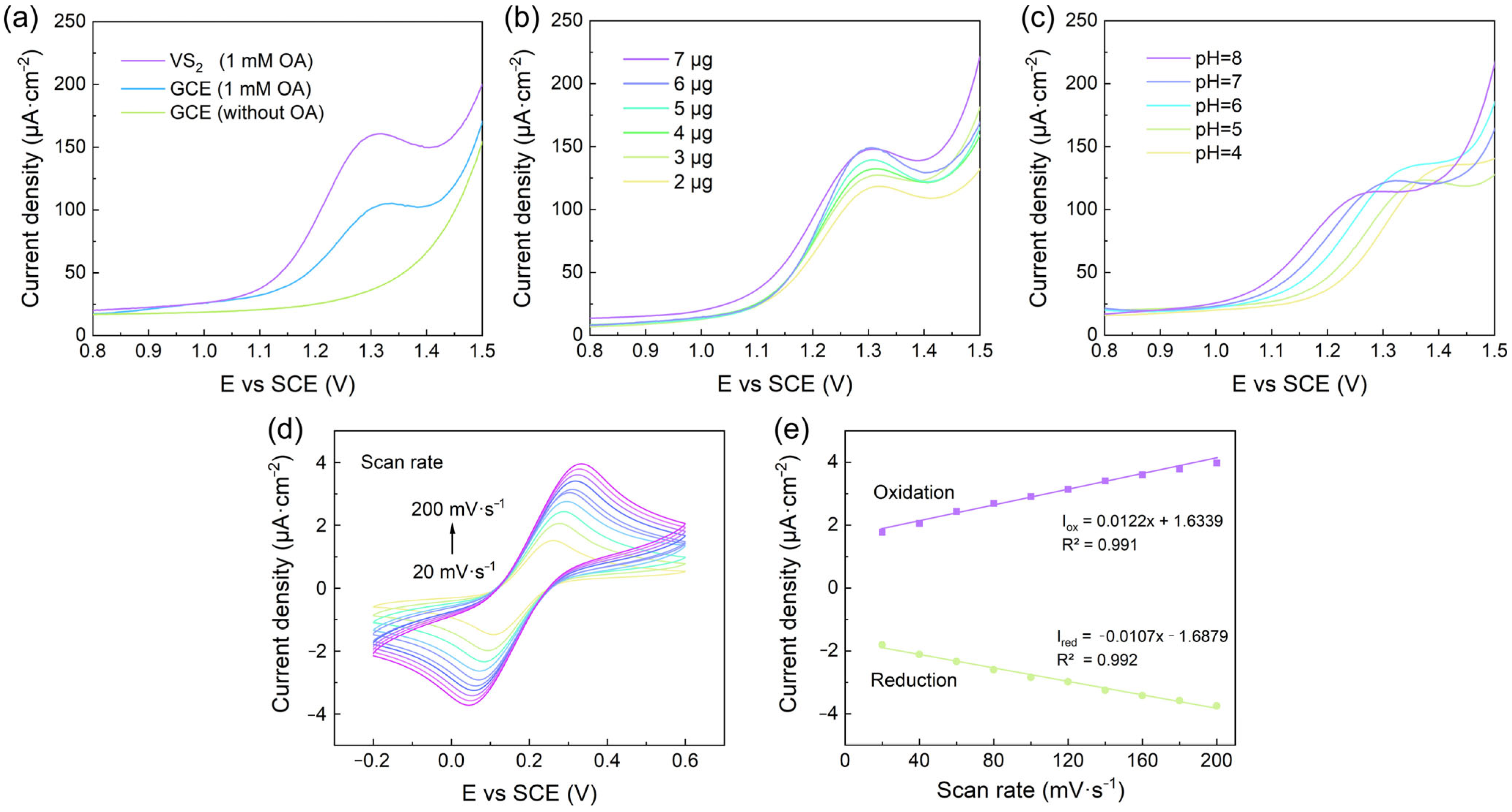
3.3. Electrochemical Behavior of VS2/GCE and Optimization of Sensing Conditions
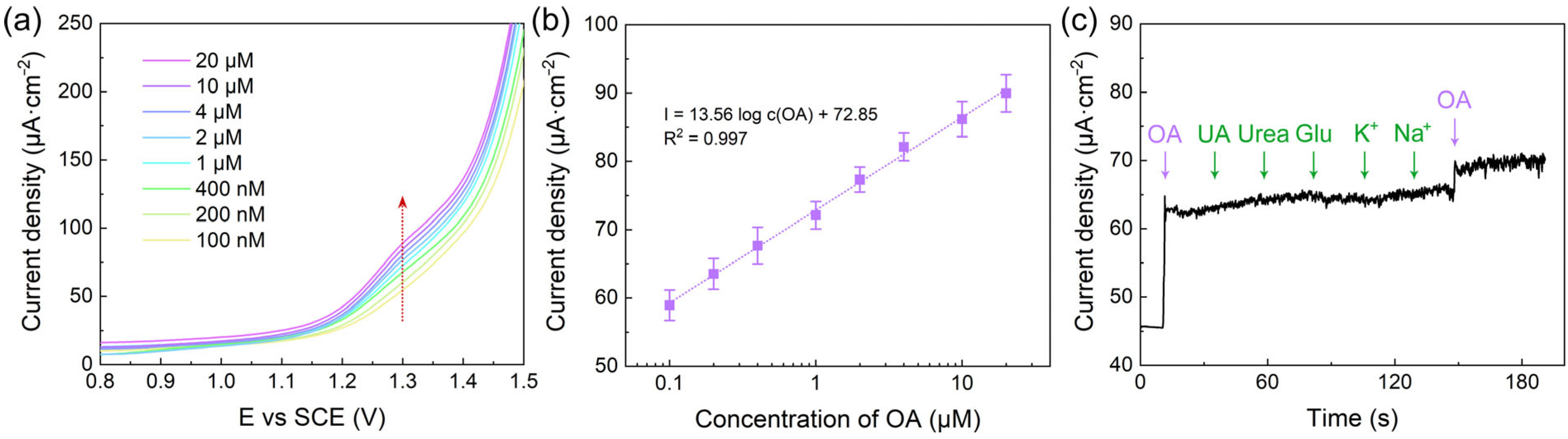
3.4. Analytical Performance and Practical Application of the VS2/GCE Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.-G. Kidney stones. Nat. Rev. Dis. Primers 2016, 2, 16008. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Dietary and Lifestyle Risk Factors Associated with Incident Kidney Stones in Men and Women. J. Urol. 2017, 198, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Selvam, R. Calcium oxalate stone disease: Role of lipid peroxidation and antioxidants. Urol. Res. 2002, 30, 35–47. [Google Scholar] [CrossRef]
- Wang, W.; Fan, J.; Huang, G.; Li, J.; Zhu, X.; Tian, Y.; Su, L. Prevalence of kidney stones in mainland China: A systematic review. Sci. Rep. 2017, 7, 41630. [Google Scholar] [CrossRef] [PubMed]
- Scherer, K.; Braig, E.; Willer, K.; Willner, M.; Fingerle, A.A.; Chabior, M.; Herzen, J.; Eiber, M.; Haller, B.; Straub, M.; et al. Non-invasive Differentiation of Kidney Stone Types using X-ray Dark-Field Radiography. Sci. Rep. 2015, 5, 9527. [Google Scholar] [CrossRef]
- Brisbane, W.; Bailey, M.R.; Sorensen, M.D. An overview of kidney stone imaging techniques. Nat. Rev. Urol. 2016, 13, 654–662. [Google Scholar] [CrossRef]
- Rao, P.N. Imaging for kidney stones. World J. Urol. 2004, 22, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Herlitz, L.C.; D’Agati, V.D.; Markowitz, G.S. Crystalline nephropathies. Arch. Pathol. Lab. Med. 2012, 136, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Hsi, R.S.; Sanford, T.; Goldfarb, D.S.; Stoller, M.L. The Role of the 24-Hour Urine Collection in the Prevention of Kidney Stone Recurrence. J. Urol. 2017, 197, 1084–1089. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Speizer, F.E.; Stampfer, M.J. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001, 59, 2290–2298. [Google Scholar] [CrossRef]
- Chung, W.-Y.; Falah Ramezani, R.; Silverio, A.A.; Tsai, V.F. Development of a Portable Multi-Sensor Urine Test and Data Collection Platform for Risk Assessment of Kidney Stone Formation. Electronics 2020, 9, 2180. [Google Scholar] [CrossRef]
- Shi, M.; Shi, P.; Yang, X.; Zhao, N.; Wu, M.; Li, J.; Ye, C.; Li, H.; Jiang, N.; Li, X.; et al. A promising electrochemical sensor based on PVP-induced shape control of a hydrothermally synthesized layered structured vanadium disulfide for the sensitive detection of a sulfamethoxazole antibiotic. Analyst 2024, 149, 386–394. [Google Scholar] [CrossRef]
- Chen, Z.-B.; Jin, H.-H.; Yang, Z.-G.; He, D.-P. Recent advances on bioreceptors and metal nanomaterials-based electrochemical impedance spectroscopy biosensors. Rare Met. 2023, 42, 1098–1117. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, H.-M.; Guo, L.; Zhang, L.; Zhao, H.-B.; Fang, X.; Li, S.; Wang, G. Facile synthesis of CuO–Co3O4 prickly-sphere-like composite for non-enzymatic glucose sensors. Rare Met. 2022, 41, 1911–1920. [Google Scholar] [CrossRef]
- Xue, J.-H.; Sun, Q.-H.; Han, C.; Yang, Y.-D.; Xu, S.-J.; Li, Q.-P.; Qian, J.-J. FeNi Prussian blue analogues on highly graphitized carbon nanosheets as efficient glucose sensors. Rare Met. 2024, 43, 2730–2738. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyazato, T.; Hatano, T. Oxalate and Urinary Stones. World J. Surg. 2000, 24, 1154–1159. [Google Scholar] [CrossRef]
- Zafar, M.A.; Liu, Y.; Allende, S.; Jacob, M.V. Electrochemical sensing of oxalic acid using silver nanoparticles loaded nitrogen-doped graphene oxide. Carbon Trends 2022, 8, 100188. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Peng, L.; Wu, C.; Sun, X.; Hu, S.; Lin, C.; Dai, J.; Yang, J.; Xie, Y. Ultrathin Nanosheets: Giant Moisture Responsiveness of VS2 Ultrathin Nanosheets for Novel Touchless Positioning Interface. Adv. Mater. 2012, 24, 1917. [Google Scholar] [CrossRef]
- Mulazzi, M.; Chainani, A.; Katayama, N.; Eguchi, R.; Matsunami, M.; Ohashi, H.; Senba, Y.; Nohara, M.; Uchida, M.; Takagi, H.; et al. Absence of nesting in the charge-density-wave system 1T-VS2 as seen by photoelectron spectroscopy. Phys. Rev. B 2010, 82, 075130. [Google Scholar] [CrossRef]
- Vilian, A.E.; Hwang, S.-K.; Lee, M.J.; Park, B.; Huh, Y.S.; Han, Y.-K. Gold nanoparticle decorated patronite on rGO for the quantification of sulfadiazine at nanomolar levels in contaminated water. Chem. Eng. J. 2022, 439, 135782. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, A.B.; Saha, N.; Bhadu, G.R.; Adhikary, B. Newly Designed Amperometric Biosensor for Hydrogen Peroxide and Glucose Based on Vanadium Sulfide Nanoparticles. ACS Appl. Nano Mater. 2018, 1, 1339–1347. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, D.; He, T.; Li, J.; Wei, L.; Wang, D.; Wang, Y.; Wang, X.; Chen, G.; Wei, Y. Vacancy engineering in VS2 nanosheets for ultrafast pseudocapacitive sodium ion storage. Chem. Eng. J. 2021, 421, 129715. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Zhuang, W.; Zhang, H.; Wang, A.; Su, W.; Yu, J.; Lin, C.-T. Enhanced electrochemical voltammetric fingerprints for plant taxonomic sensing. Biosens. Bioelectron. 2018, 120, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Xu, Y.; Zhou, J.; Zhang, H.; Karimi-Maleh, H.; Lai, G.; Zhao, S.; et al. Development of an electrochemical biosensor for phylogenetic analysis of Amaryllidaceae based on the enhanced electrochemical fingerprint recorded from plant tissue. Biosens. Bioelectron. 2020, 159, 112212. [Google Scholar] [CrossRef]
- Sun, R.; Wei, Q.; Sheng, J.; Shi, C.; An, Q.; Liu, S.; Mai, L. Novel layer-by-layer stacked VS2 nanosheets with intercalation pseudocapacitance for high-rate sodium ion charge storage. Nano Energy 2017, 35, 396–404. [Google Scholar] [CrossRef]
- Xue, X.; Chen, R.; Yan, C.; Zhao, P.; Hu, Y.; Kong, W.; Lin, H.; Wang, L.; Jin, Z. One-Step Synthesis of 2-Ethylhexylamine Pillared Vanadium Disulfide Nanoflowers with Ultralarge Interlayer Spacing for High-Performance Magnesium Storage. Adv. Energy Mater. 2019, 9, 1900145. [Google Scholar] [CrossRef]
- Liang, H.; Shi, H.; Zhang, D.; Ming, F.; Wang, R.; Zhuo, J.; Wang, Z. Solution Growth of Vertical VS2 Nanoplate Arrays for Electrocatalytic Hydrogen Evolution. Chem. Mater. 2016, 28, 5587–5591. [Google Scholar] [CrossRef]
- Liu, X.; Shuai, H.-L.; Huang, K.-J. A label-free electrochemical aptasensor based on leaf-like vanadium disulfide-Au nanoparticles for the sensitive and selective detection of platelet-derived growth factor BB. Anal. Methods 2015, 7, 8277–8284. [Google Scholar] [CrossRef]
- Alov, N.; Kutsko, D.; Spirovova, I.; Bastl, Z. XPS study of vanadium surface oxidation by oxygen ion bombardment. Surf. Sci. 2006, 600, 1628–1631. [Google Scholar] [CrossRef]
- Chia, X.; Ambrosi, A.; Lazar, P.; Sofer, Z.; Pumera, M. Electrocatalysis of layered Group 5 metallic transition metal dichalcogenides (MX2, M = V, Nb, and Ta; X = S, Se, and Te). J. Mater. Chem. A 2016, 4, 14241–14253. [Google Scholar] [CrossRef]
- Yao, K.; Zheng, K.; Liu, L.; Yu, H.; Cheng, S.; Rui, X. Chemically Binding Vanadium Sulfide in Carbon Carriers to Boost Reaction Kinetics for Potassium Storage. ACS Appl. Mater. Interfaces 2022, 14, 22389–22397. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-J.; Wang, Y.; Wang, X.-L.; Li, S.-L.; Huang, W.; Dong, L.-Z.; Liu, C.-H.; Li, Y.-F.; Lan, Y.-Q. Molybdenum Disulfide/Nitrogen-Doped Reduced Graphene Oxide Nanocomposite with Enlarged Interlayer Spacing for Electrocatalytic Hydrogen Evolution. Adv. Energy Mater. 2016, 6, 1600116. [Google Scholar] [CrossRef]
- Xue, Y.; Zuo, Z.; Li, Y.; Liu, H.; Li, Y. Graphdiyne-Supported NiCo2S4 Nanowires: A Highly Active and Stable 3D Bifunctional Electrode Material. Small 2017, 13, 1700936. [Google Scholar] [CrossRef]
- Wu, D.; Wang, C.; Wu, M.; Chao, Y.; He, P.; Ma, J. Porous bowl-shaped VS2 nanosheets/graphene composite for high-rate lithium-ion storage. J. Energy Chem. 2020, 43, 24–32. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.-C.; Hong, Y.-T.; Lee, T.-W.; Huang, J.-F. Facile fabrication of ascorbic acid reduced graphene oxide-modified electrodes toward electroanalytical determination of sulfamethoxazole in aqueous environments. Chem. Eng. J. 2018, 352, 188–197. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, Q.; Li, X.; Wu, L.; Yu, A.; Lai, G.; Fu, L.; Wei, Q.; Dai, D.; Jiang, N.; et al. A Double-Deck Structure of Reduced Graphene Oxide Modified Porous Ti3C2Tx Electrode towards Ultrasensitive and Simultaneous Detection of Dopamine and Uric Acid. Biosensors 2021, 11, 462. [Google Scholar] [CrossRef]
- Randles, J.E.B. A cathode ray polarograph. Part II—The current-voltage curves. Trans. Faraday Soc. 1948, 44, 327–338. [Google Scholar] [CrossRef]
- Ševčík, A. Oscillographic polarography with periodical triangular voltage. Collect. Czechoslov. Chem. Commun. 1948, 13, 349–377. [Google Scholar] [CrossRef]
- Kesavan, L.; Kalekar, A.M.; Damlin, P.; Kvarnström, C. Reduced graphene oxide supported palladium nano-shapes for electro-oxidation of oxalic acid. J. Electroanal. Chem. 2019, 847, 113167. [Google Scholar] [CrossRef]
- Nagarajan, R.D.; Sundramoorthy, A.K. One-pot electrosynthesis of silver nanorods/graphene nanocomposite using 4-sulphocalix [4] arene for selective detection of oxalic acid. Sens. Actuators B Chem. 2019, 301, 127132. [Google Scholar] [CrossRef]
- Venkadesh, A.; Mathiyarasu, J.; Radhakrishnan, S. Electrochemical Enzyme-free Sensing of Oxalic Acid Using an Amine-mediated Synthesis of CuS Nanosphere. Anal. Sci. 2021, 37, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Nayeri, S. Graphite/Ag/AgCl nanocomposite as a new and highly efficient electrocatalyst for selective electroxidation of oxalic acid and its assay in real samples. Mater. Sci. Eng. C 2019, 100, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, L.; Yang, J.; Li, S.; Li, M.; Mo, Q.; Li, Y.; Li, X. Electrochemically simultaneous detection of ascorbic acid, sulfite and oxalic acid on Pt-Pd nanoparticles/chitosan/nitrogen doped graphene modified glassy carbon electrode: A method for drug quality control. Microchem. J. 2021, 169, 106623. [Google Scholar] [CrossRef]
| Modified Electrodes | Measurements | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| Pd nanocubes/rGO/GCE | DPV | 49.5 μM–10 mM | 50 μM | [42] |
| Ag nanorods/graphene/GCE | CV | 3–30 mM | 40 μM | [43] |
| CuS nanosphere/GCE | DPV | 50–700 μM | 35.6 μM | [44] |
| Graphite/Ag/AgCl | DPV | 10–750 μM | 3.7 μM | [45] |
| Ag nanoparticles/N-GO/GCE | Amperometry | 10–300 μM | 2 μM | [17] |
| Pt-Pd nanoparticles/chitosan/N-GO/GCE | CV | 1.5–500 μM | 0.84 μM | [46] |
| VS2/GCE | DPV | 0.2–20 μM | 0.188 μM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Sun, Z.; Shi, P.; Zhao, N.; Sun, K.; Ye, C.; Li, H.; Jiang, N.; Fu, L.; Zhou, Y.; et al. Enhanced Electrochemical Sensing of Oxalic Acid Based on VS2 Nanoflower-Decorated Glassy Carbon Electrode Prepared by Hydrothermal Method. Biosensors 2024, 14, 387. https://doi.org/10.3390/bios14080387
Wu M, Sun Z, Shi P, Zhao N, Sun K, Ye C, Li H, Jiang N, Fu L, Zhou Y, et al. Enhanced Electrochemical Sensing of Oxalic Acid Based on VS2 Nanoflower-Decorated Glassy Carbon Electrode Prepared by Hydrothermal Method. Biosensors. 2024; 14(8):387. https://doi.org/10.3390/bios14080387
Chicago/Turabian StyleWu, Mengfan, Zhuang Sun, Peizheng Shi, Ningbin Zhao, Kaiqiang Sun, Chen Ye, He Li, Nan Jiang, Li Fu, Yunlong Zhou, and et al. 2024. "Enhanced Electrochemical Sensing of Oxalic Acid Based on VS2 Nanoflower-Decorated Glassy Carbon Electrode Prepared by Hydrothermal Method" Biosensors 14, no. 8: 387. https://doi.org/10.3390/bios14080387
APA StyleWu, M., Sun, Z., Shi, P., Zhao, N., Sun, K., Ye, C., Li, H., Jiang, N., Fu, L., Zhou, Y., & Lin, C.-T. (2024). Enhanced Electrochemical Sensing of Oxalic Acid Based on VS2 Nanoflower-Decorated Glassy Carbon Electrode Prepared by Hydrothermal Method. Biosensors, 14(8), 387. https://doi.org/10.3390/bios14080387








