Abstract
The development of tools to quickly identify the fate of damaged trees after a stress event such as a wildfire is of great importance. In this context, an innovative approach to assess irreversible physiological damage in trees could help to support the planning of management decisions for disturbed sites to restore biodiversity, protect the environment and understand the adaptations of ecosystem functionality. The vitality of trees can be estimated by several physiological indicators, such as cambium activity and the amount of starch and soluble sugars, while the accumulation of ethanol in the cambial cells and phloem is considered an alarm sign of cell death. However, their determination requires time-consuming laboratory protocols, making the approach impractical in the field. Biosensors hold considerable promise for substantially advancing this field. The general objective of this review is to define a system for quantifying the plant vitality in forest areas exposed to fire. This review describes recent electrochemical biosensors that can detect plant molecules, focusing on biosensors for glucose, fructose, and ethanol as indicators of tree vitality.
1. Introduction
Plants are exposed to various stress factors throughout their growth and development, from germination to the end of their life cycle. The ability to cope with them by modulating physiological mechanisms determines the ability to survive and maintain an ecological niche within an ecosystem under environmental constraints (i.e., resilience). The ability of plants to cope with stress is also exploited in nature-based solutions for climate change mitigation in urban areas, where plants have a pivotal role in human wellness and environmental safety [1]. Plant stressors are generally divided into two categories: abiotic and biotic stress. Abiotic stress refers to the adverse effects of non-living components on plant physiology caused by suboptimal or extreme environmental conditions such as heat, excess salt, flooding, water limitation, pollution, etc. The constant interaction between plants and environmental constraints disrupts the natural biological processes and eventually leads to increased susceptibility to disease, slowed development, or even death of the plant [2]. Trees control their functionality through acclimation mechanisms under suboptimal conditions by modulating photosynthesis, respiration, growth, nutrient transfer, and water. Over time, the acclimation response evolves into adaptive mechanisms to withstand extreme and persistent environmental stresses [3,4,5]. In this framework, there is an urgent need to explore the physiological mechanisms controlling plant acclimation and adaption strategies to develop new approaches and tools to improve ecosystem resilience and mitigation capacity, as recent forecasting models predict an increase in the frequency and severity of adverse environmental conditions.
Wildfires are one of the most important natural disturbances affecting plant ecosystems worldwide by impairing tree growth [6]. In Europe, fires are traditionally recurrent in southern countries characterized by a Mediterranean climate, and the EU Biodiversity Strategy for 2030 considers this threat as an “immediate priority” to be addressed. Recent evidence shows that 61% of wildfires affect forests, and around 25% of these areas are in the EU’s Natura 2000 biodiversity hotspots. Nevertheless, climate change is expected to further increase fire risk across Europe, as has already been the case over the last decade [7]. To tackle the problem of wildfires in these areas, the new EU Forest Strategy for 2030 strengthens measures to prevent forest fires and promote better resilience to climate change.
The damage that a fire causes to vegetation depends on the heat flows that are transferred to the different parts of the plant and is a function of several factors, such as fireline intensity, dwell time, and the rate of spread [8,9] (Figure 1). Within the same fire, fire behavior changes over time and space depending on the initiating factors (e.g., topography, meteorological conditions, fuel load, suppression measures, etc.), resulting in different heat fluxes and effects on the trees. High-intensity crown fires consume live and dead fuels, and burning of all the foliage and meristems in a tree crown can result in immediate tree death unless the tree can resprout from heat-resistant organs like epicormic sprouts (suckers) and adventitious shoots [10,11]. In contrast, low to moderate-intensity fires often do not pose a direct lethal threat to mature trees but can result in a variety of injuries that can affect their health. Cell death, caused by protein denaturation, is generally considered to be complete at 60 °C [12]. The rate of cell necrosis increases exponentially with temperature, and even lower temperatures can lead to cell death with prolonged exposure [13]. According to Bär et al. (2019), the tree may die immediately after the forest fire, or it may show signs of metabolic imbalance or progressive physical or biotic damage that may not become apparent until years later [14]. However, the response of plant functions to fire damage can vary greatly, also depending on the species, period of the year, tree age, etc. This means that trees that have survived the fire may exhibit different levels of physiological functionality, which may result in reduced growth or be more likely to succumb to delayed death [15,16].

Figure 1.
Three examples of Maritime pine plants located in the municipality of Vicopisano, Italy, damaged by fire. The plants were photographed in February 2022; the wildfire that damaged them was in August 2021. Trees that are considered alive and have green, unburnt foliage (left); trees with unknown vitality; they have both burnt and unburnt foliage (center); trees that are considered dead because they are completely burnt (right).
Currently, tree damage is assessed using empirical methods (visual or sensory assessment), anatomical observation of cambium vitality, or biochemical methods, which are not easy to apply in the field [14]. Nowadays, it is postulated that a few compounds, such as non-structural carbohydrates (soluble sugars and starch) or ethanol, can be considered valuable proxies for predicting the recovery of an injured tree after a wildfire [17,18]. Traditional biochemical monitoring methods for these compounds, such as gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC), are sensitive and effective but time-consuming and require sample pretreatment and trained personnel (Figure 2). Practical monitoring programs require fast, simple, and cost-effective screening methods for the detection of proxies of tree health. Biosensors offer all these advantages as they can be easily deployed both in the laboratory and in the field (Figure 3).

Figure 2.
(A) The GC-MS (Agilent, Santa Clara, CA, USA) and (B) the HPLC (PerkinElmer, Waltham, MA, USA) instruments used at the CNR (Florence, Italy) for sugar analysis in trees.
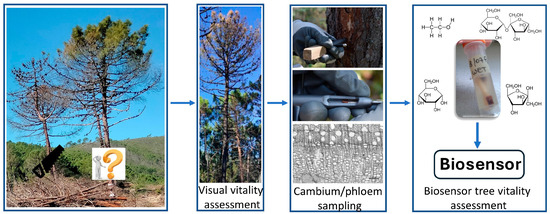
Figure 3.
Schematic approach for the use of biosensors for the analysis of target molecules to assess the vitality of fire-damaged trees. The arrows indicate the sequential steps for determining the vitality of a tree damaged by a forest fire.
The development of user-friendly new methods for assessing the vitality of fire-damaged trees should be very useful to support forestry professionals in planning and designing post-fire reforestation. In this context, this review describes some examples of enzymatic amperometric biosensors that can determine the concentration of signaling molecules in the stem tissue of injured trees and have been selected as indicators (proxies) of tree vitality to directly distinguish between damaged trees destined to die and those that are recovering.
2. Biosensors
Biosensor technology offers fast, real-time testing and online measurements at low cost. The use of this technology as a forward-looking approach to understanding and controlling biological systems is extremely promising. A biosensor is an analytical instrument where a biological recognition element (bioreceptor) is coupled to a transducer (Figure 4).
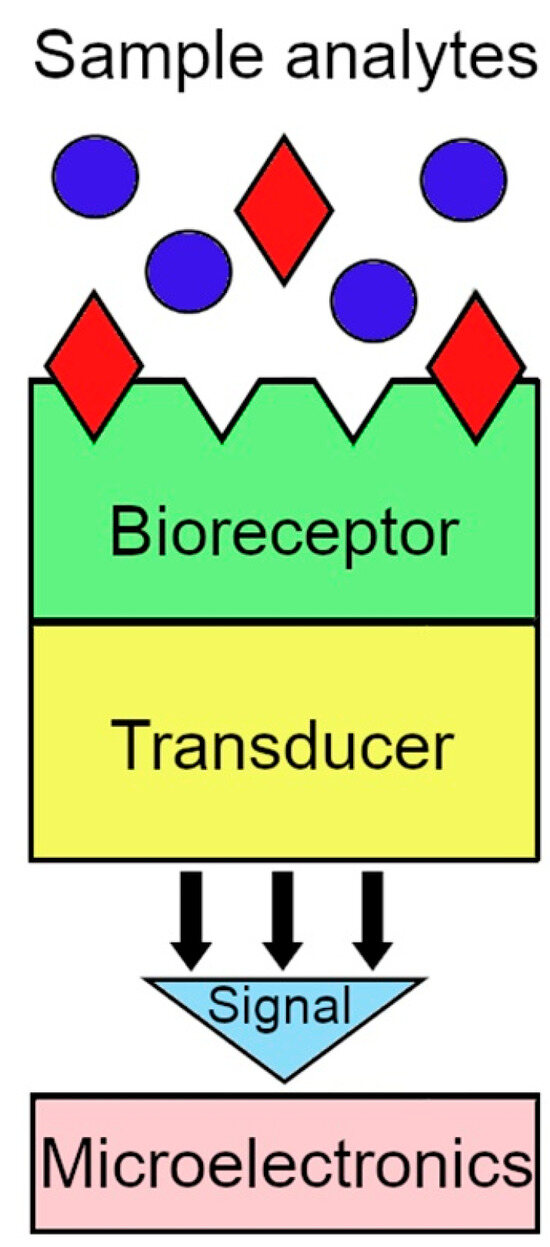
Figure 4.
General scheme of a biosensor.
The bioreceptor, also known as a biological recognition element, is generally an enzyme or a molecular receptor, such as an antibody, immobilized on the surface of the transducer. Biosensors can be categorized as immunochemical, enzymatic, non-enzymatic receptor, whole cell, or DNA biosensors based on the concept of biological recognition [19,20,21,22,23]. The role of the transducer is to convert the biological recognition event into a measurable signal that provides quantitative or semi-quantitative analytical information [24]. Specific interactions between the chemical target and the bioreceptor lead to a physico-chemical change that is recognized and measured by the transducer. In the presence of the analyte, the bioreceptor generates a signal that corresponds to its concentration. The ideal biosensor should be both selective and sensitive, have a low signal-to-noise ratio, provide a quantitative dose–response curve over physiologically relevant analyte concentrations, be non-invasive, and allow in vivo analysis [25].
The most used types of measurement are optical, electrochemical, and thermal [26,27,28,29,30,31]. The electronic circuit, the transducer, and the bioreceptor are the three basic components [32].
Biosensors have the potential to be used in a wide range of applications, such as clinical diagnosis by analyzing drugs and pharmaceuticals, mining and toxic gas monitoring, military and defensive applications, waste management by monitoring environmental pollution and microbial contamination, and personal safety in space travel [33,34,35]. Most commercially available biosensor systems are used in the pharmaceutical and healthcare industries [33,36,37,38]. A thorough understanding of biochemistry, electron flow, cofactor involvement, and interfering conditions is crucial for the development of an efficient system. A real-time safety monitoring system is ensured by the close connection between the detected signal, the triggered biological reaction, and the chemical contact. It is also important to separate the interaction of chemicals with bioreceptors from physical factors such as pH and temperature. One of the most important properties of the biosensor is the selectivity of the bioreceptor for the specific chemical target, which is maintained even in the presence of other potentially interfering species. The selectivity of biosensors, which enables real-time in-situ measurements, together with their small size, low cost, and wide range of potential applications, has attracted considerable commercial interest. Other advantages include high sensitivity, fast response time, minimal or no sample pretreatment, ease of use by non-experts, and the ability to regenerate and reuse the immobilized bioreceptor, enabling continuous or multiple testing. However, several problems associated with bioreceptors, such as their low stability and high cost, the need for cofactors, and difficulties with immobilization technology, have prevented their widespread commercialization.
3. Plant Biosensors
Monitoring the concentration of small molecules in plants is an important technique for obtaining early information on plant stress. Detecting dynamic changes in small molecule concentrations in situ is one of the most unique ways to obtain real-time plant health data. Plant health monitoring is a good strategy to support sustainable agriculture and increase crop yields while reducing the impact on the environment.
To date, novel biosensors have been proposed to monitor environmental conditions and plant growth, pesticide management, plant stress, and plant viruses [39,40,41,42,43,44,45]. Moreover, many electrochemical sensors have been developed for the in vivo detection of plant molecules [46,47,48,49].
Sneha et al. (2023) developed a biosensor based on an organic electrochemical transistor capable of detecting in vivo changes in ion concentration in plant sap [50]. The plant sap that flows through the xylem and phloem in the living plant was used as the electrolyte. In their recent study, Ruiz-Gonzalez et al. (2023) describe a sensor capable of simultaneously measuring K+ and plant sap pH in living plants using reverse iontophoresis and SPE modified by the deposition of a K+-selective membrane [51].
Plant-wearable sensors are increasingly being used in agriculture to monitor plant health. These devices can help detect diseases or abnormalities in plants at an early stage by monitoring their health in real time [52,53,54]. The ideal wearable sensor should be easily attached to different parts of the plant to monitor plant health on-site by collecting physiological data in real time. Wearable sensors for in vivo detection of small molecules in plants are either electrochemical sensors that extract molecules by reverse iontophoresis or implantable sensors that come into direct contact with the plant sap. The recorded data are converted into electrical signals and processed to monitor plant health by detecting abnormal changes in the concentration of small molecules.
Another interesting approach that has already been applied to the biosensing of plants is biosensing with microneedles [55]. Biosensors using microneedles can be non-invasive and portable, ensuring that the analysis takes place under normal plant development conditions. Microneedles can easily penetrate the plant epidermis and perform auxiliary work with high efficiency. They are less damaging to the plants, have a high monitoring sensitivity, and are easy to integrate [56]. Sensors with microneedles can detect the sap flow rate, collect the sap, and analyze its physicochemical parameters. For instance, Baek et al. developed a microneedle sap flow sensor to monitor water transport in tomato stems, and Jiao et al. (2019) developed a microneedle sensor to detect plant nitrate [57,58].
Recently, several wearable biosensors have been developed for the detection of glucose in plants [56]. They use the enzyme glucose oxidase (GOX), which catalyzes the oxidation of glucose to gluconolactone, which is then converted to gluconic acid. After electrochemical reduction to water or oxidation to oxygen, the oxygen in the solution is converted to hydrogen peroxide, which undergoes a redox reaction at the surface of the working electrode, resulting in a change in current. One of the main issues with this type of biosensor is the protection of the bioreceptors when the electrodes are inserted into the plant tissue. Researchers have used hydrogel-based microneedles as a new strategy to solve this problem [59,60]. Chen et al. (2024) recently presented an electrochemical microneedle sensor for continuous real-time monitoring of glucose in tomato and aloe vera plants. The sensor showed an LOD of 33.3 μM and a sensitivity of 17 nA/μM/cm2 [61]. Zheng et al. (2022) used hydrogel hollow silk fibroin-proline-based microneedles as a protective material for the bioreceptor [59]. During glucose detection, the shell of the hydrogel absorbs the solution and expands when it comes into contact with plant sap, allowing the liquid to penetrate the microneedles and come into contact with the electrode. The concentration ranges of glucose that the developed sensor was able to detect were 3–18 mM and 30–180 mM, with a sensitivity of 21.21 nA/mM. The sensor was able to determine the glucose content in a Solanum lycopersicum fruit without damaging it, with results similar to those of a blood glucose meter. Zhao et al. (2020) designed a microneedle-based biosensor for continuous glucose monitoring [62]. The device consisted of three pyramidal microneedles made of silk/D-sorbitol with immobilized GOx. The biosensor showed a linear range from 1.7 to 10.4 mM. The results suggest that microneedle biosensors are a promising technique for portable and continuous glucose monitoring.
Diacci et al. (2021) developed an implantable organic electrochemical transistor-based biosensor for the in vivo and real-time monitoring of glucose fluctuations in tree vascular tissue [63]. This is a proof-of-concept study in which implantable organic electrochemical transistor biosensors enable real-time monitoring of metabolites in plants and provide new insights into diurnal sugar homeostasis. The biosensor showed a linear range of 0.1 to 1.0 mM. This technology can be used to qualitatively analyze different metabolites and evaluate the effects of abiotic and biotic stress on them. In a recent work, Bukhamsin et al. (2022) presented a plant-wearable sensor with functionalized microneedle-based electrodes for the in situ detection of salicylic acid (LOD = 2.74 μM) [64]. They showed that their biosensor could serve as a promising platform for continuous and non-destructive monitoring in the field. Perdomo et al. (2023) developed a wearable electrochemical biosensor for the detection of glucose extracted from plant leaves through reverse iontophoresis [65]. This technology enables non-invasive in situ and in vivo identification of early stress responses in plants in real time and provides a unique tool for the timely agronomic management of crops. The biosensor showed a sensitivity of 22.7 nA/μM/cm2 and a limit of detection (LOD) of 9.4 μM. The same group recently presented another sensor system that enables non-invasive, real-time monitoring of salicylic acid levels in avocado trees [66]. The sensor with a reverse iontophoretic system and a graphene electrode showed a high sensitivity (82.3 nA/μM/cm2) and an LOD of 8.2 μM.
Dhanjai et al. (2020) developed a biosensor for the detection of gallic acid using a multilayer electrode prepared from layers of CNTs, cellulose nanocrystals, polyaniline, and 3-(glycidyloxypropyl)trimethoxysilane [67]. They showed that microneedles are promising for successful in situ screening of antioxidants in fruit matrices. Their biosensor showed a linear range from 0.58 to 512.6 μM with an LOD of 1.7 μM. Hossain and Tabassum (2022) developed a biosensor using a three-dimensionally printed, microneedle-based electrochemical sensor for real-time detection of salicylic acid [68]. Real-time analysis of salicylic acid in plant sap with an integrated pH correction function can help farmers respond promptly to environmental stress. The biosensor showed an LOD of 37 μM. Li et al. (2019) developed a biosensor containing a stainless-steel electrode fabricated with Au nanostructures, Pt nanoparticles, and reduced graphene oxide nanocomposite films, and a polymerized Safranine T film for in vivo detection of the phytohormone indole-3-acetic acid [69]. In vivo detection of plant signaling molecules such as indole-3-acetic acid is of great importance for precision farming, crop management, and plant phenotyping. The biosensor showed an LOD of 0.24 μΜ. Wang et al. (2021) developed a microneedle array biosensor based on Au@SnO2-vertical graphene (VG)/Ta for the detection of abscisic acid [70]. The small size, wide pH range, low LOD (0.004 μM), and wide linear concentration range (from 0.012 to 495.2 μM) allow the sensor to be used for in situ detection of abscisic acid in plants. Shao et al. (2023) developed a wireless and portable electrochemical sensor for the detection of indole-3-acetic acid in plants using screen-printed electrodes (SPEs) modified with gold nanoparticles and three-dimensionally reduced graphene oxide [71]. The sensor presented linear ranges from 0.25 to 120.0 μM and from 135 to 500 μM, and an LOD of 0.15 μM. They showed that the combination of modified SPE with small Bluetooth workstations and smartphones is very useful in creating a portable, low-cost, simple, and fast electrochemical sensing platform. Gao et al. (2021) fabricated an electrochemical sensor for the detection of free tryptophan by depositing a polydopamine-reduced graphene oxide-MnO2 nanocomposite onto a glassy carbon electrode [72]. They showed that their sensor could detect the tryptophan content in tomatoes in vitro and in vivo, demonstrating the feasibility of this research strategy for the development of electrochemical sensors for measurements in different plant tissues. The sensor presented a linear range of 1.0 to 300 μM and an LOD of 0.22 to 0.39 μM. Researchers have also developed a variety of enzyme-free glucose and fructose biosensors [73,74]. Table 1 shows some examples of electrochemical biosensors for the detection of plant molecules.

Table 1.
Examples of electrochemical biosensors for the detection of plant molecules.
4. Sugars and Ethanol as Plant Signaling Molecules in the Stress Response
The production and distribution of sugars in different tissues at different stages of plant development are tightly regulated to meet the carbon and energy needs of the system, as sugars are necessary for development and metabolism and serve as both energy sources and structural components. They influence numerous genes involved in various metabolic processes, which has led to research focusing on the identification of sugar recognition and signal transduction pathways. Sugars are formed in mature leaves and then transported via the phloem to where they are needed or stored.
Sugars in plants, such as glucose and fructose, are important signaling molecules that regulate metabolic and physiological functions [75,76,77,78,79], and they also serve as messengers for hormones during the signal transduction process [80]. Changes in the environment can result in suboptimal conditions for plants, which in turn lead to changes in metabolic processes related to acclimation response. Impairment of photosynthesis is one of the most common responses of plants to reduced water availability, high temperatures, fire, or pollution [81,82], so fluctuations in soluble sugar content in the phloem, along the axial transport system of the stem and around the sinks can be expected. Fire-induced crown scorch and bud dieback, for example, lead to a decrease in CO2 fixation, impair sugar metabolism, and lead to a depletion of non-structural carbon (soluble sugars, starch, and lipids) in the reserve compartments of the tree. If the imbalance of sugar turnover in the phloem and ray parenchyma persists for a longer period of time, the recovery of the tree may be compromised [18]. Thus, maintaining a sufficient flux of soluble sugars in the phloem plays a crucial role in maintaining the vitality of a tree after a wildfire. The lethal damage caused by crown injury and disruption of phloem flux can, therefore, be detected by changes in the amount of sugar in the phloem, ray parenchyma, and sap composition (e.g., decrease in sucrose and increase in glucose and fructose due to caramelization).
The analysis of carbohydrate content in different tissues has been used to evaluate the vitality of trees [83]. The presence of starch and glucose in the tree organs can reflect the ability of a tree to withstand severe situations [84].
In the case of fire, ethanol production is related to many physiological mechanisms, such as respiration (O2 supply), membrane destabilization (reduction of aerobic respiration and fermentation enzymes), overall enzymatic activities, sap flow (poor O2 water and accumulation), which are highly temperature dependent and lead to toxic results for the cells [17]. Therefore, quantitative analysis of these components in plants is crucial. With advances in precision agriculture, the challenge is to develop technologies for in situ and on-site detection of sugars and alcohol in plants, as researchers often need to directly detect sugar content in plants [85]. Unfortunately, existing methods for determining sugar content in plants require tissue sampling, specialized manpower, and extremely complicated and expensive equipment and procedures [18,86,87]. In addition, they often require complex pretreatment of samples and a combination of different equipment and techniques. For example, to determine the content of non-structural carbohydrates and starch, 40 mg of dried powder (from the cambial region and the mature xylem) is extracted three times with 80% ethanol solution. For each extraction, the homogenates are gently vortexed and centrifuged. The supernatant and the resulting pellet are used to determine the content of non-structural carbohydrates (HPLC) or starch (spectrophotometric method) [87]. Martinez-Trinidad et al. (2010) compared various techniques for measuring tree vitality of live oaks [88]. The results of their study suggest that a portable blood glucose meter can accurately measure glucose levels. Given the relationship between glucose and starch levels, glucose content could be used to estimate the carbohydrate content of urban trees.
Plant signaling molecules such as glucose, fructose and ethanol in plants can be determined with electrochemical biosensors as indicators of tree vitality. The following sections are dedicated to the description of recently developed biosensors that can detect glucose, fructose, and ethanol.
4.1. Amperometric Glucose Biosensors
The glucose biosensor is one of the most important bioassay devices and has already been successfully commercialized [89]. Research to develop amperometric biosensors for glucose detection is enormous as they are relatively affordable, can be easily miniaturized, and require simple electronics. Kamanina et al. (2019) developed a glucose biosensor using modified SPEs with GOx and conductive hydrogel based on a sol-gel matrix and single-walled carbon nanotubes [90]. They have shown that high-performance biosensors can be developed using enzyme-modified SPEs and conductive hydrogel. The concentration range of glucose that the developed sensor could detect was 0.045–1.04 mM. Hu et al. (2022) developed a low-cost, simple-to-manufacture, and portable electrochemical glucose biosensor with modified SPEs [91]. The GOx enzyme was immobilized in graphene aerogel and Prussian blue-modified SPEs with chitosan. The combination of graphene aerogel and Prussian blue showed good conductivity and catalytic performance. The biosensor showed a linear range of 0.5–6.0 mM with an LOD of 0.15 mM. Sakalauskiene et al. (2023) developed a reagentless amperometric glucose biosensor by combining the graphite electrode modified with gold nanostructures and Prussian blue with GOx [92]. The biosensor was easy to use and had good repeatability. The LOD (8.8 μΜ) and linear range (from 0.025 to 1.0 mΜ) were suitable for glucose determination and showed high resistance to other electroactive substances present in the real samples. Liu et al. (2023) developed a non-invasive salivary glucose sensor consisting of a Nafion-carbon nanotube nanocomposite and GOx sequentially deposited on SPEs [93]. The developed sensor showed excellent selectivity in interference tests and good performance in the artificial saliva test. Their results showed a sensitivity of 99.13 μA/mM/cm2, a linear range of 20–700 μM, and an LOD of 20 μM. Liu et al. (2023) developed an amperometric glucose biosensor on a toothbrush [94]. They coated the toothbrush with carbon-graphite ink and Ag/AgCl ink as sensor electrodes, followed by immobilization of GOx. The biosensor detected glucose in a concentration range from 0.18 mM to 5.22 mM. The biosensor is promising for non-invasive monitoring of glucose levels in the saliva of diabetic patients. Albanese et al. (2014) developed amperometric glucose biosensors by using two methods for the deposition of Prussian blue and various membranes for the immobilization of GOx [95]. The aim of their work was to develop a suitable, stable, and cost-effective glucose biosensor based on a Prussian blue-modified SPE for food analysis. The biosensors prepared using silica sol–gel immobilization showed a linear range of 0.005 to 1.0 mM and an LOD of 20 μM. Khosravi et al. (2023) developed a glucose biosensor that can be applied to a textile substrate by screen printing [96]. The biosensor showed high selectivity to glucose and excellent stability over 30 days of storage. In addition, the biosensor showed a linear response in the range of 20–1000 μM, a high sensitivity (18.41 μA/mM/cm2) and an LOD of 20 μΜ. Ang et al. (2015) developed a biosensor for the detection of glucose in fruits by immobilizing GOx on a chitosan membrane [97]. The developed biosensor showed good repeatability and reproducibility. The results of the storage stability test indicated that the immobilization process allowed the enzyme to be reused, resulting in operational stability. The wide linear detection range (0.01–15 mM) ensures good accuracy in the measurement of glucose content.
4.2. Amperometric Fructose Biosensors
Several amperometric biosensors using immobilized D-fructose dehydrogenase (FDH) for the determination of D-fructose have been reported [21,98,99,100,101]. Some of them were based on platinum electrodes [102], glassy carbon electrodes [103], or carbon paste electrodes [104]. Biscay et al. (2012) developed a fructose biosensor based on ferrocyanide-modified SPEs [105]. The biosensor showed a linear response in the range of 0.1–1.0 mM, good sensitivity (1.25 μA/mM), and an LOD of 0.05 mM. Fructose was determined in real samples with good accuracy. Suzuki et al. (2020) developed a fructose biosensor by immobilizing a variant of FDH on a porous gold microelectrode [106]. The biosensor showed an LOD of 2.0 mM and a sensitivity of 200 ± 20 μA/mM/cm2, which was only dependent on temperature. Therefore, the sensor-enabled rapid detection without calibration at constant temperature. Trivedi et al. (2009) developed a low-cost, portable, and disposable fructose biosensor using FDH [107]. The biosensor showed a linear response in the range from 3 to 13 mM and an LOD of 0.65 μΜ. Siepenkoetter et al. (2017) developed a biosensor based on FDH on nanoporous gold electrodes [108]. After a very fast response time (<5 s), the biosensor showed accurate readings (linear response in the range of 0.05–0.3 mM and an LOD of 1.2 μM) and a high specificity for d-fructose in the presence of interfering sugars. Antiochia and Gorton (2014) developed a fructose biosensor based on an osmium-polymer modified graphene SPEs [109]. They have successfully produced a simple and low-cost biosensor that uses osmium polymer as both a mediator and a support material. The biosensor showed an LOD of 0.8 μM, a linear range from 0.1 to 8.0 mM, and high sensitivity (2.15 μA/mM/cm2). Bollella et al. (2018) developed a sensitive membrane-less fructose biosensor based on FDH immobilized on a highly porous gold electrode modified with aryl thiol [110]. The biosensor responded rapidly, showed great stability (>90% of the signal remained after 90 days), high catalytic current density (920 μA/cm2), selectivity and sensitivity (175 ± 15 μA/mM/cm2) with the lowest detection limit (0.3 μM).
4.3. Amperometric Ethanol Biosensors
Alcohol biosensors commonly use alcohol dehydrogenase (ADH) and alcohol oxidase (AOX) to detect alcohol [111,112,113]. ADH and AOX catalyze processes that can be easily measured with commercially available electrochemical transducers. The most commonly used alcohol biosensors are the AOX biosensors. AOX oxidizes low molecular weight alcohols to aldehydes using molecular oxygen (O2) as an electron acceptor: RCH2OH + O2 → RCHO + H2O2. Due to the strong oxidizing properties of O2, AOX oxidizes alcohols irreversibly. The consumption of O2 or the formation of H2O2 can be measured electrochemically with amperometric electrodes by monitoring either the anodic or cathodic reaction caused by the oxidation or reduction of molecules on the surface of the working electrode.
Many amperometric biosensors that use immobilized ADH or AOX for the determination of ethanol have been reported [114,115,116,117]. Bilgi and Ayranci (2018) developed an amperometric ethanol biosensor based on ADH [111]. The biosensor exhibited the following analytical characterization parameters: linear range of 178.5 to 1000 μM, an LOD = 53.5 μM, and a sensitivity of 0.432 μA/mM. The biosensor provided good results when analyzing ethanol in a commercial alcoholic beverage. Zhang et al. (2021) proposed a screen-printed biosensor for ethanol analysis in fermentation based on the development of a well-defined nanocubic structure of a nanocomposite of gold nanoparticles and nickel hexacyanoferrate [112]. The biosensor showed excellent sensitivity with a high anti-interference capability to ensure accurate detection in a viscous and colored fermentation broth. This biosensor also showed good reproducibility and storage stability with repeated use over 30 days. Istrate et al. developed a sensitive ethanol biosensor based on ADH immobilized on the surface of a modified SPE with a nanocomposite material [113]. The biosensor showed good sensitivity (44.6 ± 0.07 µA/mM/cm2), low LOD (10 μM), good reproducibility, and stability of up to 6 weeks. Stasyuk et al. (2022) presented an amperometric biosensor based on AOX and peroxidase-like nanozymes for ethanol determination [116]. The developed biosensor showed a high sensitivity (260 μA/mM/cm2), a linear range from 5 to 100 µM, fast response, and low LOD (1.5 µM). They observed a high correlation between the ethanol content in real samples determined with the proposed biosensor and the colorimetric reference method. An amperometric bi-enzymatic ethanol biosensor based on AOX and horseradish peroxidase was presented by Hooda et al. (2018) [117]. The biosensor showed a fast (8 s) and linear response to ethanol in the range of 0.01–50 mM, an LOD of 0.1 nM, storage stability of 190 days, and a sensitivity of 155 µA/mM/cm2. Recently, the same group developed another ethanol biosensor in which AOX was immobilized on gold nanoparticles [118]. The biosensor showed a linear response from 0.01 mM to 42 mM, an LOD of 0.1 nM, and a storage stability of 180 days. Table 2 shows some examples of electrochemical biosensors for the detection of glucose, fructose, and ethanol.

Table 2.
Examples of electrochemical biosensors for the detection of glucose, fructose, and ethanol.
5. Perspectives
The responses of plants to environmental constraints can vary greatly depending on the species, tree age, intensity, and frequency of the events. This means that trees that survive a fire may exhibit different physiological functions, resulting in reduced growth or, more likely, delayed death [15,119,120]. On the other hand, it is known that damaged trees can also benefit from reduced competition in the short and medium term [121,122,123]. Therefore, assessing fire injury to trees and irreversible physiological damage by identifying reliable proxies is a crucial step in planning the best practices to mitigate the consequences of fires and accelerate the regeneration processes of trees and/or restore biodiversity. For example, immediate detection of injury to trees could improve the knowledge of the compounds’ dynamics of post-fire tree mortality and forest recovery, and in this contest, biosensors are the best tool.
6. Conclusions
The aim of this review was to define a system to quantify plant vitality in forest areas exposed to fire. The review describes recent electrochemical biosensors that can determine plant molecules, focusing on the biosensing of glucose, fructose, and ethanol as indicators of tree vitality. Based on a comprehensive review of the current literature on biosensor technology, we conclude that electrochemical biosensors could be useful in quantifying the effects of forest fires on plant vitality.
Author Contributions
Conceptualization, E.T. and A.G.; resources, E.T. and A.G.; data curation, E.T., I.C.M., R.M.Z. and A.G.; writing—original draft preparation, E.T. and A.G.; writing—review and editing, T.D.L., C.C., N.F., E.M., C.F., N.R., V.G.M. and M.L.T.; supervision, E.T., C.C., E.M. and A.G.; funding acquisition, E.T., C.C., E.M. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Recovery and Resilience Plan (NRRP), Mission 4, Component C2, Investment 1.1—Call for tender No. 1409 of 14 September 2022—"Progetti di Ricerca di Rilevante interesse Nazionale—PRIN” of Italian Ministry of University and Research funded by the European Union—NextGenerationEU, Grant No. P2022Z5742, CUP B53D23023780001, Project title “Developing of innovative methods to assess tree vitality after a wildfire through analyses of cambium sugars metabolism—DIVAS”; by the National Recovery and Resilience Plan, Mission 4 Component 2—Investment 1.4—National Center for HPC, Big Data and Quantum Computing—funded by the European Union—NextGenerationEU—CUP (B83C22002830001) and by the European Union’s Horizon Europe—the Framework Programme for Research and Innovation [grant number 101093150], project LIBRA (Light Based Multisensing Device for Screening of Pathogens and Nutrients in Bioreactors), CUP B53C23000560006.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Goodwin, S.; Olazabal, M.; Castro, A.J.; Pascual, U. Global mapping of urban nature-based solutions for climate change adaptation. Nat. Sustain. 2023, 6, 458–469. [Google Scholar] [CrossRef]
- Anzano, A.; Bonanomi, G.; Mazzoleni, S.; Lanzotti, V. Plant metabolomics in biotic and abiotic stress: A critical overview. Phytochem. Rev. 2022, 21, 503–524. [Google Scholar] [CrossRef]
- van Zanten, M.; Ai, H.; Quint, M. Plant thermotropism: An underexplored thermal engagement and avoidance strategy. J. Exp. Bot. 2021, 72, 7414–7420. [Google Scholar] [CrossRef] [PubMed]
- Cocozza, C.; Traversi, M.L.; Giovannelli, A. Tree growth conditions are demanded when optimal, are unwanted when limited, but when are they suboptimal? Plants 2021, 10, 1943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ response to abiotic stress: Mechanisms and strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.-L.; Gao, Q.-X. Contributions of natural systems and human activity to greenhouse gas emissions. Adv. Clim. Chang. Res. 2018, 9, 243–252. [Google Scholar] [CrossRef]
- Carnicer, J.; Alegria, A.; Giannakopoulos, C.; Di Giuseppe, F.; Karali, A.; Koutsias, N.; Lionello, P.; Parrington, M.; Vitolo, C. Global warming is shifting the relationships between fire weather and realized fire-induced CO2 emissions in Europe. Sci. Rep. 2022, 12, 10365. [Google Scholar] [CrossRef] [PubMed]
- Michaletz, S.T.; Johnson, E.A. How forest fires kill trees: A review of the fundamental biophysical processes. Scand. J. For. Res. 2007, 22, 500–515. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Hiers, J.K.; Varner, J.M.; Hoffman, C.M.; Dickinson, M.B.; Michaletz, S.T.; Loudermilk, E.L.; Butler, B.W. Advances in mechanistic approaches to quantifying biophysical fire effects. Curr. For. Rep. 2018, 4, 161–177. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Epicormic resprouting in fire-prone ecosystems. Trends Plant Sci. 2017, 22, 1008–1015. [Google Scholar] [CrossRef]
- Rosenberg, B.; Kemeny, G.; Switzer, R.C.; Hamilton, T.C. Quantitative evidence for protein denaturation as the cause of thermal death. Nature 1971, 232, 471–473. [Google Scholar] [CrossRef]
- Dickinson, M.B.; Johnson, E.A. Temperature-dependent rate models of vascular cambium cell mortality. Can. J. For. Res. 2004, 34, 546–559. [Google Scholar] [CrossRef]
- Bär, A.; Michaletz, S.T.; Mayr, S. Fire effects on tree physiology. New Phytol. 2019, 223, 1728–1741. [Google Scholar] [CrossRef]
- Nesmith, J.; Das, A.; O’Hara, K.; van Mantgem, P. The influence of pre-fire tree growth and crown condition on post-fire mortality of sugar pine following prescribed fire in Sequoia National Park. Can. J. For. Res. 2015, 45, 910–919. [Google Scholar] [CrossRef]
- Maringer, J.; Ascoli, D.; Küffer, N.; Schmidtlein, S.; Conedera, M. What drives European beech (Fagus sylvatica L.) mortality after forest fires of varying severity? For. Ecol. Manag. 2016, 368, 81–93. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Douglas, W.J. Physiological stress and ethanol accumulation in tree stems and woody tissues at sublethal temperatures from fire. BioScience 2017, 67, 443–451. [Google Scholar] [CrossRef]
- Reed, C.C.; Hood, S.M. Nonstructural carbohydrates explain post-fire tree mortality and recovery patterns. Tree Physiol. 2024, 44, tpad155. [Google Scholar] [CrossRef]
- Moraskie, M.; Roshid, M.H.O.; O’Connor, G.; Dikici, E.; Zingg, J.M.; Deo, S.; Daunert, S. Microbial whole-cell biosensors: Current applications, challenges, and future perspectives. Biosens. Bioelectron. 2021, 191, 113359. [Google Scholar] [CrossRef] [PubMed]
- Wijayanti, S.D.; Tsvik, L.; Haltrich, D. Recent advances in electrochemical enzyme-based biosensors for food and beverage analysis. Foods 2023, 12, 3355. [Google Scholar] [CrossRef] [PubMed]
- Sadani, K.; Nag, P.; Thian, X.Y.; Mukherji, S. Enzymatic optical biosensors for healthcare applications. Biosens. Bioelectron. X 2022, 12, 100278. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, J.; Chao, J. Recent advances in DNA-based electrogenerated chemiluminescence biosensors. Sens. Diagn. 2023, 2, 582–599. [Google Scholar] [CrossRef]
- Shan, C.W.; Chen, Z.; Han, G.C.; Feng, X.Z.; Kraatz, H.B. Electrochemical immuno-biosensors for the detection of the tumor marker alpha-fetoprotein: A review. Talanta 2024, 271, 125638. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Sadanandom, A.; Napier, R.M. Biosensors in plants. Curr. Opin. Plant Biol. 2010, 13, 736–743. [Google Scholar] [CrossRef]
- Danielsson, B. Calorimetric biosensors. Biochem. Soc. Trans. 1991, 19, 26–28. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.J.; Yang, Z.; Wilkinson, S.; Zhou, X. Optical biosensors based on refractometric sensing schemes: A review. Biosens. Bioelectron. 2019, 144, 111693. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.S. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Ong, J.J.; Pollard, T.D.; Goyanes, A.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Optical biosensors—Illuminating the path to personalized drug dosing. Biosens. Bioelectron. 2021, 188, 113331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, W.; Lv, C.; Liu, X.; Yang, M.; Guo, M.; Fu, Q. Electrochemical biosensors represent promising detection tools in medical field. Adv. Sens. Energ. Mat. 2023, 2, 100081. [Google Scholar] [CrossRef]
- Sumitha, M.S.; Xavier, T.S. Recent advances in electrochemical biosensors—A brief review. Hybrid Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Singh, A.K.; Mittal, S.; Das, M.; Saharia, A.; Tiwari, M. Optical biosensors: A decade in review. Alex. Eng. J. 2023, 67, 673–691. [Google Scholar] [CrossRef]
- Roda, A.; Mirasoli, M.; Guardigli, M.; Zangheri, M.; Caliceti, C.; Calabria, D.; Simoni, P. Advanced biosensors for monitoring astronauts’ health during long-duration space missions. Biosens. Bioelectron. 2018, 111, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Gavrilaș, S.; Ursachi, C.Ș.; Perța-Crișan, S.; Munteanu, F.D. Recent trends in biosensors for environmental quality monitoring. Sensors 2022, 22, 1513. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhu, J.; Wu, W.; Wang, N.; Wang, J.; Wu, J.; Wu, Q.; Wang, X.; Yu, C.; Wei, G.; et al. Wearable sweat biosensors refresh personalized health/medical diagnostics. Research 2021, 2021, 9757126. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Sayson, D.; Nguyen, B.H.; Tran, S.D. Advances in medical wearable biosensors: Design, fabrication and materials strategies in healthcare monitoring. Molecules 2022, 27, 165. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Brito, N.F.; Szulczyński, B.; Wojciechowski, M.; Buda, N.; Melo, A.C.A.; Kamysz, W.; Gębicki, J. Olfactory receptor-based biosensors as potential future tools in medical diagnosis. TrAC Trends Anal. Chem. 2022, 150, 116599. [Google Scholar] [CrossRef]
- Nassar, J.M.; Khan, S.M.; Villalva, D.R.; Nour, M.M.; Almuslem, A.S.; Hussain, M.M. Compliant plant wearables for localized microclimate and plant growth monitoring. Npj Flex. Electron. 2018, 2, 24. [Google Scholar] [CrossRef]
- Zhao, F.; He, J.; Li, X.; Bai, Y.; Ying, Y.; Ping, J. Smart plant-wearable biosensor for in-situ pesticide analysis. Biosens. Bioelectron. 2020, 170, 112636. [Google Scholar] [CrossRef]
- Barbosa, J.A.; Freitas, V.M.S.; Vidotto, L.H.B.; Schleder, G.R.; de Oliveira, R.A.G.; da Rocha, J.F.; Kubota, L.T.; Vieira, L.C.S.; Tolentino, H.C.N.; Neckel, I.T.; et al. Biocompatible wearable electrodes on leaves toward the on-site monitoring of water loss from plants. ACS Appl. Mater. Interfaces 2022, 14, 22989–23001. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Aguirre, I.; Hodnik, V.; Glais, L.; Rupar, M.; Jacquot, E.; Anderluh, G.; Ravnikar, M. Surface plasmon resonance for monitoring the interaction of Potato virus Y with monoclonal antibodies. Anal. Biochem. 2014, 447, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Huang, X.; Xu, J.; Li, G.; Ma, J.; Ji, H.F.; Zhu, S.; Chen, H. Rapid and sensitive detection of maize chlorotic mottle virus using surface plasmon resonance-based biosensor. Anal. Biochem. 2013, 440, 18–22. [Google Scholar] [CrossRef]
- Kumar, V.; Arora, K. Trends in nano-inspired biosensors for plants. Mater. Sci. Energy Technol. 2020, 3, 255–273. [Google Scholar] [CrossRef]
- Bharti, A.; Jain, U.; Chauhan, N. From lab to field: Nano-biosensors for real-time plant nutrient tracking. Plant Nano Biol. 2024, 9, 100079. [Google Scholar] [CrossRef]
- Crespo-Rosa, J.R.; Foca, G.; Ulrici, A.; Pigani, L.; Zanfrognini, B.; Cubillana-Aguilera, L.; Palacios-Santander, J.M.; Zanardi, C. Simultaneous detection of glucose and fructose in synthetic musts by multivariate analysis of silica-based amperometric sensor signals. Sensors 2021, 21, 4190. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Lee, K.H. Electrochemical sensors for sustainable precision agriculture—A review. Front Chem. 2022, 10, 848320. [Google Scholar] [CrossRef] [PubMed]
- Ai, G.; Zhou, Y.; Zhang, H.; Wei, Q.; Luo, B.; Xie, Y.; Wang, C.; Xue, X.; Li, A. Ultrasensitive molecular imprinted electrochemical sensor for in vivo determination of glycine betaine in plants. Food Chem. 2024, 435, 137554. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, T.; Li, A.; Zhao, C. A novel electrochemical sensor for in situ and in vivo detection of sugars based on boronic acid-diol recognition. Comput. Electron. Agric. 2024, 218, 108714. [Google Scholar] [CrossRef]
- Sneha, M.; Ravindranath, N.A.; Murugesan, N.; Jayaraman, V. A biosensor for monitoring of salt stress in plants. Org. Electron. 2023, 113, 106698. [Google Scholar] [CrossRef]
- Ruiz-Gonzalez, A.; Kempson, H.; Haseloff, J. A simple reversed iontophoresis-based sensor to enable in vivo multiplexed measurement of plant biomarkers using screen-printed electrodes. Sensors 2023, 23, 780. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, F.; Wang, M.; Zhang, Y.; Fu, S. Flexible wearable sensors for crop monitoring: A review. Front. Plant Sci. 2024, 15, 1406074. [Google Scholar] [CrossRef]
- Qu, C.-C.; Sun, X.-Y.; Sun, W.-X.; Cao, L.-X.; Wang, X.-Q.; He, Z.-Z. Flexible wearables for plants. Small 2021, 17, 2104482. [Google Scholar] [CrossRef] [PubMed]
- Dufil, G.; Bernacka-Wojcik, I.; Armada-Moreira, A.; Stavrinidou, E. Plant bioelectronics and biohybrids: The growing contribution of organic electronic and carbon-based materials. Chem. Rev. 2022, 122, 4847–4883. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lu, H.; Jiang, S.; Gao, B. Recent advances of microneedles biosensors for plants. Anal. Bioanal. Chem. 2024, 416, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.H.; Zhou, J.; Pan, Y.X.; Ping, J.F. Wearable electrochemical sensors for plant small-molecule detection. Trends Plant Sci. 2024, 29, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Baek, E.; Jeon, K.S.; Park, K.H.; Yeo, J.; Lee, J. Monitoring of water transportation in plant stem with microneedle sap flow sensor. J. Microelectromech. Syst. 2018, 27, 440–447. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, X.; Chen, Y.; Castellano, M.J.; Schnable, J.C.; Schnable, P.S.; Dong, L. In-planta nitrate detection using insertable plant microsensor. In Proceedings of the 20th International Conference on Solid-State Sensors, Actuators and Microsystems, Berlin, Germany, 23–27 June 2019. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, D.; Wang, W.; Liu, J.; Guan Thng, S.T.; Chen, P. A silk-microneedle patch to detect glucose in the interstitial fluid of skin or plant tissue. Sens. Actuator B-Chem. 2022, 372, 132626. [Google Scholar] [CrossRef]
- GhavamiNejad, P.; GhavamiNejad, A.; Zheng, H.; Dhingra, K.; Samarikhalaj, M.; Poudineh, M. A conductive hydrogel microneedle based assay integrating PEDOT:PSS and Ag-Pt nanoparticles for real-time, enzyme-less, and electrochemical sensing of glucose. Adv. Healthc. Mater. 2023, 12, e2202362. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, S.H.; Chen, J.B.; Zhou, J.; Fan, K.; Pan, Y.X. An integrated plant glucose monitoring system based on microneedle-enabled electrochemical sensor. Biosens. Bioelectron. 2024, 248, 115964. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, Z.Z.; Jiang, F.J.; Zheng, Z.Z.; Lu, S.Z. Silk/polyols/GOD microneedle based electrochemical biosensor for continuous glucose monitoring. Rsc Adv. 2020, 10, 6163–6171. [Google Scholar] [CrossRef] [PubMed]
- Diacci, C.; Abedi, T.; Lee, J.W.; Gabrielsson, E.O.; Berggren, M.; Simon, D.T.; Niittylä, T.; Stavrinidou, E. Diurnal in vivo xylem sap glucose and sucrose monitoring using implantable organic electrochemical transistor sensors. iScience 2021, 24, 101966. [Google Scholar] [CrossRef] [PubMed]
- Bukhamsin, A.; Ait Lahcen, A.; Filho, J.D.O.; Shetty, S.; Blilou, I.; Kosel, J.; Salama, K.N. Minimally-invasive, real-time, non-destructive, species-independent phytohormone biosensor for precision farming. Biosens. Bioelectron. 2022, 214, 114515. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, S.A.; De la Paz, E.; Del Caño, R.; Seker, S.; Saha, T.; Wang, J.; Jaramillo-Botero, A. Non-invasive in vivo glucose-based stress monitoring in plants. Biosens. Bioelectron. 2023, 231, 115300. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, S.A.; Valencia, D.P.; Velez, G.E.; Jaramillo-Botero, A. Advancing abiotic stress monitoring in plants with a wearable non-destructive real-time salicylic acid laser-induced-graphene sensor. Biosens. Bioelectron. 2024, 255, chen116261. [Google Scholar] [CrossRef]
- Dhanjai; Mugo, S.M.; Lu, W. Modified stainless steel microneedle electrode for polyphenolics detection. Anal. Bioanal. Chem. 2020, 412, 7063–7072. [Google Scholar] [CrossRef]
- Hossain, N.I.; Tabassum, S. Stem-FIT: A microneedle based multi-parametric sensor for in situ monitoring of salicylic acid and pH levels in live plants. In Proceedings of the 2022 IEEE 17th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Taoyuan, Taiwan, 14–17 April 2022; pp. 312–316. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Wang, X.; Hou, P.; Luo, B.; Song, P.; Pan, D.; Li, A.; Chen, L. Disposable stainless steel-based electrochemical microsensor for in vivo determination of indole-3-acetic acid in soybean seedlings. Biosens. Bioelectron. 2019, 126, 193–199. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, L.; Li, M.; Li, C.; Li, P.; Li, H. Au@ SnO2-vertical graphene-based microneedle sensor for in-situ determination of abscisic acid in plants. Mater. Sci. Eng. C 2021, 127, 112237. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Ai, Y.; Yan, L.; Wang, B.; Huang, Y.; Zou, Q.; Fu, H.; Niu, X.; Sun, W. Wireless electrochemical sensor for the detection of phytoregulator indole-3-acetic acid using gold nanoparticles and three-dimensional reduced graphene oxide modified screen printed carbon electrode. Talanta 2023, 253, 124030. [Google Scholar] [CrossRef]
- Gao, J.; Li, H.; Li, M.; Wang, G.; Long, Y.; Li, P.; Li, C.; Yang, B. Polydopamine/graphene/MnO2 composite-based electrochemical sensor for in situ determination of free tryptophan in plants. Anal. Chim. Acta 2021, 1145, 103–113. [Google Scholar] [CrossRef]
- Gota, T.; Chowdhury, M.; Ojumu, T. Non-enzymatic fructose sensor based on Co3O4 thin film. Electroanalysis 2017, 29, 2855–2862. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Luo, B.; Wang, C.; Hou, P.; Dong, H.; Li, A.; Zhao, C. Enzyme-free electrochemical sensors for in situ quantification of reducing sugars based on carboxylated graphene–carboxylated multiwalled carbon nanotubes–gold nanoparticle–modified electrode. Front. Plant Sci. 2022, 13, 872190. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.-H.; Pasriga, R.; Yoon, J.; Jeon, J.-S.; An, G. Roles of sugars in controlling flowering time. J. Plant Biol. 2018, 61, 121–130. [Google Scholar] [CrossRef]
- Ahmad, I.Z. Role of sugars in abiotic stress signaling in plants. In Plant Signaling Molecules. Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing Sawston: Cambridge, UK, 2019; pp. 207–217. [Google Scholar] [CrossRef]
- D’Andrea, E.; Rezaie, N.; Battistelli, A.; Gavrichkova, O.; Kuhlmann, I.; Matteucci, G.; Moscatello, S.; Proietti, S.; Scartazza, A.; Trumbore, S.; et al. Winter’s bite: Beech trees survive complete defoliation due to spring late-frost damage by mobilizing old C reserves. New Phytol. 2019, 224, 625–631. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, E.; Scartazza, A.; Battistelli, A.; Collalti, A.; Proietti, S.; Rezaie, N.; Matteucci, G.; Moscatello, S. Unravelling resilience mechanisms in forests: Role of non-structural carbohydrates in responding to extreme weather events. Tree Physiol. 2021, 41, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sheen, J. Dynamic and diverse sugar signaling. Curr. Opin. Plant Biol. 2016, 33, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, A.; Traversi, M.L.; Anichini, M.; Hoshika, Y.; Fares, S.; Paoletti, E. Effect of long-tTerm vs. short-term ambient ozone exposure on radial stem growth, sap flux and xylem morphology of O3-sensitive poplar trees. Forests 2019, 10, 396. [Google Scholar] [CrossRef]
- Giovannelli, A.; Mattana, S.; Emiliani, G.; Anichini, M.; Traversi, M.L.; Pavone, F.S.; Cicchi, R. Localized stem heating from the rest to growth phase induces latewood-like cell formation and slower stem radial growth in Norway spruce saplings. Tree Physiol. 2022, 42, 1149–1163. [Google Scholar] [CrossRef]
- Barbaroux, C.; Breda, N.; Dufrene, E. Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petrea and Fagus sylvatica). New Phytol. 2003, 157, 605–615. [Google Scholar] [CrossRef]
- Martinez-Trinidad, T.; Watson, W.T.; Arnold, M.A.; Lombardini, L. Investigations of exogenous applications of carbohydrates on the growth and vitality of live oaks. Urban For. Urban Green. 2009, 8, 41–48. [Google Scholar] [CrossRef]
- Lo Presti, D.; Di Tocco, J.; Massaroni, C.; Cimini, S.; De Gara, L.; Singh, S.; Raucci, A.; Manganiello, G.; Woo, S.L.; Schena, E.; et al. Current understanding, challenges and perspective on portable systems applied to plant monitoring and precision agriculture. Biosens. Bioelectron. 2023, 222, 115005. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.M.; Cluck, D.R.; Smith, S.L.; Ryan, K.C. Using bark char codes to predict post-fire cambium mortality. Fire Ecol. 2008, 4, 57–73. [Google Scholar] [CrossRef]
- Giovannelli, A.; Emiliani, G.; Traversi, M.L.; Deslauriers, A.; Rossi, S. Sampling cambial region and mature xylem for non structural carbohydrates and starch analyses. Dendrochronologia 2011, 29, 177–182. [Google Scholar] [CrossRef]
- Martinez-Trinidad, T.; Watson, W.T.; Arnold, M.A.; Lombardini, L.; Appel, D.N. Comparing various techniques to measure tree vitality of live oaks. Urban For. Urban Green. 2010, 9, 199–203. [Google Scholar] [CrossRef]
- Xue, Y.; Thalmayer, A.S.; Zeising, S.; Fischer, G.; Lübke, M. Commercial and scientific solutions for blood glucose monitoring—A review. Sensors 2022, 22, 425. [Google Scholar] [CrossRef] [PubMed]
- Kamanina, O.A.; Kamanin, S.S.; Kharkova, A.S.; Arlyapov, V.A. Glucose biosensor based on screen-printed electrode modified with silicone sol–gel conducting matrix containing carbon nanotubes. 3 Biotech 2019, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wang, D.; Xu, J.; Chen, K.; Li, X.; Yi, H.; Ni, Z. Glucose sensing on screen-printed electrochemical electrodes based on porous graphene aerogel @prussian blue. Biomed. Microdevices 2022, 24, 14. [Google Scholar] [CrossRef]
- Sakalauskiene, L.; Brasiunas, B.; Popov, A.; Kausaite-Minkstimiene, A.; Ramanaviciene, A. The development of reagentless amperometric glucose biosensor based on gold nanostructures, prussian blue and glucose oxidase. Biosensors 2023, 13, 942. [Google Scholar] [CrossRef]
- Liu, C.-T.; Liu, C.-H.; Lai, Y.-T.; Lee, C.-Y.; Gupta, S.; Tai, N.-H. A salivary glucose biosensor based on immobilization of glucose oxidase in nafion-carbon nanotubes nanocomposites modified on screen printed electrode. Microchem. J. 2023, 91, 108872. [Google Scholar] [CrossRef]
- Liu, Y.; Yue, W.; Cui, Y. Development of an amperometric biosensor on a toothbrush for glucose. Sens. Actuators Rep. 2023, 5, 100133. [Google Scholar] [CrossRef]
- Albanese, D.; Sannini, A.; Malvano, F.; Pilloton, R.; Di Matteo, M. Optimisation of glucose biosensors based on sol–gel entrapment and prussian blue-modified screen-printed electrodes for real food analysis. Food Anal. Methods 2014, 7, 1002–1008. [Google Scholar] [CrossRef]
- Khosravi, S.; Soltanian, S.; Servati, A.; Khademhosseini, A.; Zhu, Y.; Servati, P. Screen-printed textile-based electrochemical biosensor for noninvasive monitoring of glucose in sweat. Biosensors 2023, 13, 684. [Google Scholar] [CrossRef]
- Ang, L.F.; Por, L.Y.; Yam, M.F. Development of an amperometric-based glucose biosensor to measure the glucose content of fruit. PLoS ONE 2015, 10, e0111859. [Google Scholar] [CrossRef]
- Šakinyte, I.; Barkauskas, J.; Gaidukevič, J.; Razumiene, J. Thermally reduced graphene oxide: The study and use for reagentless amperometric d-fructose biosensors. Talanta 2015, 144, 1096–1103. [Google Scholar] [CrossRef]
- Bollella, P.; Hibino, Y.; Conejo-valverde, P.; Soto-cruz, J.; Bergueiro, J. The influence of the shape of Au nanoparticles on the catalytic current of fructose dehydrogenase. Anal. Bioanal. Chem. 2019, 411, 7645–7657. [Google Scholar] [CrossRef]
- Bollella, P. Enzyme-based amperometric biosensors: 60 years later, Quo Vadis? Anal. Chim. Acta 2022, 1234, 340517. [Google Scholar] [CrossRef]
- Silveri, F.; Paolini, D.; Della Pelle, F.; Bollella, P.; Scroccarello, A.; Suzuki, Y.; Fukawa, E.; Sowa, K.; Di Franco, C.; Torsi, L.; et al. Lab-made flexible third-generation fructose biosensors based on 0D-nanostructured transducers. Biosens. Bioelectron. 2023, 237, 115450. [Google Scholar] [CrossRef]
- Antiochia, R.; Palleschi, G. A tri-enzyme electrode probe for the sequential determination of fructose and glucose in the same sample. Anal. Lett. 1997, 30, 683–697. [Google Scholar] [CrossRef]
- Begun, A.; Kobatake, E.; Suzawa, T.; Ikariyama, Y.; Aizawa, M. New electrocatalytic biomolecular interface for fabricating a fructose dehydrogenase-based sensing system. Anal. Chim. Acta 1993, 280, 31–36. [Google Scholar] [CrossRef]
- Paredes, P.A.; Parellada, J.; Fernndez, V.M.; Katakis, I.; Dominguez, E. Amperometric mediated carbon paste biosensor based on D-fructose dehydrogenase for the determination of fructose in food analysis. Biosens. Bioelectron. 1997, 12, 1233–1243. [Google Scholar] [CrossRef]
- Biscay, J.; Rama, E.C.; García, M.B.G.; Reviejo, A.J.; Carrazón, J.M.P.; García, A.C. Amperometric fructose sensor based on ferrocyanide modified screen-printed carbon electrode. Talanta 2012, 88, 432–438. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kano, K.; Shirai, O.; Kitazumi, Y. Diffusion-limited electrochemical D-fructose sensor based on direct electron transfer-type bioelectrocatalysis by a variant of D-fructose dehydrogenase at a porous gold microelectrode. J. Electroanal. Chem. 2020, 877, 114651. [Google Scholar] [CrossRef]
- Trivedi, U.B.; Lakshminarayana, D.; Kothari, I.L.; Patel, P.B.; Panchal, C.J. Amperometric fructose biosensor based on fructose dehydrogenase enzyme. Sens. Actuators B 2009, 136, 45–51. [Google Scholar] [CrossRef]
- Siepenkoetter, T.; Salaj-Kosla, U.; Magner, E. The immobilization of fructose dehydrogenase on nanoporous gold electrodes for the detection of fructose. ChemElectroChem 2017, 4, 905–912. [Google Scholar] [CrossRef]
- Antiochia, R.; Gorton, L. A new osmium-polymer modified screen-printed graphene electrode for fructose detection. Sensor. Actuator. B Chem. 2014, 195, 287–293. [Google Scholar] [CrossRef]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. Highly sensitive membraneless fructose biosensor based on fructose dehydrogenase immobilized onto aryl thiol modified highly porous gold electrode: Characterization and application in food samples. Anal. Chem. 2018, 90, 12131–12136. [Google Scholar] [CrossRef]
- Bilgi, M.; Ayranci, E. Development of amperometric biosensors using screen-printed carbon electrodes modified with conducting polymer and nanomaterials for the analysis of ethanol, methanol and their mixtures. J. Electroanal. Chem. 2018, 823, 588–592. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Y.; Feng, J.; Chu, Z.; Jin, W. Screen-printing of nanocube-based flexible microchips for the precise biosensing of ethanol during fermentation. AIChE J. 2021, 67, e1714. [Google Scholar] [CrossRef]
- Istrate, O.M.; Bala, C.; Rotariu, L. A new highly sensitive electrochemical biosensor for ethanol detection based on gold nanoparticles/reduced graphene oxide/polyallylamine hydrochloride nanocomposite. Biosensors 2023, 13, 954. [Google Scholar] [CrossRef]
- Azevedo, A.M.; Prazeres, D.M.F.; Cabral, J.M.S.; Fonseca, L.P. Ethanol biosensors based on alcohol oxidase. Biosens. Bioelectron. 2005, 21, 235–247. [Google Scholar] [CrossRef]
- Akyilmaz, E.; Dinckaya, E. A mushroom (Agaricus bisporus) tissue homogenate based alcohol oxidase electrode for alcohol determinationin serum. Talanta 2000, 53, 505–509. [Google Scholar] [CrossRef]
- Stasyuk, N.; Demkiv, O.; Gayda, G.; Zakalska, O.; Nogala, W.; Gonchar, M. Amperometric biosensors based on alcohol oxidase and peroxidase-like nanozymes for ethanol determination. Mikrochim. Acta. 2022, 189, 474. [Google Scholar] [CrossRef]
- Hooda, V.; Kumar, V.; Gahlaut, A.; Hooda, V. A novel amperometric bienzymatic biosensor based on alcohol oxidase coupled PVC reaction cell and nanomaterials modified working electrode for rapid quantification of alcohol. Prep. Biochem. Biotechnol. 2018, 48, 877–886. [Google Scholar] [CrossRef]
- Hooda, V.; Gahlaut, A.; Hooda, V. A novel amperometric biosensor for rapid detection of ethanol utilizing gold nanoparticles and enzyme coupled PVC reaction cell. Environ. Technol. 2021, 42, 3318–3328. [Google Scholar] [CrossRef]
- Maringer, J.; Ascoli, D.; Dorren, L.; Bebi, P.; Conedera, M. Temporal trends in the protective capacity of burnt beech forests (Fagus sylvatica L.) against rockfall. Eur. J. For. Res. 2016, 135, 657–673. [Google Scholar] [CrossRef]
- Thompson, M.T.; Koyama, A.; Kavanagh, K.L. Wildfire effects on physiological properties in conifers of central Idaho forests, USA. Trees 2017, 31, 545–555. [Google Scholar] [CrossRef]
- Battipaglia, G.; Tognetti, R.; Valese, E.; Ascoli, D.; De Luca, P.F.; Basile, S.; Ottaviano, M.; Mazzoleni, S.; Marchetti, M.; Esposito, A. Incendi 2017: Un’importante lezione. Forest 2017, 14, 231–236. [Google Scholar] [CrossRef]
- Valor, T.; Casals, P.; Altieri, S.; González-Olabarria, J.R.; Piqué, M.; Battipaglia, G. Disentangling the effects of crown scorch and competition release on the physiological and growth response of Pinus halepensis Mill. using δ13C and δ18O isotopes. For. Ecol. Manag. 2018, 424, 276–287. [Google Scholar] [CrossRef]
- Valor, T.; González-Olabarria, J.R.; Piqué, M. Assessing the impact of prescribed burning on the growth of European pines. For. Ecol. Manag. 2015, 343, 101–109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).