SERS-Based Microneedle Biosensor for In Situ and Sensitive Detection of Tyrosinase
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Au NPs
2.3. Synthesis of Ag NPs and Ag NPs-4MPBA
2.4. Synthesis of the Au-Coated PMMA MN Array
2.5. Synthesis of Functionalized MN Arrays for SERS
2.6. Preparation of Skin Phantoms
2.7. Cytotoxicity Assessment
2.8. Characterizations
2.9. Raman Measurements
3. Results and Discussion
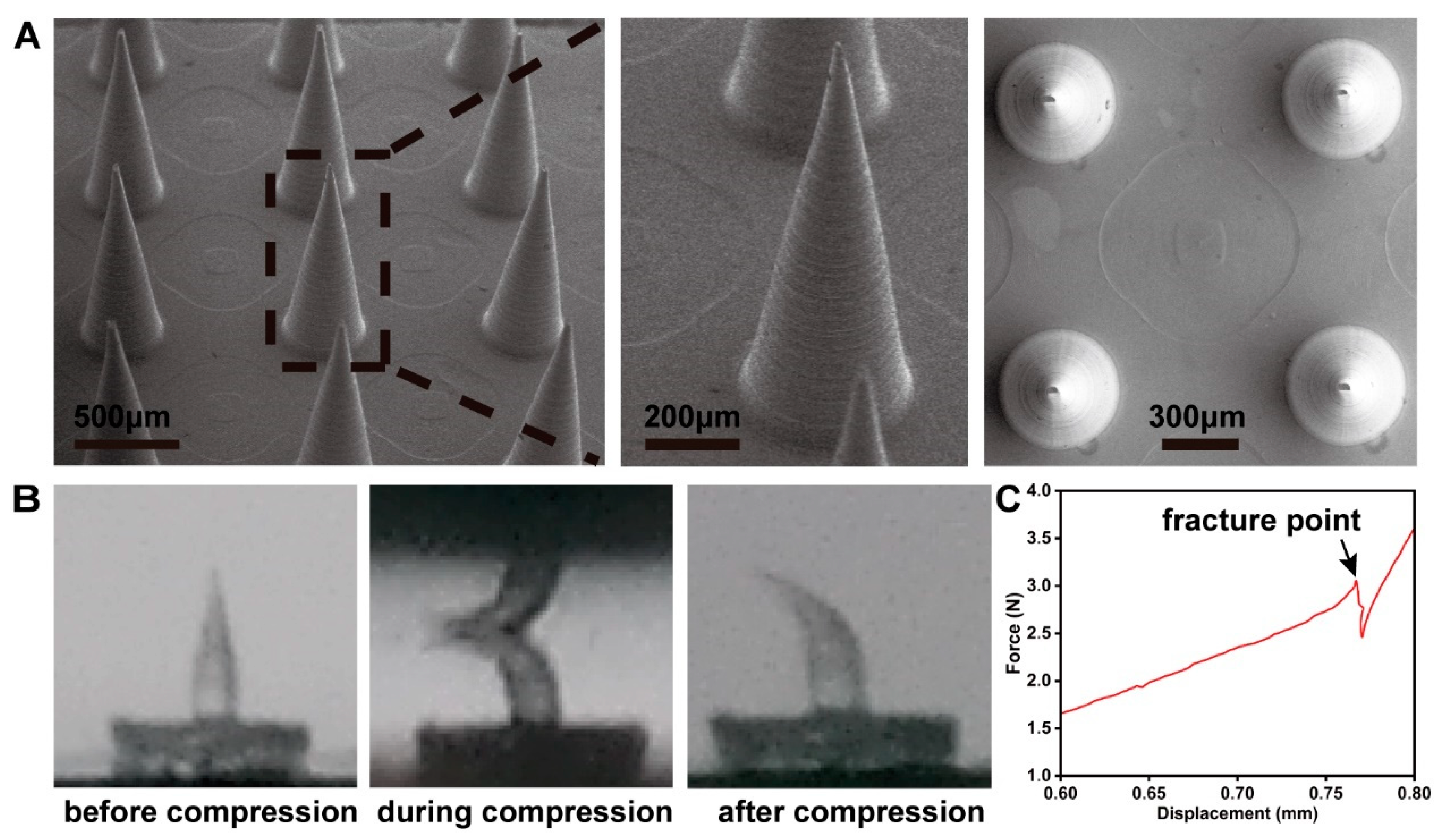
3.1. Characterization of the MN
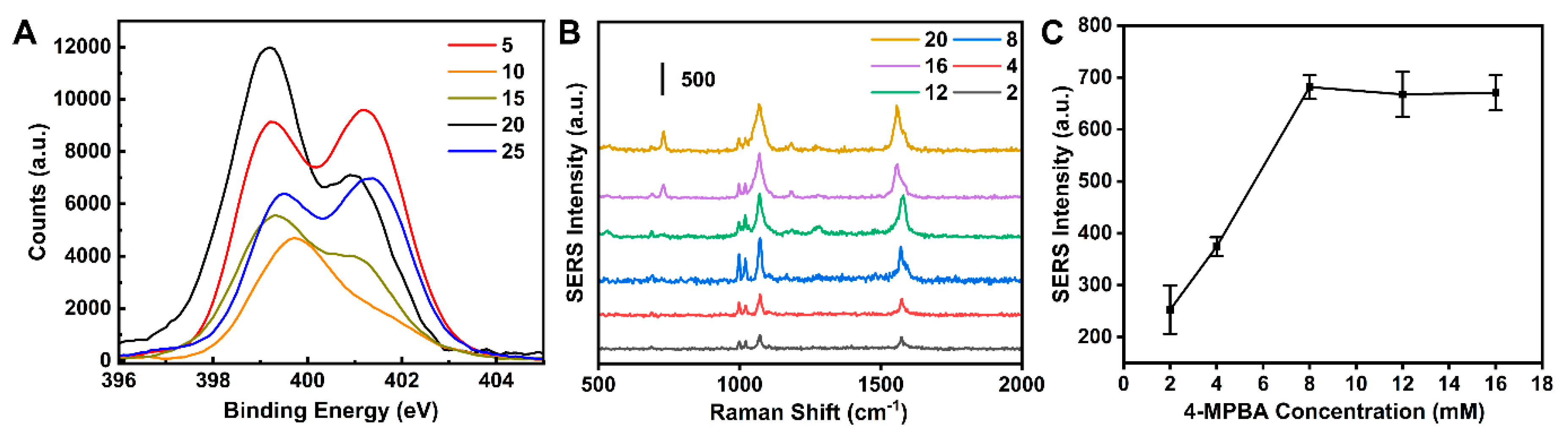
3.2. Optimization of the SERS Sensor
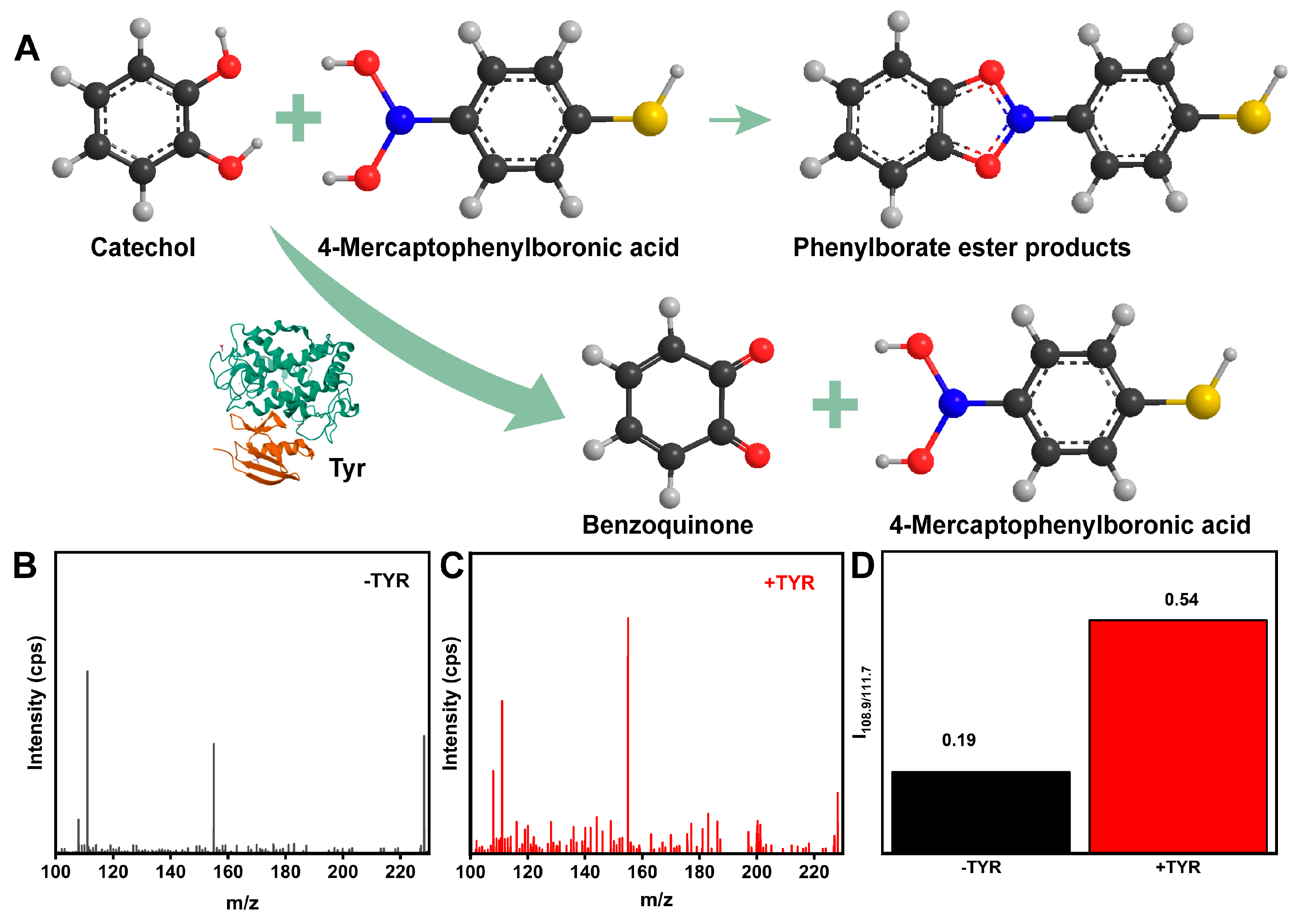
3.3. Feasibility of the MN SERS Sensor for TYR Detection
3.4. Development for the Standard Working Curve of TYR
3.5. Interference and Selectivity Studies
3.6. Stability Assessment
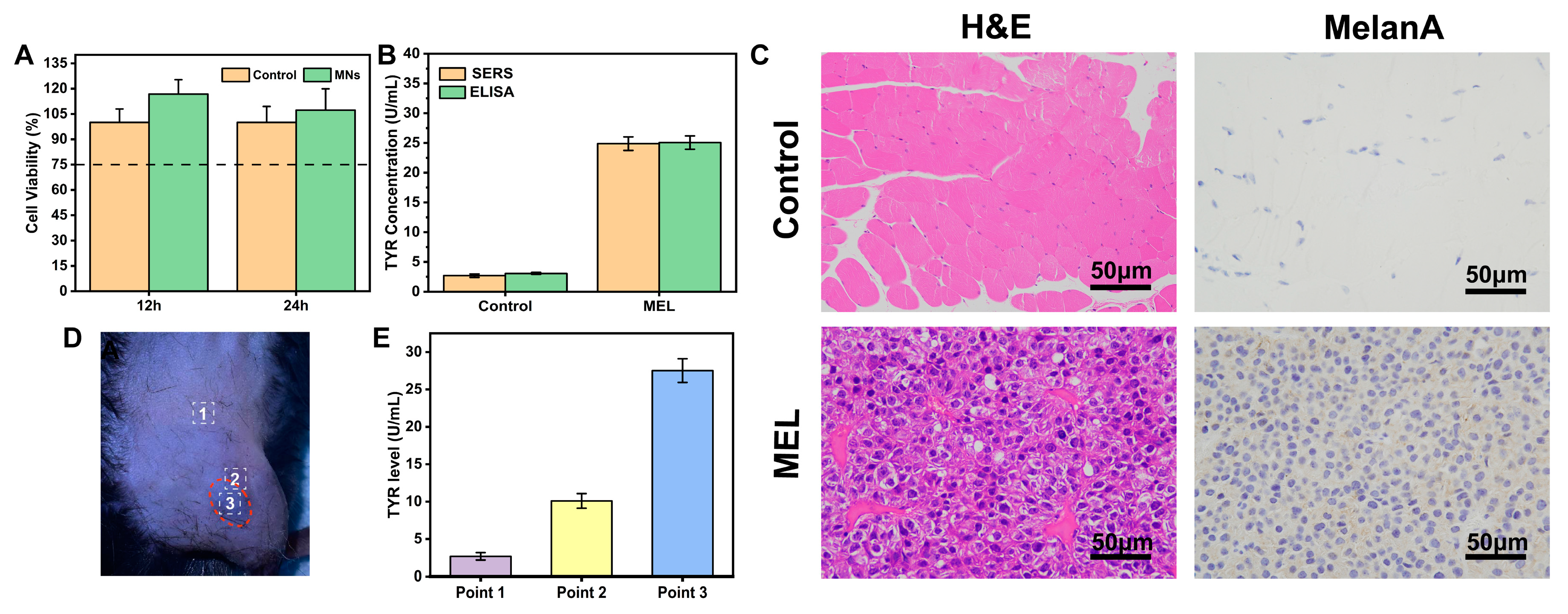
3.7. Comparison of SERS Results with ELISA Analysis
3.8. In Situ Skin Model for TYR Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Xu, T.; Liang, K.; Li, J.; Fang, Y.; Li, F.; Chen, Y.; Zhang, H.; Li, D.; Tang, Y.; et al. Self-assembled peptides-modified flexible field-effect transistors for tyrosinase detection. iScience 2022, 25, 103673. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Yeh, Y.-C. Detection of tyrosine and monitoring tyrosinase activity using an enzyme cascade-triggered colorimetric reaction. RSC Adv. 2020, 10, 29745–29750. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-W.; Huang, P.-C.; Li, J.-F.; Wu, F.-Y. Colorimetric detection of tyrosinase during the synthesis of kojic acid/silver nanoparticles under illumination. Sens. Actuators B Chem. 2017, 251, 836–841. [Google Scholar] [CrossRef]
- Fayad, S.; Morin, P.; Nehmé, R. Use of chromatographic and electrophoretic tools for assaying elastase, collagenase, hyaluronidase, and tyrosinase activity. J. Chromatogr. A 2017, 1529, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, Z.; Zhang, L.; Tian, Y. Electrochemical Detection of Tyrosinase in Cell Lysates at Functionalized Nanochannels via Amplifying of Ionic Current Response. Electroanalysis 2022, 34, 1021–1026. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, B.; Cui, Y.; Li, L.; Nian, F.; Zhang, Z. A self-ratiometric and selective electrochemical sensor for the detection of tyrosinase in mouse brain homogenate. Analyst 2022, 147, 4092–4097. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, J.; Lu, S.; Wang, S.; Sun, J.; Yang, X. Polymethyldopa Nanoparticles-Based Fluorescent Sensor for Detection of Tyrosinase Activity. ACS Sens. 2018, 3, 1855–1862. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.-J.; Wei, T.; Ma, X.; Zheng, X.-S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Fan, M.; Andrade, G.F.S.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Gong, K.; Liu, Y.; Zhang, L. Strategies and Challenges of Identifying Nanoplastics in Environment by Surface-Enhanced Raman Spectroscopy. Environ. Sci. Technol. 2023, 57, 25–43. [Google Scholar] [CrossRef]
- Guven, B.; Basaran-Akgul, N.; Temur, E.; Tamer, U.; Boyacı, İ.H. SERS-based sandwich immunoassay using antibody coated magnetic nanoparticles for Escherichia coli enumeration. Analyst 2011, 136, 740–748. [Google Scholar] [CrossRef]
- Guan, H.; Huang, C.; Lu, D.; Chen, G.; Lin, J.; Hu, J.; He, Y.; Huang, Z. Label-free Raman spectroscopy: A potential tool for early diagnosis of diabetic keratopathy. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2021, 256, 119731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, H.; Hu, Z.; Yu, G.; Yang, D.; Zhao, J. Label and label-free based surface-enhanced Raman scattering for pathogen bacteria detection: A review. Biosens. Bioelectron. 2017, 94, 131–140. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Wang, Y.; Mei, R.; Fu, L.; Li, J.; Wang, X.; Chen, L. Chiral molecular imprinting-based SERS detection strategy for absolute enantiomeric discrimination. Nat. Commun. 2022, 13, 5757. [Google Scholar] [CrossRef]
- Shin, H.; Jeong, H.; Park, J.; Hong, S.; Choi, Y. Correlation between Cancerous Exosomes and Protein Markers Based on Surface-Enhanced Raman Spectroscopy (SERS) and Principal Component Analysis (PCA). ACS Sens. 2018, 3, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hwang, M.; Choi, B.; Jeong, H.; Jung, J.-H.; Kim, H.K.; Hong, S.; Park, J.-H.; Choi, Y. Exosome Classification by Pattern Analysis of Surface-Enhanced Raman Spectroscopy Data for Lung Cancer Diagnosis. Anal. Chem. 2017, 89, 6695–6701. [Google Scholar] [CrossRef]
- Lin, C.-C.; Yang, Y.-M.; Chen, Y.-F.; Yang, T.-S.; Chang, H.-C. A new protein A assay based on Raman reporter labeled immunogold nanoparticles. Biosens. Bioelectron. 2008, 24, 178–183. [Google Scholar] [CrossRef]
- Gong, J.-L.; Liang, Y.; Huang, Y.; Chen, J.-W.; Jiang, J.-H.; Shen, G.-L.; Yu, R.-Q. Ag/SiO2 core-shell nanoparticle-based surface-enhanced Raman probes for immunoassay of cancer marker using silica-coated magnetic nanoparticles as separation tools. Biosens. Bioelectron. 2007, 22, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Lin, X.; Chen, C.; Lu, Y.; Feng, S.; Huang, Z.; You, R.; Chen, J.; Wu, Y. Interference-free SERS tags for ultrasensitive quantitative detection of tyrosinase in human serum based on magnetic bead separation. Anal. Chim. Acta 2020, 1138, 150–157. [Google Scholar] [CrossRef]
- Wang, L.; Gan, Z.-F.; Guo, D.; Xia, H.-L.; Patrice, F.T.; Hafez, M.E.; Li, D.-W. Electrochemistry-Regulated Recyclable SERS Sensor for Sensitive and Selective Detection of Tyrosinase Activity. Anal. Chem. 2019, 91, 6507–6513. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, T.; McGettigan, S.; Kumar, S.; Liu, S.; Speicher, D.; Schuchter, L.; Xu, X. IL-8 and cathepsin B as melanoma serum biomarkers. Int. J. Mol. Sci. 2011, 12, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Jin, L.; Wang, F.; Yang, H.; He, H. Laser transparent multiplexed SERS microneedles for in situ and real-time detection of inflammation. Biosens. Bioelectron. 2023, 225, 115079. [Google Scholar] [CrossRef]
- Parrilla, M.; Cuartero, M.; Padrell Sánchez, S.; Rajabi, M.; Roxhed, N.; Niklaus, F.; Crespo, G.A. Wearable All-Solid-State Potentiometric Microneedle Patch for Intradermal Potassium Detection. Anal. Chem. 2019, 91, 1578–1586. [Google Scholar] [CrossRef]
- Zhu, D.D.; Zheng, L.W.; Duong, P.K.; Cheah, R.H.; Liu, X.Y.; Wong, J.R.; Wang, W.J.; Tien Guan, S.T.; Zheng, X.T.; Chen, P. Colorimetric microneedle patches for multiplexed transdermal detection of metabolites. Biosens. Bioelectron. 2022, 212, 114412. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Jin, Y.; Li, R.R.; Jiang, C.; Ping, J.; Charles, C.J.; Kong, Y.L.; Ho, J.S. Needle-integrated ultrathin bioimpedance microsensor array for early detection of extravasation. Biosens. Bioelectron. 2022, 216, 114651. [Google Scholar] [CrossRef]
- Lin, S.; Ouyang, Y.; Lin, W.; Zhou, X.; Miao, M.; Cheng, E.; Jiang, Y.; Meng, Z.; Jin, M.; Zhang, S.; et al. Microenvironment-optimized GelMA microneedles for interstitial fluid extraction and real-time glucose detection. Surf. Interfaces 2024, 45, 103847. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Microneedle-based biosensor for minimally-invasive lactate detection. Biosens. Bioelectron. 2019, 123, 152–159. [Google Scholar] [CrossRef]
- Panicker, L.R.; Shamsheera, F.; Narayan, R.; Kotagiri, Y.G. Wearable Electrochemical Microneedle Sensors Based on the Graphene-Silver-Chitosan Nanocomposite for Real-Time Continuous Monitoring of the Depression Biomarker Serotonin. ACS Appl. Nano Mater. 2023, 6, 20601–20611. [Google Scholar] [CrossRef]
- Li, Z.; Kadian, S.; Mishra, R.K.; Huang, T.; Zhou, C.; Liu, S.; Wang, Z.; Narayan, R.; Zhu, Z. Electrochemical detection of cholesterol in human biofluid using microneedle sensor. J. Mater. Chem. B 2023, 11, 6075–6081. [Google Scholar] [CrossRef]
- Wang, Q.; Molinero-Fernandez, A.; Casanova, A.; Titulaer, J.; Campillo-Brocal, J.C.; Konradsson-Geuken, Å.; Crespo, G.A.; Cuartero, M. Intradermal Glycine Detection with a Wearable Microneedle Biosensor: The First In Vivo Assay. Anal. Chem. 2022, 94, 11856–11864. [Google Scholar] [CrossRef] [PubMed]
- García-Guzmán, J.J.; Pérez-Ràfols, C.; Cuartero, M.; Crespo, G.A. Toward In Vivo Transdermal pH Sensing with a Validated Microneedle Membrane Electrode. ACS Sens. 2021, 6, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhong, X.; Li, Z.; Xia, Y. Successive, Seed-Mediated Growth for the Synthesis of Single-Crystal Gold Nanospheres with Uniform Diameters Controlled in the Range of 5–150 nm. Part. Part. Syst. Charact. 2014, 31, 266–273. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Lin, X.; Fang, G.; Liu, Y.; He, Y.; Wang, L.; Dong, B. Marangoni Effect-Driven Transfer and Compression at Three-Phase Interfaces for Highly Reproducible Nanoparticle Monolayers. J. Phys. Chem. Lett. 2020, 11, 3573–3581. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.; Liu, Q. Hollow agarose microneedle with silver coating for intradermal surface-enhanced Raman measurements: A skin-mimicking phantom study. J. Biomed. Opt. 2015, 20, 61102. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; Zhao, D.; He, H.; Wang, Z. SERS-Based Microneedle Biosensor for In Situ and Sensitive Detection of Tyrosinase. Biosensors 2024, 14, 202. https://doi.org/10.3390/bios14040202
Gu Z, Zhao D, He H, Wang Z. SERS-Based Microneedle Biosensor for In Situ and Sensitive Detection of Tyrosinase. Biosensors. 2024; 14(4):202. https://doi.org/10.3390/bios14040202
Chicago/Turabian StyleGu, Zimeng, Di Zhao, Hongyan He, and Zhenhui Wang. 2024. "SERS-Based Microneedle Biosensor for In Situ and Sensitive Detection of Tyrosinase" Biosensors 14, no. 4: 202. https://doi.org/10.3390/bios14040202
APA StyleGu, Z., Zhao, D., He, H., & Wang, Z. (2024). SERS-Based Microneedle Biosensor for In Situ and Sensitive Detection of Tyrosinase. Biosensors, 14(4), 202. https://doi.org/10.3390/bios14040202




