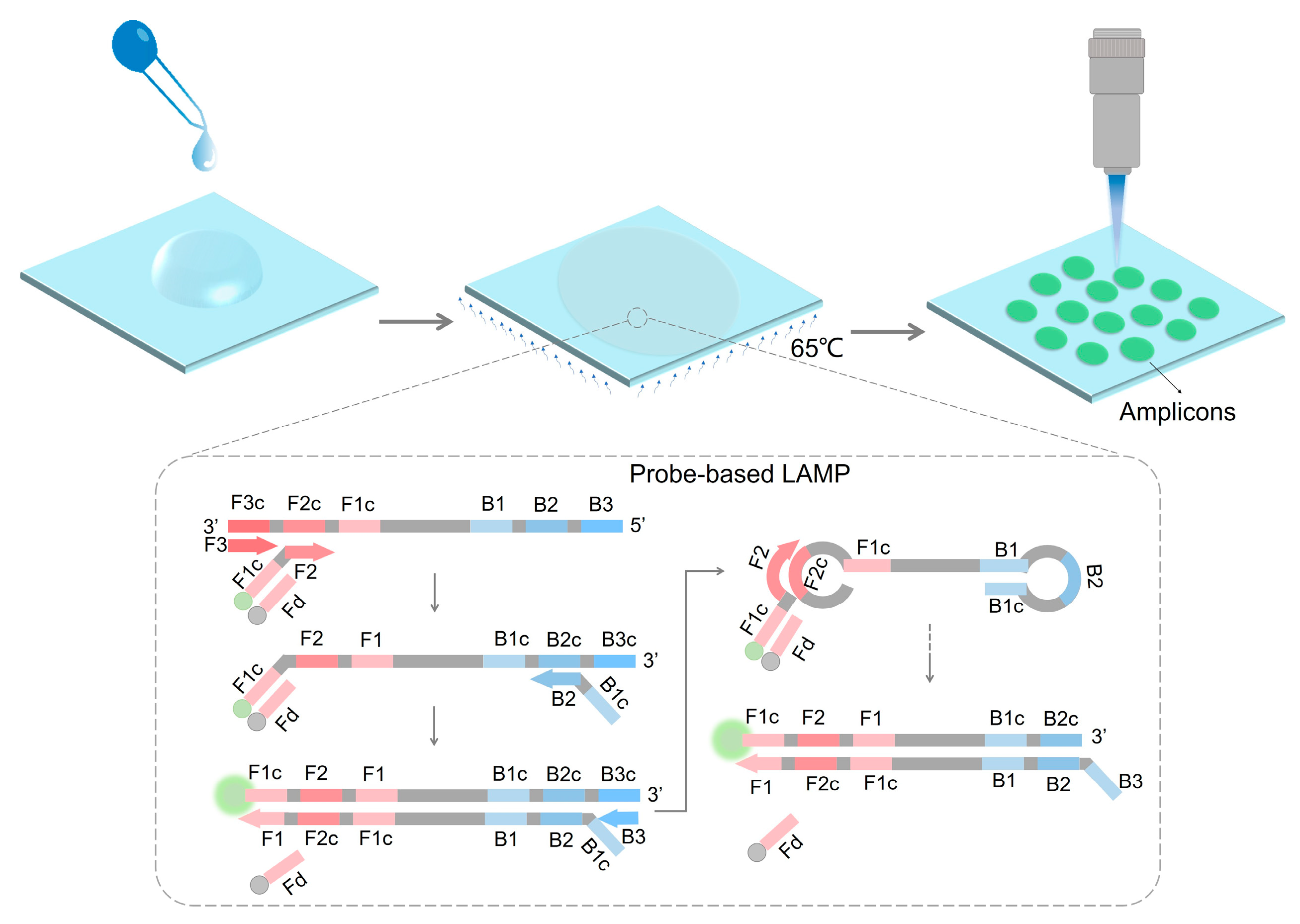
A Point-of-Care Nucleic Acid Quantification Method by Counting Light Spots Formed by LAMP Amplicons on a Paper Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
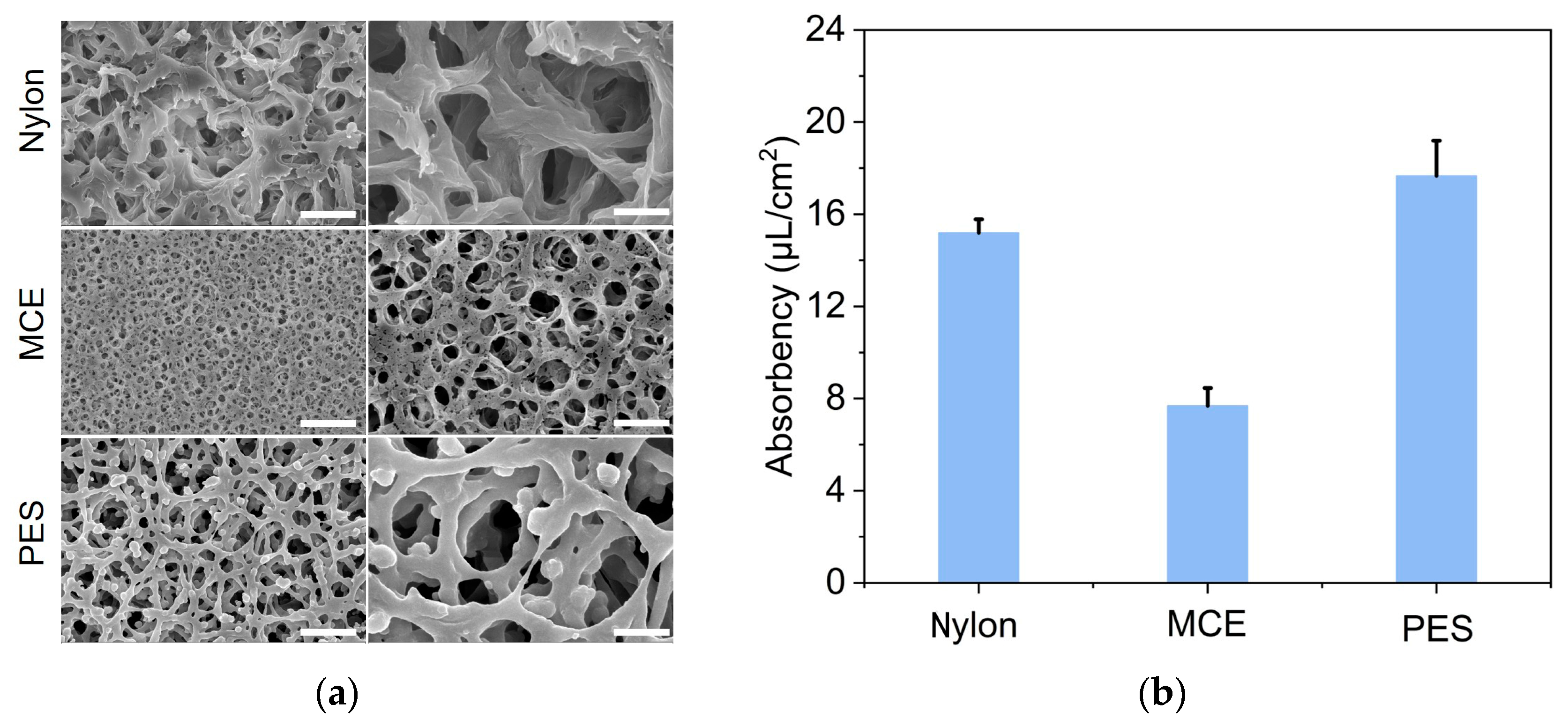
2.2. Membrane Characterization and Water Absorbency Testing
2.3. LAMP Reaction System
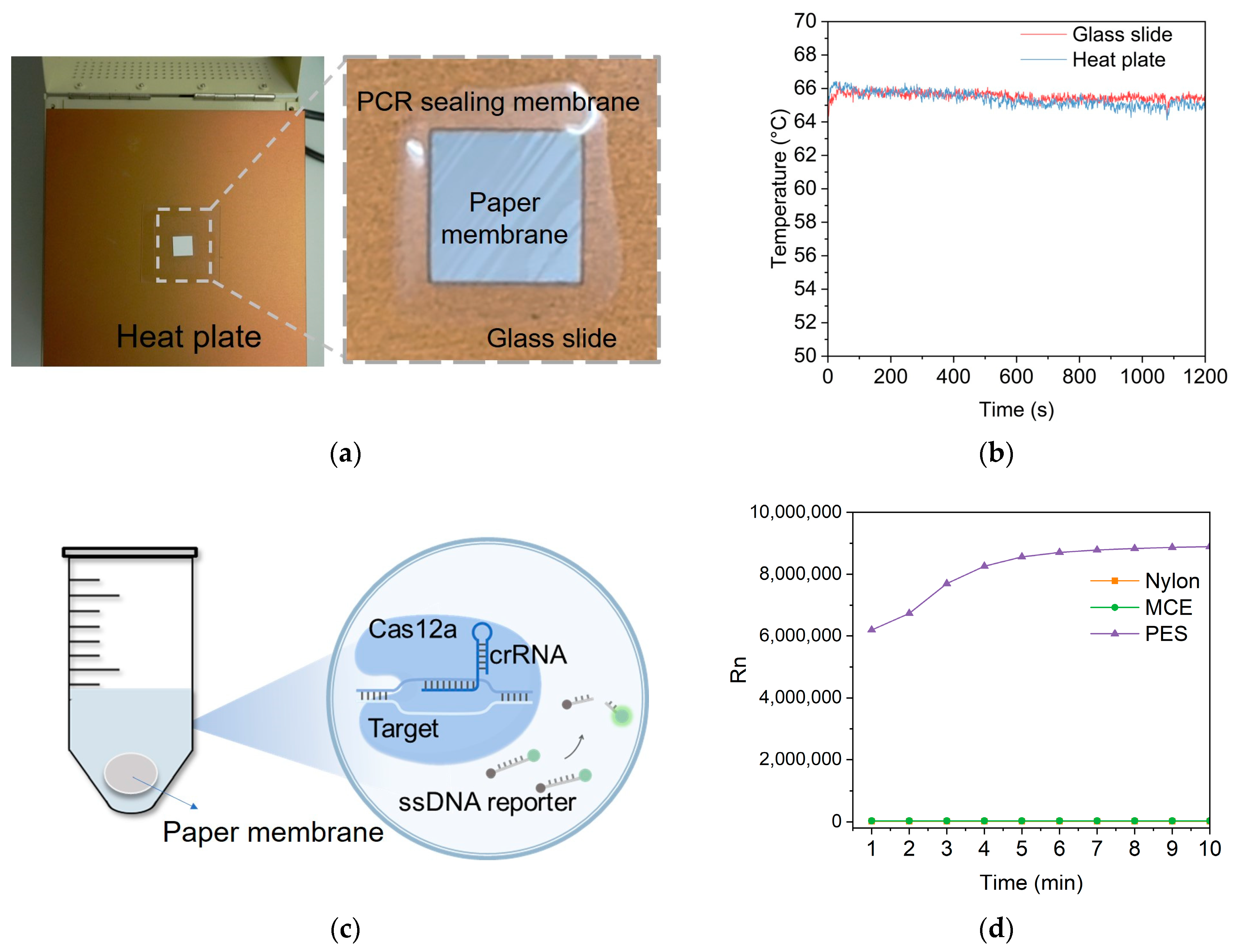
2.4. LAMP Reaction on the Paper Membranes
2.5. Feasibility of LAMP Reaction on the Paper Membranes
2.6. Light Spot Observation on the Paper Membrane
3. Results and Discussion
3.1. Characterization of Paper Membrane
3.2. Feasibility of LAMP Amplification on the Paper Membrane
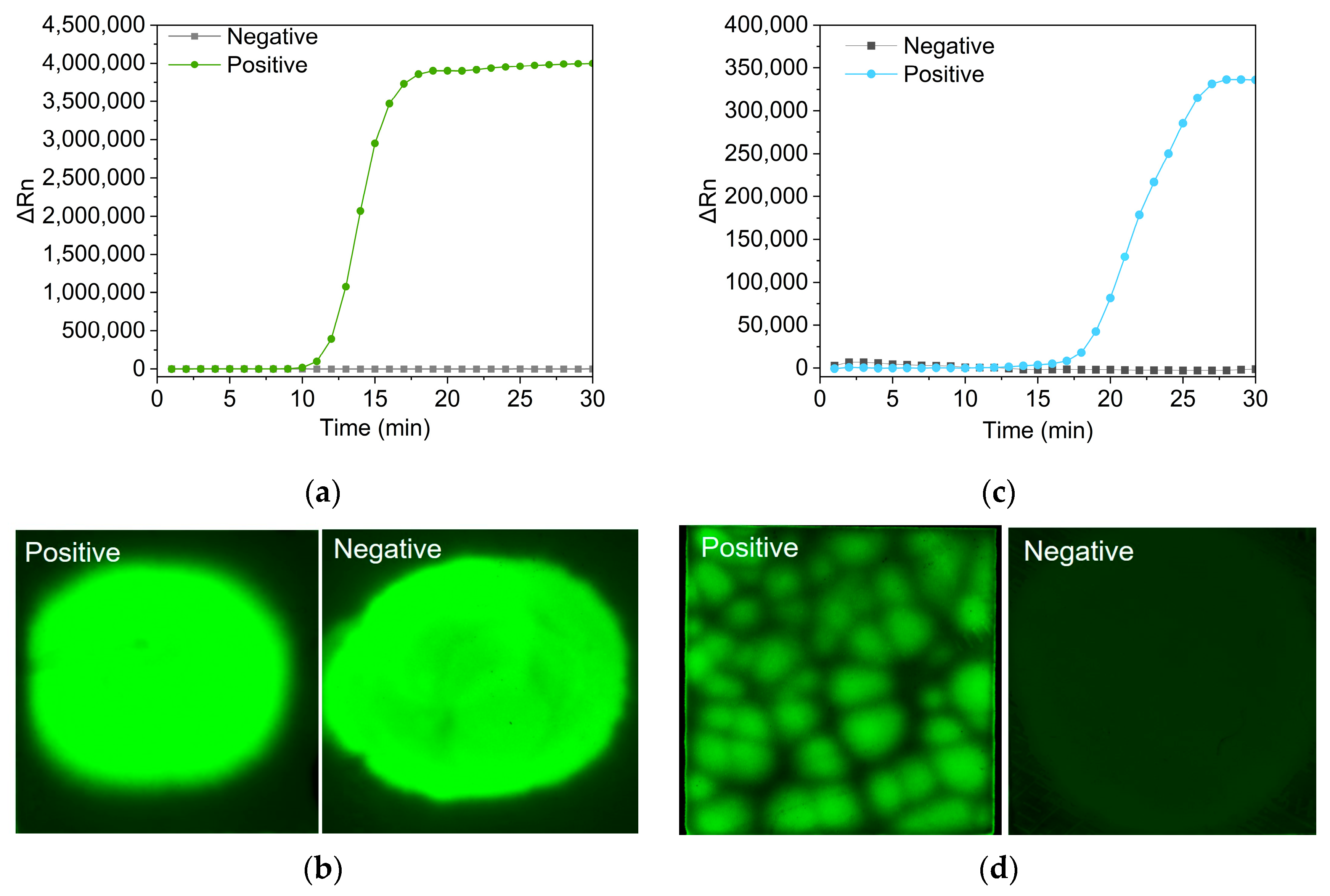
3.3. LAMP Reaction on the PES Membrane
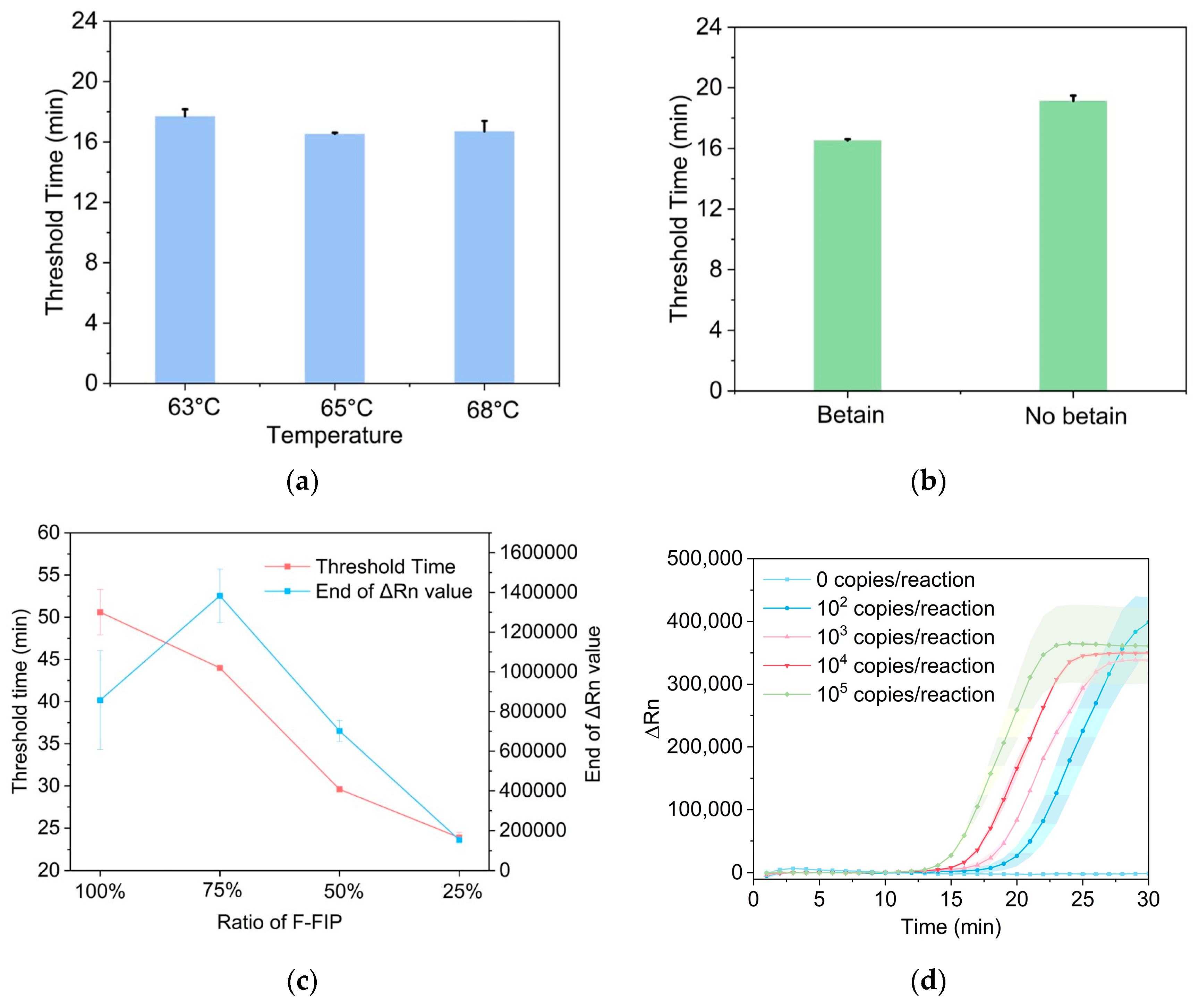
3.4. Optimization of Probe-Based LAMP Reaction
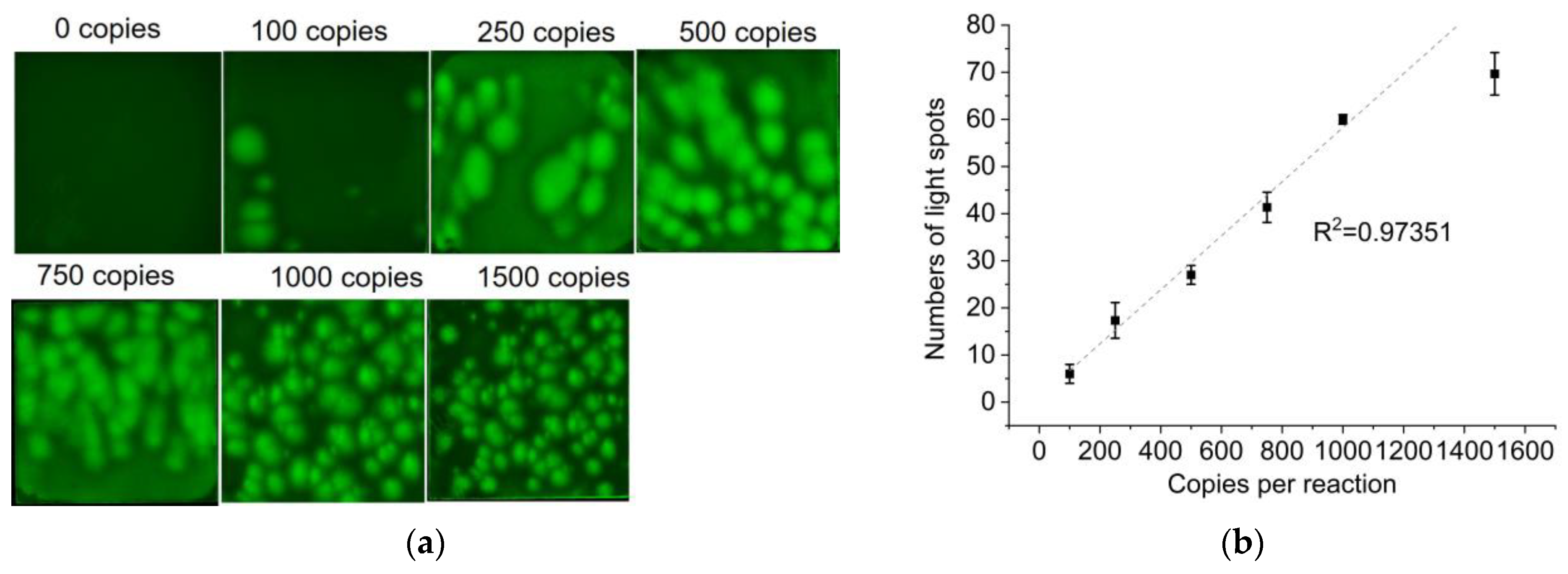
3.5. Vibrio Parahemolyticus DNA Quantification on the PES Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.J.; Qian, C.; Liu, C.Z.; Shen, H.; Wang, Z.J.; Ping, J.F.; Wu, J.; Chen, H. Nucleic acid amplification free biosensors for pathogen detection. Biosens. Bioelectron. 2020, 153, 112049. [Google Scholar] [CrossRef]
- Dehaut, A.; Krzewinski, F.; Grard, T.; Chollet, M.; Jacques, P.; Brisabois, A.; Duflos, G. Monitoring the freshness of fish: Development of a qPCR method applied to MAP chilled whiting. J. Sci. Food Agric. 2016, 96, 2080–2089. [Google Scholar] [CrossRef]
- Jaafar, R.; Aherfi, S.; Wurtz, N.; Grimaldier, C.; Hoang, V.T.; Colson, P.; Raoult, D.; La Scola, B. Correlation Between 3790 Quantitative Polymerase Chain Reaction-Positives Samples and Positive Cell Cultures, Including 1941 Severe Acute Respiratory Syndrome Coronavirus 2 Isolates. Clin. Infect. Dis. 2021, 72, E921–E922. [Google Scholar] [CrossRef]
- Ellison, S.L.R.; Emslie, K.R.; Kassir, Z. A standard additions method reduces inhibitor-induced bias in quantitative real-time PCR. Anal. Bioanal. Chem. 2011, 401, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.; et al. The need for transparency and good practices in the qPCR literature. Nat. Meth. 2013, 10, 1063–1067. [Google Scholar] [CrossRef]
- Takeuchi, K.; Yanagisawa, H.; Kurosawa, Y.; Iida, Y.; Kawai, K.; Fujimaki, S. Degradation of SARS-CoV-2 specific ribonucleic acid in samples for nucleic acid amplification detection. PLoS ONE 2022, 17, e0264541. [Google Scholar] [CrossRef] [PubMed]
- Alikian, M.; Whale, A.S.; Akiki, S.; Piechocki, K.; Torrado, C.; Myint, T.; Cowen, S.; Griffiths, M.; Reid, A.G.; Apperley, J.; et al. RT-qPCR and RT-Digital PCR: A Comparison of Different Platforms for the Evaluation of Residual Disease in Chronic Myeloid Leukemia. Clin. Chem. 2017, 63, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Loganathan, N.; Agarwalla, S.; Yang, C.; Yuan, W.Y.; Zeng, J.; Wu, R.G.; Wang, W.; Duraiswamy, S. Current commercial dPCR platforms: Technology and market review. Crit. Rev. Biotechnol. 2023, 43, 433–464. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Ahmed, W.; Oikarinen, S.; Sherchan, S.P.; Heikinheimo, A.; Jiang, G.M.; Simpson, S.L.; Greaves, J.; Bivins, A. Application of digital PCR for public health-related water quality monitoring. Sci. Total Environ. 2022, 837, 155663. [Google Scholar] [CrossRef]
- Liao, P.Y.; Huang, Y.Y. Digital PCR: Endless Frontier of ‘Divide and Conquer’. Micromachines 2017, 8, 231. [Google Scholar] [CrossRef]
- Oliveira, B.B.; Veigas, B.; Baptista, P.V. Isothermal amplification of nucleic acids: The race for the next “gold standard”. Front. Sens. 2021, 2, 752600. [Google Scholar] [CrossRef]
- Akhmetzianova, L.U.; Davletkulov, T.M.; Sakhabutdinova, A.R.; Chemeris, A.V.; Gubaydullin, I.M.; Garafutdinov, R.R. LAMPrimers iQ: New primer design software for loop-mediated isothermal amplification (LAMP). Anal. Biochem. 2024, 684, 115376. [Google Scholar] [CrossRef] [PubMed]
- Parida, M.; Sannarangaiah, S.; Dash, P.K.; Rao, P.V.L.; Morita, K. Loop mediated isothermal amplification (LAMP): A new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 2008, 18, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Moehling, T.J.; Choi, G.; Dugan, L.C.; Salit, M.; Meagher, R.J. LAMP Diagnostics at the point-of-care: Emerging trends and perspectives for the developer community. Expert Rev. Mol. Diagn. 2021, 21, 1. [Google Scholar]
- Yuan, H.; Chao, Y.C.; Shum, H.C. Droplet and Microchamber-Based Digital Loop-Mediated Isothermal Amplification (dLAMP). Small 2020, 16, e1904469. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Zaferani, M.; Cheong, S.H.; Abbaspourrad, A. Pathogenic Bacteria Detection Using RNA-Based Loop-Mediated Isothermal-Amplification-Assisted Nucleic Acid Amplification via Droplet Microfluidics. ACS Sens. 2019, 4, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Zheng, K.X.; Ye, Y.; Ji, J.L.; Cheng, X.Y.; He, S.L. Pipette-Tip-Enabled Digital Nucleic Acid Analyzer for COVID-19 Testing with Isothermal Amplification. Anal. Chem. 2021, 93, 15288–15294. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, L.B.; Ye, Z.Z.; Gong, J.J.; Hao, P.; Ping, J.F.; Ying, Y.B. TriD-LAMP: A pump-free microfluidic chip for duplex droplet digital loop-mediated isothermal amplification analysis. Anal. Chim. Acta 2022, 1233, 340513. [Google Scholar] [CrossRef]
- Ono, T.; Ichiki, T.; Noji, H. Digital enzyme assay using attoliter droplet array. Analyst 2018, 143, 4923–4929. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, S.W.; Matei, R.; Marx, W.; Beisel, C.L.; Wei, Q.S. Coupling smartphone and CRISPR-Cas12a for digital and multiplexed nucleic acid detection. AIChE J. 2021, 67, e17365. [Google Scholar] [CrossRef]
- Bao, L.; Rezk, A.R.; Yeo, L.Y.; Zhang, X.H. Highly Ordered Arrays of Femtoliter Surface Droplets. Small 2015, 11, 4850–4855. [Google Scholar] [CrossRef]
- Molter, T.W.; McQuaide, S.C.; Suchorolski, M.T.; Strovas, T.J.; Burgess, L.W.; Meldrum, D.R.; Lidstrom, M.E. A microwell array device capable of measuring single-cell oxygen consumption rates. Sens. Actuators B 2009, 135, 678–686. [Google Scholar] [CrossRef]
- Huang, X.; Lin, X.Y.; Urmann, K.; Li, L.J.; Xie, X.; Jiang, S.; Hoffmann, M.R. Smartphone-Based in-Gel Loop-Mediated Isothermal Amplification (gLAMP) System Enables Rapid Coliphage MS2 Quantification in Environmental Waters. Environ. Sci. Technol. 2018, 52, 6399–6407. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Li, J.; Lin, X.Y.; Huang, X.; Hoffmann, M.R. Single-Cell Phenotypic Analysis and Digital Molecular Detection Linkable by a Hydrogel Bead-Based Platform. ACS Appl. Bio Mater. 2021, 4, 2664–2674. [Google Scholar] [CrossRef]
- Lin, X.Y.; Huang, X.; Urmann, K.; Xie, X.; Hoffmann, M.R. Digital Loop-Mediated Isothermal Amplification on a Commercial Membrane. ACS Sens. 2019, 4, 242–249. [Google Scholar] [CrossRef]
- Chen, Y.J.; Qian, S.W.J.; Yu, X.P.; Wu, J.; Xu, J.F. Microfluidics: The propellant of CRISPR-based nucleic acid detection. Trends Biotechnol. 2023, 41, 557–574. [Google Scholar] [CrossRef]
- Magro, L.; Escadafal, C.; Garneret, P.; Jacquelin, B.; Kwasiborski, A.; Manuguerra, J.C.; Monti, F.; Sakuntabhai, A.; Vanhomwegen, J.; Lafaye, P.; et al. Paper microfluidics for nucleic acid amplification testing (NAAT) of infectious diseases. Lab Chip 2017, 17, 2347–2371. [Google Scholar] [CrossRef]
- Sritong, N.; de Medeiros, M.S.; Basing, L.A.; Linnes, J.C. Promise and perils of paper-based point-of-care nucleic acid detection for endemic and pandemic pathogens. Lab Chip 2023, 23, 888–912. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Xie, X.; Shi, X.L.; Lin, Y.M.; Mou, J.; Chen, Q.C.; Lu, Y.; Zhou, L.; Jiang, M.; Sun, H.H.; et al. Vibrio parahaemolyticus, Southern Coastal Region of China, 2007–2012. Emerg. Infect. Dis. 2014, 20, 685–688. [Google Scholar] [CrossRef]
- Linnes, J.C.; Rodriguez, N.M.; Liu, L.; Klapperich, C.M. Polyethersulfone improves isothermal nucleic acid amplification compared to current paper-based diagnostics. Biomed. Microdevices 2016, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Quyen, T.L.; Ngo, T.A.; Bang, D.D.; Madsen, M.; Wolff, A. Classification of Multiple DNA Dyes Based on Inhibition Effects on Real-Time Loop-Mediated Isothermal Amplification (LAMP): Prospect for Point of Care Setting. Front. Microbiol. 2019, 10, 2234. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.A.; Zhang, Y.H.; Evans, T.C. Simultaneous multiple target detection in real-time loop-mediated isothermal amplification. BioTechniques 2012, 53, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Hu, W.L.; Li, Y.; Li, Y.; Chen, S.Y.; Wang, J.G. Development of an RT-LAMP assay for the detection of maize yellow mosaic virus in maize. J. Virol. Methods 2022, 300, 114384. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhu, Y.; Peng, C.; Wang, X.; Wu, J.; Chen, H.; Xu, J. A Point-of-Care Nucleic Acid Quantification Method by Counting Light Spots Formed by LAMP Amplicons on a Paper Membrane. Biosensors 2024, 14, 139. https://doi.org/10.3390/bios14030139
Chen Y, Zhu Y, Peng C, Wang X, Wu J, Chen H, Xu J. A Point-of-Care Nucleic Acid Quantification Method by Counting Light Spots Formed by LAMP Amplicons on a Paper Membrane. Biosensors. 2024; 14(3):139. https://doi.org/10.3390/bios14030139
Chicago/Turabian StyleChen, Yanju, Yuanyuan Zhu, Cheng Peng, Xiaofu Wang, Jian Wu, Huan Chen, and Junfeng Xu. 2024. "A Point-of-Care Nucleic Acid Quantification Method by Counting Light Spots Formed by LAMP Amplicons on a Paper Membrane" Biosensors 14, no. 3: 139. https://doi.org/10.3390/bios14030139
APA StyleChen, Y., Zhu, Y., Peng, C., Wang, X., Wu, J., Chen, H., & Xu, J. (2024). A Point-of-Care Nucleic Acid Quantification Method by Counting Light Spots Formed by LAMP Amplicons on a Paper Membrane. Biosensors, 14(3), 139. https://doi.org/10.3390/bios14030139




