Application of Fluorescence- and Bioluminescence-Based Biosensors in Cancer Drug Discovery
Abstract
1. Introduction
1.1. Brief Overview of Cancer Drug Discovery
1.2. Significance of Biosensors in Cancer Research
2. Types of Fluorescence- and Bioluminescence-Based Biosensors
2.1. Fluorescence Biosensors
2.2. Bioluminescence Biosensors
2.3. Software Tools and Data Analysis for Biosensors
3. Application of Fluorescence- and Bioluminescence-Based Biosensors in Drug Discovery Through HTS
3.1. HTS Using Fluorescence-Based Biosensors
3.1.1. FRET Biosensors
3.1.2. TR-FRET Biosensors
| Biosensor Type | Target/Pathway | Key Findings | Reference |
|---|---|---|---|
| FRET | ERK and AKT kinases |
| He et al. (2019) [57] |
| hNTH1-YB1 PPI |
| Senarisoy et al. (2020) [58] | |
| ZAP70 |
| Liu et al. (2021) [59] | |
| H2O2-mediated pathways |
| Hao et al. (2022) [60] | |
| TR-FRET | CBP bromodomain |
| Zhang et al. (2020) [61] |
| SMAD4R361H-SMAD3 |
| Tang et al. (2021) [64] | |
| NSD3-MYC PPI |
| Xiong et al. (2018) [65] | |
| MKK3-MYC PPI |
| Yang et al. (2021) [29] | |
| SMAD4-SMAD3 PPI |
| Ouyang et al. (2024) [28] | |
| SYK-FCER1G |
| Du et al. (2024) [66] | |
| KDM |
| Singh et al. (2020) [67] | |
| KRAS GTPase |
| Larson et al. (2023) [68] | |
| BRET/ NanoBRET | RAS-RAF PPI |
| Durrant et al. (2021) [33] |
| PTK7-β-catinin |
| Ganier et al. (2022) [69] | |
| NanoBiT | RAF |
| Miyamoto et al. (2019) [70] |
| PP1 and PP2A holoenzymes |
| Claes and Bollen (2023) [71] | |
| YAP/TAZ-TEAD |
| Nouri et al. (2019) [24] | |
| RAS-effector PPI |
| Cooley et al. (2020) [72] | |
| HiBiT | PD-L1 |
| Uchida et al. (2021) [73] |
| YAP/TAZ |
| Wu et al. (2023) [40] |
3.1.3. BRET and NanoBRET Biosensors
3.2. HTS Using Bioluminescent NanoBiT Biosensors
4. Application of Biosensors in Drug Validation
4.1. FRET and TR-FRET Biosensors
4.2. BRET and NanoBRET Biosensors
4.2.1. Ligand–Receptor Binding Inhibitor Validation
4.2.2. Kinase Inhibitor Validation
4.2.3. PROTAC and Molecular Glue Validation
4.2.4. Covalent Inhibitor Validation
4.2.5. Validation of Candidate Inhibitors from DEL Screening
4.3. NanoBiT Biosensors
5. Biosensors in Studying Cancer Cell Signaling Pathways
5.1. FRET-Based Biosensors
5.2. NanoBRET Biosensors
5.3. Firefly Luciferase Biosensor and NanoBiT Biosensors
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| AI | Artificial intelligence |
| BiP | Binding immunoglobulin protein |
| BRET | Bioluminescence resonance energy transfer |
| CFP | Cyan fluorescent protein |
| CLuc | C-terminal luciferase |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CSK | C-terminal Src kinase |
| CTC | Circulating tumor cells |
| ctDNA | Circulating tumor DNA |
| DEL | DNA encoded library |
| EGFR | Epithelial growth factor receptor |
| Eu | Europium |
| FDA | Federal drug administration |
| FP | Fluorescence polarization |
| FRET | Fluorescent resonance energy transfer |
| GFP | Green fluorescent protein |
| GGTase I | Geranylgeranyltransferase Type I |
| GPCR | G protein-coupled receptors |
| HTS | High throughput screening |
| ITC | Isothermal titration calorimetry |
| KDM | Histone lysine demethylase |
| LAST-BS | LATS biosensor |
| LgBiT | Large BiT |
| NanoBiT | NanoLuc binary technology |
| NanoBRET | NanoLuc BRET |
| NGS | Next generation sequencing |
| NLuc | N-terminal luciferase |
| NSCLC | None-small cell lung cancer |
| PhALC | Phosphorylation-assisted luciferase complementation |
| PI3K α | Phosphoinositide 3-kinase α |
| PLK | Polo-like kinases |
| PPI | Protein–protein interaction |
| PROTAC | Proteolysis targeting chimera |
| Prx2 | Peroxiredoxin-2 |
| RLuc | Renilla luciferase |
| Ser | Serine |
| SLCA | Split-luciferase complementation assays |
| SM | Small molecule |
| SmBiT | Small BiT |
| SPR | Surface plasmon resonance |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| Thr | Threonine |
| TNBC | Triple negative breast cancer |
| TPD | Targeted protein degradation |
| TR-FRET | Time-lapse FRET |
| uHTS | Ultra HTS |
| YAP | Yes-associated protein |
| YFP | Yellow fluorescent protein |
References
- Gershell, L.J.; Atkins, J.H. A brief history of novel drug discovery technologies. Nat. Rev. Drug Discov. 2003, 2, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Li, H.; Ver Steeg, G.; Godzik, A. Advances in AI for Protein Structure Prediction: Implications for Cancer Drug Discovery and Development. Biomolecules 2024, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cuozzo, J. Review article: High-throughput affinity-based technologies for small-molecule drug discovery. J. Biomol. Screen. 2009, 14, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Blay, V.; Tolani, B.; Ho, S.P.; Arkin, M.R. High-Throughput Screening: Today’s biochemical and cell-based approaches. Drug Discov. Today 2020, 25, 1807–1821. [Google Scholar] [CrossRef]
- Salame, N.; Fooks, K.; El-Hachem, N.; Bikorimana, J.P.; Mercier, F.E.; Rafei, M. Recent Advances in Cancer Drug Discovery Through the Use of Phenotypic Reporter Systems, Connectivity Mapping, and Pooled CRISPR Screening. Front. Pharmacol. 2022, 13, 852143. [Google Scholar] [CrossRef]
- Guo, H.; Xu, X.; Zhang, J.; Du, Y.; Yang, X.; He, Z.; Zhao, L.; Liang, T.; Guo, L. The Pivotal Role of Preclinical Animal Models in Anti-Cancer Drug Discovery and Personalized Cancer Therapy Strategies. Pharmaceuticals 2024, 17, 1048. [Google Scholar] [CrossRef]
- Calpe, B.; Kovacs, W.J. High-throughput screening in multicellular spheroids for target discovery in the tumor microenvironment. Expert Opin. Drug Discov. 2020, 15, 955–967. [Google Scholar] [CrossRef]
- Sun, G.; Rong, D.; Li, Z.; Sun, G.; Wu, F.; Li, X.; Cao, H.; Cheng, Y.; Tang, W.; Sun, Y. Role of Small Molecule Targeted Compounds in Cancer: Progress, Opportunities, and Challenges. Front. Cell Dev. Biol. 2021, 9, 694363. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Brown, D.G.; Boström, J. Where Do Recent Small Molecule Clinical Development Candidates Come From? J. Med. Chem. 2018, 61, 9442–9468. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Crown, J. Drugging “undruggable” genes for cancer treatment: Are we making progress? Int. J. Cancer 2021, 148, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.; Tashakor, A.; Hosseinkhani, S. Split-luciferase complementary assay: Applications, recent developments, and future perspectives. Anal. Bioanal. Chem. 2014, 406, 5541–5560. [Google Scholar] [CrossRef]
- Quazi, S. Application of biosensors in cancers, an overview. Front. Bioeng. Biotechnol. 2023, 11, 1193493. [Google Scholar] [CrossRef]
- Wehr, M.C.; Rossner, M.J. Split protein biosensor assays in molecular pharmacological studies. Drug Discov. Today 2016, 21, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Zhou, M.; Wu, B.; Zhou, J. Application of Biosensors in Detecting Breast Cancer Metastasis. Sensors 2023, 23, 8813. [Google Scholar] [CrossRef] [PubMed]
- Abdul Wahab, M.R.; Palaniyandi, T.; Viswanathan, S.; Baskar, G.; Surendran, H.; Gangadharan, S.G.D.; Sugumaran, A.; Sivaji, A.; Kaliamoorthy, S.; Kumarasamy, S. Biomarker-specific biosensors revolutionise breast cancer diagnosis. Clin. Chim. Acta 2024, 555, 117792. [Google Scholar] [CrossRef]
- Sanko, V.; Kuralay, F. Label-Free Electrochemical Biosensor Platforms for Cancer Diagnosis: Recent Achievements and Challenges. Biosensors 2023, 13, 333. [Google Scholar] [CrossRef]
- Das, S.; Devireddy, R.; Gartia, M.R. Surface Plasmon Resonance (SPR) Sensor for Cancer Biomarker Detection. Biosensors 2023, 13, 396. [Google Scholar] [CrossRef]
- Simard, J.R.; Lee, L.; Vieux, E.; Improgo, R.; Tieu, T.; Phillips, A.J.; Fisher, S.L.; Pollock, R.M.; Park, E. High-Throughput Quantitative Assay Technologies for Accelerating the Discovery and Optimization of Targeted Protein Degradation Therapeutics. SLAS Discov. 2021, 26, 503–517. [Google Scholar] [CrossRef]
- Reyes-Alcaraz, A.; Lucero Garcia-Rojas, E.Y.; Merlinsky, E.A.; Seong, J.Y.; Bond, R.A.; McConnell, B.K. A NanoBiT assay to monitor membrane proteins trafficking for drug discovery and drug development. Commun. Biol. 2022, 5, 212. [Google Scholar] [CrossRef] [PubMed]
- Pipchuk, A.; Kelly, T.; Carew, M.; Nicol, C.; Yang, X. Development of Novel Bioluminescent Biosensors Monitoring the Conformation and Activity of the Merlin Tumour Suppressor. Int. J. Mol. Sci. 2024, 25, 1527. [Google Scholar] [CrossRef]
- Pipchuk, A.; Yang, X. Using Biosensors to Study Protein-Protein Interaction in the Hippo Pathway. Front. Cell Dev. Biol. 2021, 9, 660137. [Google Scholar] [CrossRef]
- Nouri, K.; Azad, T.; Ling, M.; van Rensburg, H.J.J.; Pipchuk, A.; Shen, H.; Hao, Y.; Zhang, J.; Yang, X. Identification of celastrol as a novel YAP-TEAD inhibitor for cancer therapy by high throughput screening with ultrasensitive YAP/TAZ-TEAD biosensors. Cancers 2019, 11, 1596. [Google Scholar] [CrossRef] [PubMed]
- Kozielewicz, P.; Schihada, H.; Schulte, G. Employing Genetically Encoded, Biophysical Sensors to Understand WNT/Frizzled Interaction and Receptor Complex Activation. Handb. Exp. Pharmacol. 2021, 269, 101–115. [Google Scholar] [CrossRef]
- Dale, N.C.; Johnstone, E.K.M.; White, C.W.; Pfleger, K.D.G. NanoBRET: The Bright Future of Proximity-Based Assays. Front. Bioeng. Biotechnol. 2019, 7, 56. [Google Scholar] [CrossRef]
- Verma, A.K.; Noumani, A.; Yadav, A.K.; Solanki, P.R. FRET Based Biosensor: Principle Applications Recent Advances and Challenges. Diagnostics 2023, 13, 1375. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Li, Q.; Niu, Q.; Qui, M.; Fu, H.; Du, Y.; Mo, X. A multiplexed time-resolved fluorescence resonance energy transfer ultrahigh-throughput screening assay for targeting the SMAD4-SMAD3-DNA complex. J. Mol. Cell Biol. 2024, 15, mjad068. [Google Scholar] [CrossRef]
- Yang, X.; Fan, D.; Troha, A.H.; Ahn, H.M.; Qian, K.; Liang, B.; Du, Y.; Fu, H.; Ivanov, A.A. Discovery of the first chemical tools to regulate MKK3-mediated MYC activation in cancer. Bioorg. Med. Chem. 2021, 45, 116324. [Google Scholar] [CrossRef]
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent Advances in Biosensor Technology for Potential Applications—An Overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef]
- Machleidt, T.; Woodroofe, C.C.; Schwinn, M.K.; Méndez, J.; Robers, M.B.; Zimmerman, K.; Otto, P.; Daniels, D.L.; Kirkland, T.A.; Wood, K.V. NanoBRET--A Novel BRET Platform for the Analysis of Protein-Protein Interactions. ACS Chem. Biol. 2015, 10, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Vunnam, N.; Lewis, A.K.; Chiu, T.L.; Brummel, B.E.; Schaaf, T.M.; Grant, B.D.; Bawaskar, P.; Thomas, D.D.; Sachs, J.N. An Innovative High-Throughput Screening Approach for Discovery of Small Molecules That Inhibit TNF Receptors. SLAS Discov. 2017, 22, 950–961. [Google Scholar] [CrossRef]
- Durrant, D.E.; Smith, E.A.; Goncharova, E.I.; Sharma, N.; Alexander, P.A.; Stephen, A.G.; Henrich, C.J.; Morrison, D.K. Development of a High-throughput NanoBRET Screening Platform to Identify Modulators of the RAS/RAF Interaction. Mol. Cancer Ther. 2021, 20, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Teske, K.A.; Su, W.; Corona, C.R.; Wen, J.; Deng, J.; Ping, Y.; Zhang, Z.; Zhang, Q.; Wilkinson, J.; Beck, M.T.; et al. DELs enable the development of BRET probes for target engagement studies in cells. Cell Chem. Biol. 2023, 30, 987–998.e924. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.S.; Schwinn, M.K.; Hall, M.P.; Zimmerman, K.; Otto, P.; Lubben, T.H.; Butler, B.L.; Binkowski, B.F.; Machleidt, T.; Kirkland, T.A.; et al. NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chem. Biol. 2016, 11, 400–408. [Google Scholar] [CrossRef] [PubMed]
- England, C.G.; Ehlerding, E.B.; Cai, W. NanoLuc: A Small Luciferase Is Brightening Up the Field of Bioluminescence. Bioconjugate Chem. 2016, 27, 1175–1187. [Google Scholar] [CrossRef]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef]
- Azad, T.; Janse van Rensburg, H.J.; Lightbody, E.D.; Neveu, B.; Champagne, A.; Ghaffari, A.; Kay, V.R.; Hao, Y.; Shen, H.; Yeung, B.; et al. A LATS biosensor functional screen identifies VEGFR as a novel regulator of the Hippo pathway in angiogenesis. Nat.Commun. 2018, 9, 1061. [Google Scholar] [CrossRef]
- Azad, T.; Nouri, K.; Janse van Rensburg, H.J.; Hao, Y.; Yang, X. Monitoring Hippo signaling pathway activity using a luciferase-based large tumor suppressor (LATS) biosensor. J. Vis. Exp. 2018, 139, 58416. [Google Scholar] [CrossRef]
- Wu, L.; Ge, A.; Hao, Y.; Yang, X. Development of a New HiBiT Biosensor Monitoring Stability of YAP/TAZ Proteins in Cells. Chemosensors 2023, 11, 492. [Google Scholar] [CrossRef]
- Broussard, J.A.; Rappaz, B.; Webb, D.J.; Brown, C.M. Fluorescence resonance energy transfer microscopy as demonstrated by measuring the activation of the serine/threonine kinase Akt. Nat. Protoc. 2013, 8, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Hachet-Haas, M.; Converset, N.; Marchal, O.; Matthes, H.; Gioria, S.; Galzi, J.L.; Lecat, S. FRET and colocalization analyzer—A method to validate measurements of sensitized emission FRET acquired by confocal microscopy and available as an ImageJ Plug-in. Microsc. Res. Tech. 2006, 69, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Degorce, F.; Card, A.; Soh, S.; Trinquet, E.; Knapik, G.P.; Xie, B. HTRF: A technology tailored for drug discovery—A review of theoretical aspects and recent applications. Curr. Chem. Genom. 2009, 3, 22–32. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Macarron, R.; Banks, M.N.; Bojanic, D.; Burns, D.J.; Cirovic, D.A.; Garyantes, T.; Green, D.V.; Hertzberg, R.P.; Janzen, W.P.; Paslay, J.W.; et al. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov. 2011, 10, 188–195. [Google Scholar] [CrossRef]
- Srinivasan, B.; Flórez Weidinger, J.D.; Zhai, X.; Lemercier, G.; Ikeda, T.; Brewer, M.; Zhang, B.; Heyse, S.; Wingfield, J.; Steigele, S. High-throughput mechanistic screening of non-equilibrium inhibitors by a fully automated data analysis pipeline in early drug-discovery. SLAS Discov. 2022, 27, 460–470. [Google Scholar] [CrossRef]
- Shaterabadi, D.; Zamani Sani, M.; Rahdan, F.; Taghizadeh, M.; Rafiee, M.; Dorosti, N.; Dianatinasab, A.; Taheri-Anganeh, M.; Asadi, P.; Khatami, S.H.; et al. MicroRNA biosensors in lung cancer. Clin. Chim. Acta 2024, 552, 117676. [Google Scholar] [CrossRef]
- Kang, M.J.; Cho, Y.W.; Kim, T.H. Progress in Nano-Biosensors for Non-Invasive Monitoring of Stem Cell Differentiation. Biosensors 2023, 13, 501. [Google Scholar] [CrossRef]
- Lin, X.; Wang, K.; Luo, C.; Yang, M.; Wu, J. MicroRNA Biosensors for Early Detection of Hepatocellular Carcinoma. Chemosensors 2023, 11, 504. [Google Scholar] [CrossRef]
- Meng, X.; Pang, X.; Yang, J.; Zhang, X.; Dong, H. Recent Advances in Electrochemiluminescence Biosensors for MicroRNA Detection. Small 2023, 20, e2307701. [Google Scholar] [CrossRef]
- Mi, D.; Li, Y.; Gu, H.; Li, Y.; Chen, Y. Current advances of small molecule E3 ligands for proteolysis-targeting chimeras design. Eur. J. Med. Chem. 2023, 256, 115444. [Google Scholar] [CrossRef] [PubMed]
- Dogheim, G.M.; Amralla, M.T. Proteolysis Targeting Chimera (PROTAC) as a promising novel therapeutic modality for the treatment of triple-negative breast cancer (TNBC). Drug Dev. Res. 2023, 84, 629–653. [Google Scholar] [CrossRef]
- Xue, Y.; Bolinger, A.A.; Zhou, J. Novel approaches to targeted protein degradation technologies in drug discovery. Expert Opin. Drug Discov. 2023, 18, 467–483. [Google Scholar] [CrossRef]
- You, Y.; Lai, X.; Pan, Y.; Zheng, H.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial intelligence in cancer target identification and drug discovery. Signal Transduct. Target. Ther. 2022, 7, 156. [Google Scholar] [CrossRef]
- Wu, T.; Yoon, H.; Xiong, Y.; Dixon-Clarke, S.E.; Nowak, R.P.; Fischer, E.S. Targeted protein degradation as a powerful research tool in basic biology and drug target discovery. Nat. Struct. Mol. Biol. 2020, 27, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wink, S.; de Bont, H.; Le Dévédec, S.; Zhang, Y.; van de Water, B. FRET biosensor-based kinase inhibitor screen for ERK and AKT activity reveals differential kinase dependencies for proliferation in TNBC cells. Biochem. Pharmacol. 2019, 169, 113640. [Google Scholar] [CrossRef]
- Senarisoy, M.; Barette, C.; Lacroix, F.; De Bonis, S.; Stelter, M.; Hans, F.; Kleman, J.P.; Fauvarque, M.O.; Timmins, J. Förster Resonance Energy Transfer Based Biosensor for Targeting the hNTH1-YB1 Interface as a Potential Anticancer Drug Target. ACS Chem. Biol. 2020, 15, 990–1003. [Google Scholar] [CrossRef]
- Liu, L.; Limsakul, P.; Meng, X.; Huang, Y.; Harrison, R.E.S.; Huang, T.S.; Shi, Y.; Yu, Y.; Charupanit, K.; Zhong, S.; et al. Integration of FRET and sequencing to engineer kinase biosensors from mammalian cell libraries. Nat. Commun. 2021, 12, 5031. [Google Scholar] [CrossRef]
- Hao, Y.; Langford, T.F.; Moon, S.J.; Eller, K.A.; Sikes, H.D. Screening compound libraries for H(2)O(2)-mediated cancer therapeutics using a peroxiredoxin-based sensor. Cell Chem. Biol. 2022, 29, 625–635.e623. [Google Scholar] [CrossRef]
- Zhang, F.C.; Sun, Z.Y.; Liao, L.P.; Zuo, Y.; Zhang, D.; Wang, J.; Chen, Y.T.; Xiao, S.H.; Jiang, H.; Lu, T.; et al. Discovery of novel CBP bromodomain inhibitors through TR-FRET-based high-throughput screening. Acta Pharmacol. Sin. 2020, 41, 286–292. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Mishra, L.; Deng, C.X. The role of TGF-β/SMAD4 signaling in cancer. Int. J. Biol. Sci. 2018, 14, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Mo, X.; Niu, Q.; Wahafu, A.; Yang, X.; Qui, M.; Ivanov, A.; Du, Y.; Fu, H. Hypomorph mutation-directed small-molecule protein-protein interaction inducers to restore mutant SMAD4-suppressed TGF-β signaling. Cell Chem. Biol. 2021, 28, 636–647. [Google Scholar] [CrossRef]
- Xiong, J.; Pecchi, V.G.; Qui, M.; Ivanov, A.A.; Mo, X.; Niu, Q.; Chen, X.; Fu, H.; Du, Y. Development of a Time-Resolved Fluorescence Resonance Energy Transfer Ultrahigh-Throughput Screening Assay for Targeting the NSD3 and MYC Interaction. Assay. Drug Dev. Technol. 2018, 16, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, D.; Katis, V.L.; Zoeller, E.L.; Qui, M.; Levey, A.I.; Gileadi, O.; Fu, H. Development of a time-resolved fluorescence resonance energy transfer ultra-high throughput screening assay targeting SYK and FCER1G interaction. SLAS Discov. 2024, 29, 100177. [Google Scholar] [CrossRef]
- Singh, S.; Abu-Zaid, A.; Lin, W.; Low, J.; Abdolvahabi, A.; Jin, H.; Wu, Q.; Cooke, B.; Fang, J.; Bowling, J.; et al. 17-DMAG dually inhibits Hsp90 and histone lysine demethylases in alveolar rhabdomyosarcoma. iScience 2021, 24, 101996. [Google Scholar] [CrossRef]
- Larson, J.E.; Hardy, P.B.; Schomburg, N.K.; Wang, X.; Kireev, D.; Rossman, K.L.; Pearce, K.H. Development of a high-throughput TR-FRET screening assay for a fast-cycling KRAS mutant. SLAS Discov. 2023, 28, 39–47. [Google Scholar] [CrossRef]
- Ganier, L.; Betzi, S.; Derviaux, C.; Roche, P.; Dessaux, C.; Muller, C.; Hoffer, L.; Morelli, X.; Borg, J.P. Discovery of Small-Molecule Inhibitors of the PTK7/β-Catenin Interaction Targeting the Wnt Signaling Pathway in Colorectal Cancer. ACS Chem. Biol. 2022, 17, 1061–1072. [Google Scholar] [CrossRef]
- Miyamoto, K.; Sawa, M. Development of Highly Sensitive Biosensors of RAF Dimerization in Cells. Sci. Rep. 2019, 9, 636. [Google Scholar] [CrossRef]
- Claes, Z.; Bollen, M. A split-luciferase lysate-based approach to identify small-molecule modulators of phosphatase subunit interactions. Cell Chem. Biol. 2023, 30, 1666–1679.e1666. [Google Scholar] [CrossRef] [PubMed]
- Cooley, R.; Kara, N.; Hui, N.S.; Tart, J.; Roustan, C.; George, R.; Hancock, D.C.; Binkowski, B.F.; Wood, K.V.; Ismail, M.; et al. Development of a cell-free split-luciferase biochemical assay as a tool for screening for inhibitors of challenging protein-protein interaction targets. Wellcome Open Res. 2020, 5, 20. [Google Scholar] [CrossRef]
- Uchida, Y.; Matsushima, T.; Kurimoto, R.; Chiba, T.; Inutani, Y.; Asahara, H. Identification of chemical compounds regulating PD-L1 by introducing HiBiT-tagged cells. FEBS Lett. 2021, 595, 563–576. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yonezawa, T.; Sakamoto, J.; Furuya, T.; Osawa, M.; Ikeda, K. Identification of novel inhibitors of Keap1/Nrf2 by a promising method combining protein-protein interaction-oriented library and machine learning. Sci. Rep. 2021, 11, 7420. [Google Scholar] [CrossRef]
- Cartwright, T.N.; Meyer, S.K.; Higgins, J.M.G. Robustness of NanoBiT luciferase complementation technology in the presence of widely used kinase inhibitors. SLAS Discov. 2022, 27, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Wood, K.V. Bioluminescent assays for high-throughput screening. Assay Drug Dev. Technol. 2007, 5, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.J. YAP/TAZ: Drivers of tumor growth, metastasis, and resistance to therapy. Bioessays 2020, 42, e1900162. [Google Scholar] [CrossRef]
- Kong, N.R.; Jones, L.H. Clinical Translation of Targeted Protein Degraders. Clin. Pharmacol. Ther. 2023, 114, 558–568. [Google Scholar] [CrossRef]
- Bhole, R.P.; Kute, P.R.; Chikhale, R.V.; Bonde, C.G.; Pant, A.; Gurav, S.S. Unlocking the potential of PROTACs: A comprehensive review of protein degradation strategies in disease therapy. Bioorg. Chem. 2023, 139, 106720. [Google Scholar] [CrossRef]
- Lankford, K.P.; Hulleman, J.D. Protocol for HiBiT tagging endogenous proteins using CRISPR-Cas9 gene editing. STAR Protoc. 2024, 5, 103000. [Google Scholar] [CrossRef]
- Schwinn, M.K.; Machleidt, T.; Zimmerman, K.; Eggers, C.T.; Dixon, A.S.; Hurst, R.; Hall, M.P.; Encell, L.P.; Binkowski, B.F.; Wood, K.V. CRISPR-Mediated Tagging of Endogenous Proteins with a Luminescent Peptide. ACS Chem. Biol. 2018, 13, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Riching, K.; Lai, M.P.; Lu, D.; Cheng, R.; Qi, X.; Wang, J. Lysineless HiBiT and NanoLuc Tagging Systems as Alternative Tools for Monitoring Targeted Protein Degradation. ACS Med. Chem. Lett. 2024, 15, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Orhan, M.D.; Avsar, T.; Durdagi, S. Hybrid In Silico and TR-FRET-Guided Discovery of Novel BCL-2 Inhibitors. ACS Pharmacol. Transl. Sci. 2021, 4, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Borysko, P.; Moroz, Y.S.; Vasylchenko, O.V.; Hurmach, V.V.; Starodubtseva, A.; Stefanishena, N.; Nesteruk, K.; Zozulya, S.; Kondratov, I.S.; Grygorenko, O.O. Straightforward hit identification approach in fragment-based discovery of bromodomain-containing protein 4 (BRD4) inhibitors. Bioorg Med. Chem. 2018, 26, 3399–3405. [Google Scholar] [CrossRef]
- Farmer, J.P.; Mistry, S.N.; Laughton, C.A.; Holliday, N.D. Development of fluorescent peptide G protein-coupled receptor activation biosensors for NanoBRET characterization of intracellular allosteric modulators. FASEB J. 2022, 36, e22576. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Chen, T. General Stepwise Approach to Optimize a TR-FRET Assay for Characterizing the BRD/PROTAC/CRBN Ternary Complex. ACS Pharmacol. Transl. Sci. 2021, 4, 941–952. [Google Scholar] [CrossRef]
- Abed, D.A.; Ali, A.R.; Lee, S.; Nguyen, M.U.; Verzi, M.P.; Hu, L. Optimization of the C2 substituents on the 1,4-bis(arylsulfonamido)naphthalene-N,N’-diacetic acid scaffold for better inhibition of Keap1-Nrf2 protein-protein interaction. Eur. J. Med. Chem. 2023, 252, 115302. [Google Scholar] [CrossRef]
- Payne, N.C.; Maksoud, S.; Tannous, B.A.; Mazitschek, R. A direct high-throughput protein quantification strategy facilitates discovery and characterization of a celastrol-derived BRD4 degrader. Cell Chem. Biol. 2022, 29, 1333–1340.e1335. [Google Scholar] [CrossRef]
- Kozielewicz, P.; Schulte, G. NanoBRET and NanoBiT/BRET-Based Ligand Binding Assays Permit Quantitative Assessment of Small Molecule Ligand Binding to Smoothened. Methods Mol. Biol. 2022, 2374, 195–204. [Google Scholar] [CrossRef]
- Lay, C.S.; Thomas, D.A.; Evans, J.P.; Campbell, M.; McCombe, K.; Phillipou, A.N.; Gordon, L.J.; Jones, E.J.; Riching, K.; Mahmood, M.; et al. Development of an intracellular quantitative assay to measure compound binding kinetics. Cell Chem. Biol. 2023, 30, 1692. [Google Scholar] [CrossRef]
- Chiappa, M.; Petrella, S.; Damia, G.; Broggini, M.; Guffanti, F.; Ricci, F. Present and Future Perspective on PLK1 Inhibition in Cancer Treatment. Front. Oncol. 2022, 12, 903016. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Smith, J.L.; Beck, M.T.; Wilkinson, J.M.; Michaud, A.; Vasta, J.D.; Robers, M.B.; Willson, T.M. Development of Cell Permeable NanoBRET Probes for the Measurement of PLK1 Target Engagement in Live Cells. Molecules 2023, 28, 2950. [Google Scholar] [CrossRef]
- Kong, D.; Tian, Q.; Chen, Z.; Zheng, H.; Stashko, M.A.; Yan, D.; Earp, H.S.; Frye, S.V.; DeRyckere, D.; Kireev, D.; et al. Discovery of Novel Macrocyclic MERTK/AXL Dual Inhibitors. J. Med. Chem. 2024, 67, 5866–5882. [Google Scholar] [CrossRef]
- Chi, Z.; Chen, S.; Yang, D.; Cui, W.; Lu, Y.; Wang, Z.; Li, M.; Yu, W.; Zhang, J.; Jiang, Y.; et al. Gasdermin D-mediated metabolic crosstalk promotes tissue repair. Nature 2024, 634, 1168–1177. [Google Scholar] [CrossRef]
- Bai, N.; Riching, K.M.; Makaju, A.; Wu, H.; Acker, T.M.; Ou, S.C.; Zhang, Y.; Shen, X.; Bulloch, D.N.; Rui, H.; et al. Modeling the CRL4A ligase complex to predict target protein ubiquitination induced by cereblon-recruiting PROTACs. J. Biol. Chem. 2022, 298, 101653. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Xiao, Y.; Liu, X.; Hu, W.; Sobh, A.; Yuan, Y.; Zhou, S.; Hua, N.; Mackintosh, S.G.; Zhang, X.; et al. Piperlongumine conjugates induce targeted protein degradation. Cell Chem. Biol. 2023, 30, 203–213.e217. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J. Quantitative measurement of PROTAC intracellular accumulation. Methods Enzymol. 2023, 681, 189–214. [Google Scholar] [CrossRef] [PubMed]
- Zerfas, B.L.; Huerta, F.; Liu, H.; Du, G.; Gray, N.S.; Jones, L.H.; Nowak, R.P. Advancing targeted protein degrader discovery by measuring cereblon engagement in cells. Methods Enzymol. 2023, 681, 169–188. [Google Scholar] [CrossRef]
- Borsari, C.; Keles, E.; McPhail, J.A.; Schaefer, A.; Sriramaratnam, R.; Goch, W.; Schaefer, T.; De Pascale, M.; Bal, W.; Gstaiger, M.; et al. Covalent Proximity Scanning of a Distal Cysteine to Target PI3Kα. J. Am. Chem. Soc. 2022, 144, 6326–6342. [Google Scholar] [CrossRef]
- Weeks, R.; Zhou, X.; Yuan, T.L.; Zhang, J. Fluorescent Biosensor for Measuring Ras Activity in Living Cells. J. Am. Chem. Soc. 2022, 144, 17432–17440. [Google Scholar] [CrossRef]
- Sunkari, Y.K.; Siripuram, V.K.; Nguyen, T.L.; Flajolet, M. High-power screening (HPS) empowered by DNA-encoded libraries. Trends Pharmacol. Sci. 2022, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Madasu, C.; Liao, Z.; Parks, S.E.; Sharma, K.L.; Bohren, K.M.; Ye, Q.; Li, F.; Palaniappan, M.; Tan, Z.; Yuan, F.; et al. Identification of potent pan-ephrin receptor kinase inhibitors using DNA-encoded chemistry technology. Proc. Natl. Acad. Sci. USA 2024, 121, e2322934121. [Google Scholar] [CrossRef] [PubMed]
- Hinz, S.; Jung, D.; Hauert, D.; Bachmann, H.S. Molecular and Pharmacological Characterization of the Interaction between Human Geranylgeranyltransferase Type I and Ras-Related Protein Rap1B. Int. J. Mol. Sci. 2021, 22, 2501. [Google Scholar] [CrossRef] [PubMed]
- Morató, X.; Fernández-Dueñas, V.; Pérez-Villamor, P.; Valle-León, M.; Vela, J.M.; Merlos, M.; Burgueño, J.; Ciruela, F. Development of a Novel σ(1) Receptor Biosensor Based on Its Heterodimerization with Binding Immunoglobulin Protein in Living Cells. ACS Chem. Neurosci. 2023, 14, 2201–2207. [Google Scholar] [CrossRef]
- Campbell, E.; Adamson, H.; Luxton, T.; Tiede, C.; Wälti, C.; Tomlinson, D.C.; Jeuken, L.J.C. Therapeutic drug monitoring of immunotherapies with novel Affimer-NanoBiT sensor construct. Sens. Diagn. 2024, 3, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, L.; Spohr, C.; Beha, C.; Griffin, R.; Braun, S.; Halbach, S.; Brummer, T. Analysis of RAS and drug induced homo- and heterodimerization of RAF and KSR1 proteins in living cells using split Nanoluc luciferase. Cell Commun. Signal 2023, 21, 136. [Google Scholar] [CrossRef]
- Kelly, T.; Bhandari, S.; Carew, M.; Rubino, R.; Nicol, C.; Yang, X. A Novel Bioluminescent Biosensor Quantifying Intramolecular Interaction and Levels of Pyroptosis Effector GSDMD. Cells 2024, 13, 1606. [Google Scholar] [CrossRef]
- Hsu, W.C.; Nenov, M.N.; Shavkunov, A.; Panova, N.; Zhan, M.; Laezza, F. Identifying a kinase network regulating FGF14:Nav1.6 complex assembly using split-luciferase complementation. PLoS ONE 2015, 10, e0117246. [Google Scholar] [CrossRef]
- Alcobia, D.C.; Ziegler, A.I.; Kondrashov, A.; Comeo, E.; Mistry, S.; Kellam, B.; Chang, A.; Woolard, J.; Hill, S.J.; Sloan, E.K. Visualizing Ligand Binding to a GPCR In Vivo Using NanoBRET. iScience 2018, 6, 280–288. [Google Scholar] [CrossRef]
- Stoddart, L.A.; Kilpatrick, L.E.; Hill, S.J. NanoBRET Approaches to Study Ligand Binding to GPCRs and RTKs. Trends Pharmacol. Sci. 2018, 39, 136–147. [Google Scholar] [CrossRef]
- Mo, X.; Niu, Q.; Ivanov, A.A.; Tsang, Y.H.; Tang, C.; Shu, C.; Li, Q.; Qian, K.; Wahafu, A.; Doyle, S.P.; et al. Systematic discovery of mutation-directed neo-protein-protein interactions in cancer. Cell 2022, 185, 1974–1985.e1912. [Google Scholar] [CrossRef] [PubMed]
- Dosquet, H.; Neirinckx, V.; Meyrath, M.; Wantz, M.; Haan, S.; Niclou, S.P.; Szpakowska, M.; Chevigné, A. Nanoluciferase-based complementation assays to monitor activation, modulation and signaling of receptor tyrosine kinases (RTKs). Methods Enzymol. 2023, 682, 1–16. [Google Scholar] [CrossRef]
- Boon, K.; Vanalken, N.; Meyen, E.; Schols, D.; Van Loy, T. REGA-SIGN: Development of a Novel Set of NanoBRET-Based G Protein Biosensors. Biosensors 2023, 13, 767. [Google Scholar] [CrossRef]
- Kulkarni, A.; Chang, M.T.; Vissers, J.H.A.; Dey, A.; Harvey, K.F. The Hippo Pathway as a Driver of Select Human Cancers. Trends Cancer 2020, 6, 781–796. [Google Scholar] [CrossRef]
- Visser, S.; Yang, X. LATS tumor suppressor: A new governor of cellular homeostasis. Cell Cycle 2010, 9, 3892–3903. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.; Nouri, K.; van Rensburg, H.J.J.; Maritan, S.M.; Wu, L.; Hao, Y.; Montminy, T.; Yu, J.; Khanal, P.; Mulligan, L.M.; et al. A gain-of-functional screen identifies the Hippo pathway as a central mediator of receptor tyrosine kinases during tumorigenesis. Oncogene 2020, 39, 334–355. [Google Scholar] [CrossRef] [PubMed]
- Nouri, K.; Azad, T.; Lightbody, E.; Khanal, P.; Nicol, C.J.; Yang, X. A kinome-wide screen using a NanoLuc LATS luminescent biosensor identifies ALK as a novel regulator of the Hippo pathway in tumorigenesis and immune evasion. FASEB J. 2019, 33, 12487–12499. [Google Scholar] [CrossRef]
- Sarmasti Emami, S.; Ge, A.; Zhang, D.; Hao, Y.; Ling, M.; Rubino, R.; Nicol, C.J.B.; Wang, W.; Yang, X. Identification of PTPN12 Phosphatase as a Novel Negative Regulator of Hippo Pathway Effectors YAP/TAZ in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 4064. [Google Scholar] [CrossRef]
- Póti, Á.L.; Dénes, L.; Papp, K.; Bató, C.; Bánóczi, Z.; Reményi, A.; Alexa, A. Phosphorylation-Assisted Luciferase Complementation Assay Designed to Monitor Kinase Activity and Kinase-Domain-Mediated Protein-Protein Binding. Int. J. Mol. Sci. 2023, 24, 4854. [Google Scholar] [CrossRef]
- Kupcho, K.; Shultz, J.; Hurst, R.; Hartnett, J.; Zhou, W.; Machleidt, T.; Grailer, J.; Worzella, T.; Riss, T.; Lazar, D.; et al. A real-time, bioluminescent annexin V assay for the assessment of apoptosis. Apoptosis 2019, 24, 184–197. [Google Scholar] [CrossRef]
- Inoue, A.; Raimondi, F.; Kadji, F.M.N.; Singh, G.; Kishi, T.; Uwamizu, A.; Ono, Y.; Shinjo, Y.; Ishida, S.; Arang, N.; et al. Illuminating G-protein-coupling selectivity of GPCRs. Cell 2019, 177, 1933–1947.e1925. [Google Scholar] [CrossRef] [PubMed]
- Zeghal, M.; Matte, K.; Venes, A.; Patel, S.; Laroche, G.; Sarvan, S.; Joshi, M.; Rain, J.C.; Couture, J.F.; Giguère, P.M. Development of a V5-tag-directed nanobody and its implementation as an intracellular biosensor of GPCR signaling. J. Biol. Chem. 2023, 299, 105107. [Google Scholar] [CrossRef] [PubMed]

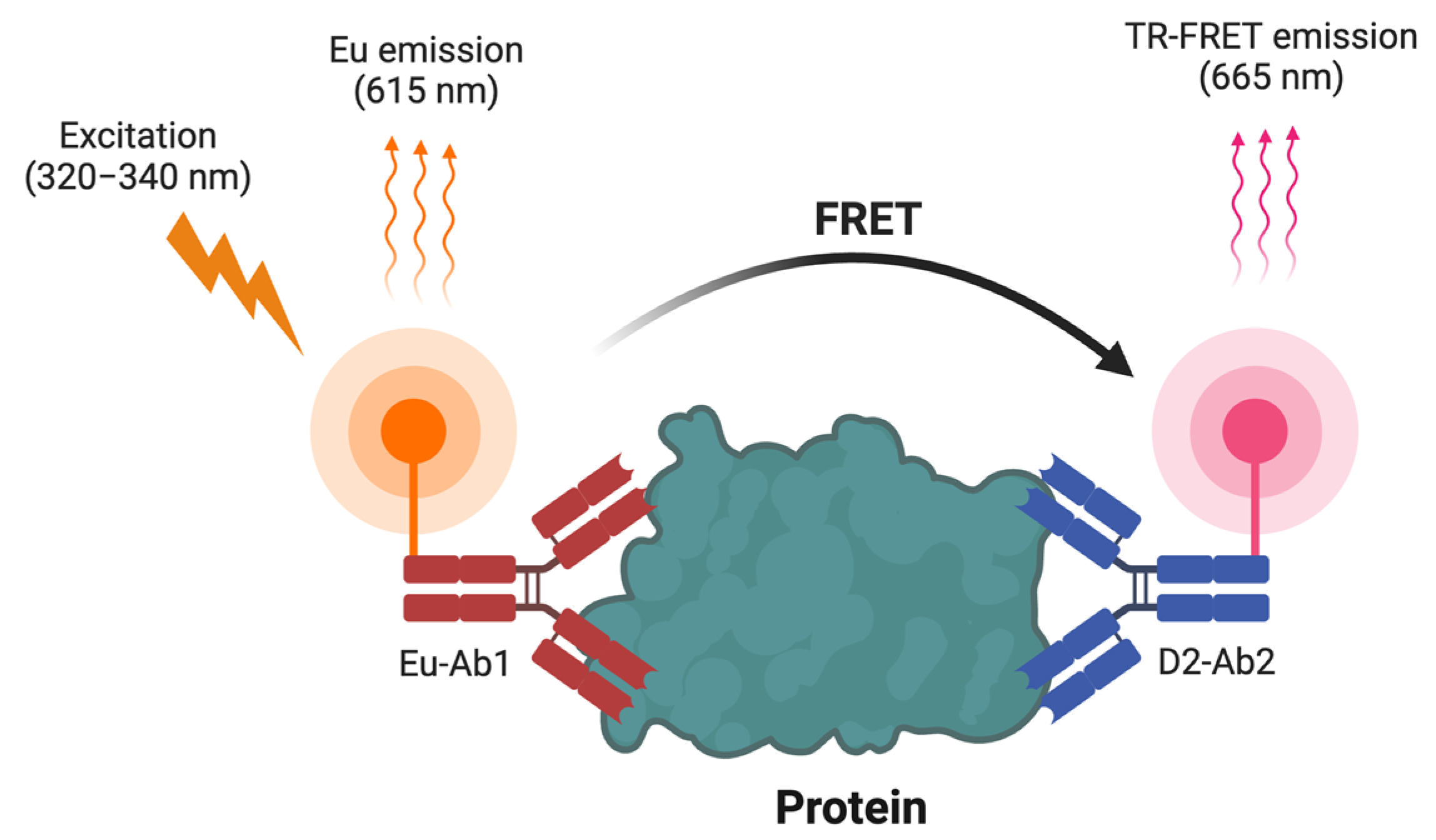
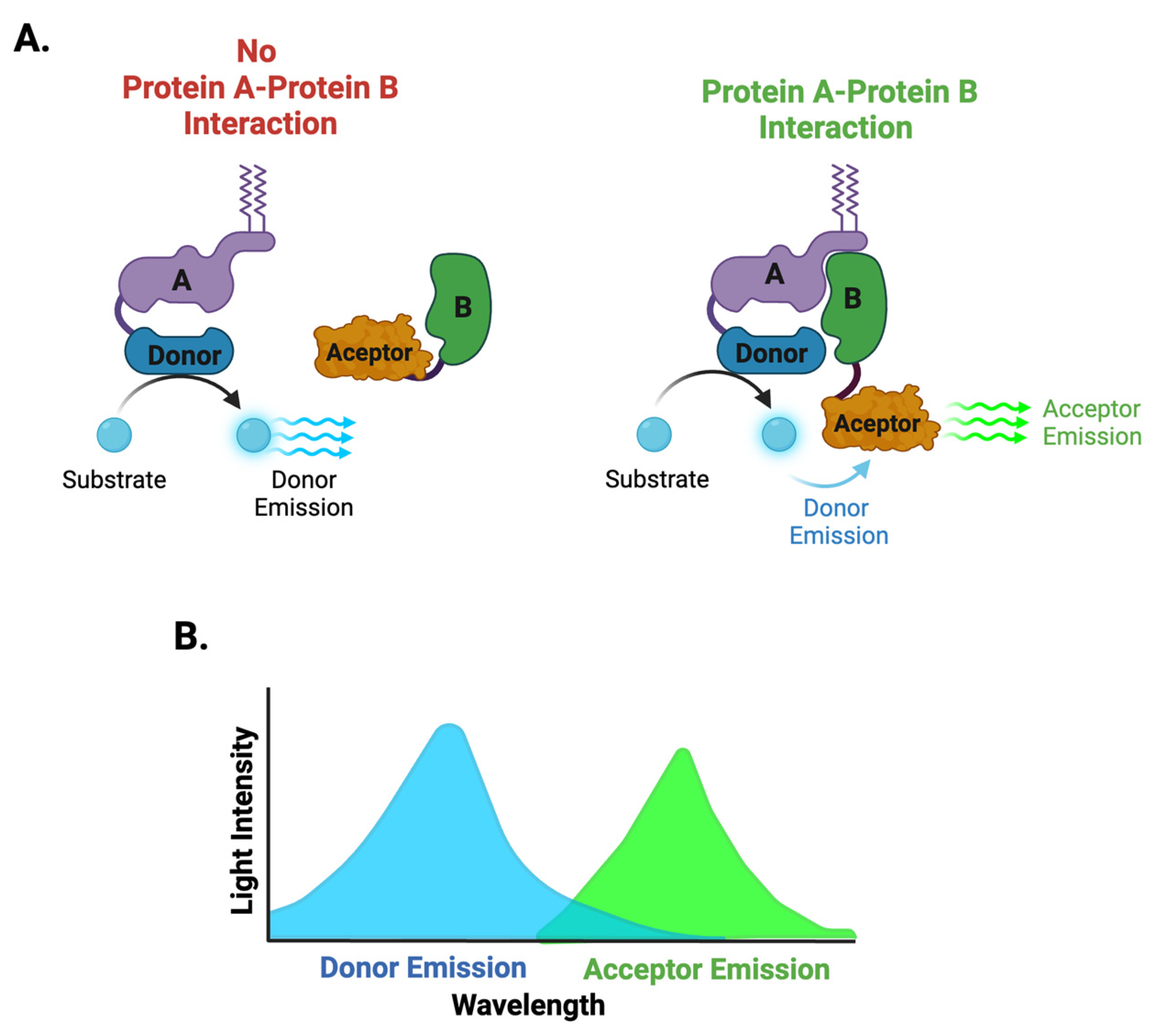
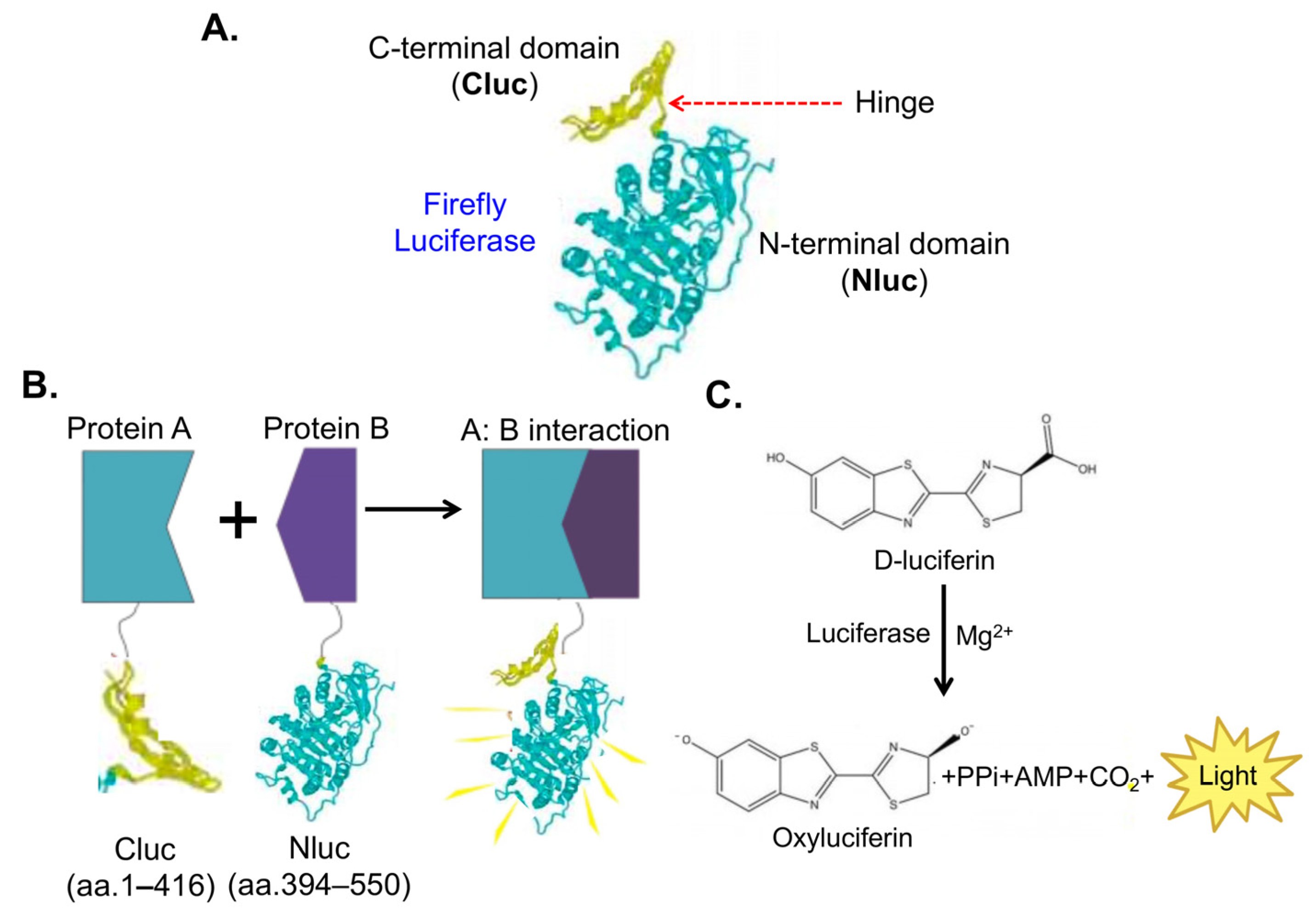
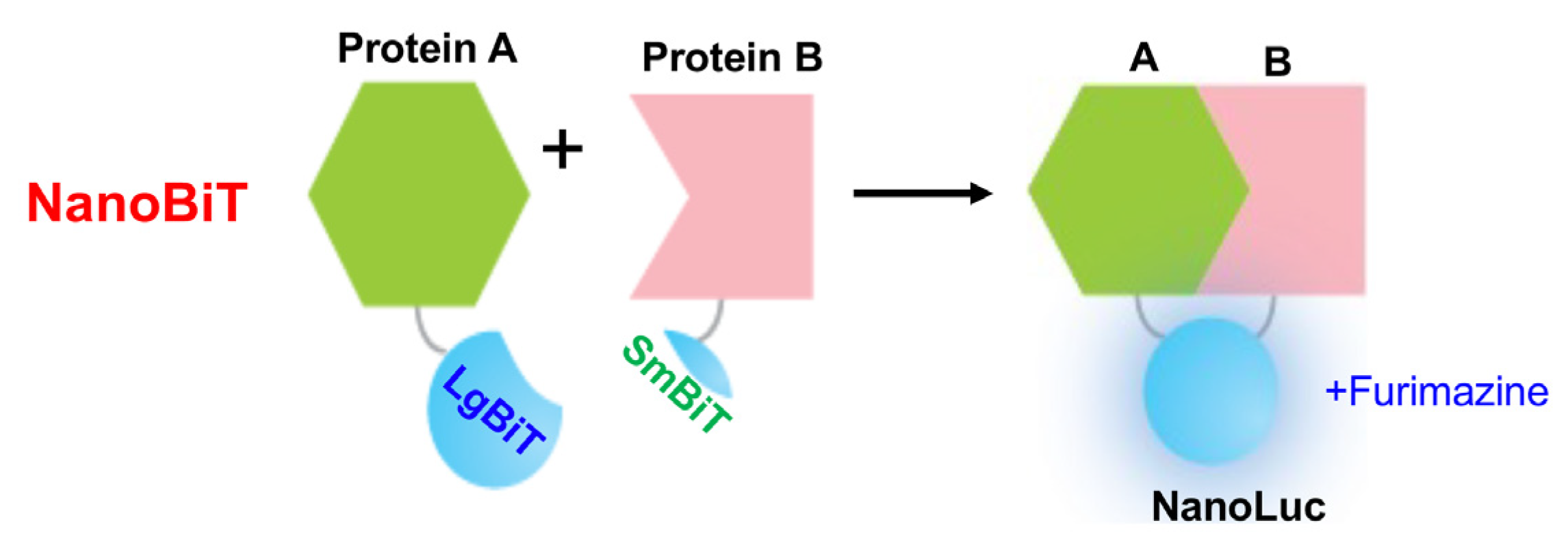
| Biosensors | Advantages | Limitations |
|---|---|---|
| FRET/TR-FRET |
|
|
| BRET/NanoBRET |
|
|
| NanoBiT |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, T.; Yang, X. Application of Fluorescence- and Bioluminescence-Based Biosensors in Cancer Drug Discovery. Biosensors 2024, 14, 570. https://doi.org/10.3390/bios14120570
Kelly T, Yang X. Application of Fluorescence- and Bioluminescence-Based Biosensors in Cancer Drug Discovery. Biosensors. 2024; 14(12):570. https://doi.org/10.3390/bios14120570
Chicago/Turabian StyleKelly, Tynan, and Xiaolong Yang. 2024. "Application of Fluorescence- and Bioluminescence-Based Biosensors in Cancer Drug Discovery" Biosensors 14, no. 12: 570. https://doi.org/10.3390/bios14120570
APA StyleKelly, T., & Yang, X. (2024). Application of Fluorescence- and Bioluminescence-Based Biosensors in Cancer Drug Discovery. Biosensors, 14(12), 570. https://doi.org/10.3390/bios14120570






