Preliminary Investigation of a Potential Optical Biosensor Using the Diamond™ Nucleic Acid Dye Applied to DNA and Friction Ridge Analysis from Fingerprint Traces
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Preparation of the Solutions
2.2. Methods
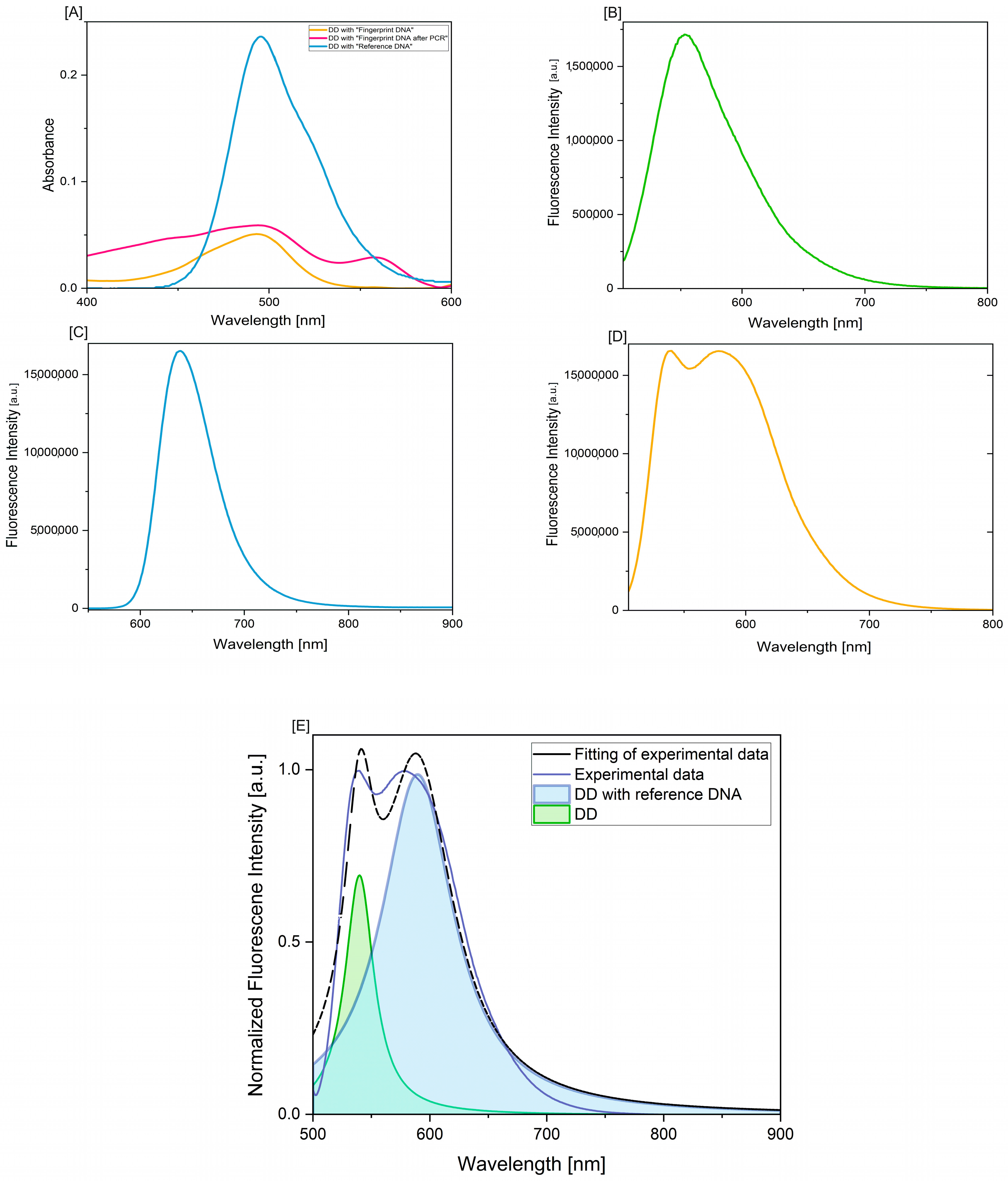
2.2.1. UV-VIS/Fluorescence Spectral Measurements
2.2.2. Fingerprint Collection/Observation
2.2.3. Direct PCR
2.2.4. Research Permission and Ethics Declarations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, S.L. Forensic science identification evidence: Tensions between law and science. J. Philos. Sci. Law 2016, 16, 1–35. [Google Scholar] [CrossRef]
- Shelton, D.E. Juror expectations for scientific evidence in criminal cases: Perceptions and reality about the CSI effect myth. TM Cool. L. Rev. 2010, 27, 1. [Google Scholar]
- Allen, R.; Sankar, P.; Prabhakar, S. Fingerprint identification technology. In Biometric Systems: Technology, Design and Performance Evaluation; Springer: London, UK, 2005; pp. 22–61. [Google Scholar] [CrossRef]
- McCormack, H.B.M. 3. Scientific evidence. In Science Bench Book for Judges; The National Judicial College: Reno, NV, USA; Justice Speakers Institute, LLC: Northville, MI, USA, 2019; pp. 51–52. Available online: https://supremecourt.nmcourts.gov/wp-content/uploads/sites/32/2023/11/Science-Bench-Book-for-Judges_2019.pdf (accessed on 28 October 2024).
- Maltoni, D.; Maio, D.; Jain, A.K.; Prabhakar, S. Handbook of Fingerprint Recognition; Springer: London, UK, 2009; Volume 2. [Google Scholar]
- Sun, Z.; Paulino, A.A.; Feng, J.; Chai, Z.; Tan, T.; Jain, A.K. A study of multibiometric traits of identical twins. In Biometric Technology for Human Identification VII; SPIE: Bellingham, WA, USA, 2010; Volume 7667, pp. 283–294. [Google Scholar] [CrossRef]
- Lennard, C. The detection and enhancement of latent fingerprints. In Proceedings of the 13th INTERPOL Forensic Science Symposium, Lyon, France, 16–19 October 2001; US Department of Justice: Washington, DC, USA, 2001; p. D2-88. [Google Scholar]
- Jain, A.K.; Feng, J. Latent Fingerprint Matching. IEEE Trans. Pattern Anal. Mach. Intell. 2010, 33, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Sewell, J.; Quinones, I.; Ames, C.; Multaney, B.; Curtis, S.; Seeboruth, H.; Moore, S.; Daniel, B. Recovery of DNA and fingerprints from touched documents. Forensic Sci. Int. Genet. 2008, 2, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Balogh, M.; Burger, J.; Bender, K.; Schneider, P.M.; Alt, K.W. STR genotyping and mtDNA sequencing of latent fingerprint on paper. Forensic Sci. Int. 2003, 137, 188–195. [Google Scholar] [CrossRef]
- Datta, A.K.; Lee, H.C.; Ramotowski, R.; Gaensslen, R.E. Advances in fingerprint technology. In History and Development of Fingerprinting; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Raymond, J.J.; Du Pasquier, E. The effect of common fingerprint detection techniques on the DNA typing of fingerprints deposited on different. J. Forensic Identif. 2004, 54, V23. [Google Scholar]
- Pokupcic, K. Blood as an important tool in criminal investigation. J. Forensic Sci. Crim. Investig. 2017, 3, 1–3. [Google Scholar] [CrossRef]
- Hochmeister, M.; Budowle, B.; Borer, U.; Eggmann, U.; Comey, C.; Dirnhofer, R. Typing of deoxyribonucleic acid (DNA) extracted from compact bone from human remains. J. Forensic Sci. 1991, 36, 1649–1661. [Google Scholar] [CrossRef]
- Higuchi, R.; von Beroldingen, C.H.; Sensabaugh, G.F.; Erlich, H.A. DNA typing from single hairs. Nature 1988, 332, 543–546. [Google Scholar] [CrossRef]
- Higuchi, R.; Krummel, B.; Saiki, R. A general method of in vitro preparation and specific mutagenesis of DNA fragments: Study of protein and DNA interactions. Nucleic Acids Res. 1988, 16, 7351–7367. [Google Scholar] [CrossRef]
- van Oorschot, R.; Gutowski, S.; Robinson, S.; Hedley, J.; Andrew, I. HUMTH01 validation studies: Effect of substrate, environment, and mixtures. J. Forensic Sci. 1996, 41, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Hagelberg, E.; Gray, I.C.; Jeffreys, A.J. Identification of the skeletal remains of a murder victim by DNA analysis. Nature 1991, 352, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, B.; Rand, S.; Bajanowski, T. Forensic identification of urine samples. Int. J. Leg. Med. 1992, 105, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Van Oorschot, R.A.; Jones, M.K. DNA fingerprints from fingerprints. Nature 1997, 387, 767. [Google Scholar] [CrossRef] [PubMed]
- Subhani, Z.; Daniel, B.; Frascione, N. DNA Profiles from fingerprint lifts—Enhancing the evidential value of fingermarks through successful DNA typing. J. Forensic Sci. 2019, 64, 201–206. [Google Scholar] [CrossRef]
- Fieldhouse, S.; Parsons, R.; Bleay, S.; Walton-Williams, L. The effect of DNA recovery on the subsequent quality of latent fingermarks: A pseudo-operational trial. Forensic Sci. Int. 2020, 307, 110076. [Google Scholar] [CrossRef]
- Buś, M.M.; Allen, M. Collecting and preserving biological samples from challenging environments for DNA analysis. Biopreservation Biobank. 2014, 12, 17–22. [Google Scholar] [CrossRef]
- Alessandrini, F.; Cecati, M.; Pesaresi, M.; Turchi, C.; Carle, F.; Tagliabracci, A. Fingerprints as evidence for a genetic profile: Morphological study on fingerprints and analysis of exogenous and individual factors affecting DNA typing. J. Forensic Sci. 2003, 48, 586–592. [Google Scholar] [CrossRef]
- DiamondTM Nucleic Acid Dye Is a Safe and Economical Alternative to Ethidium Bromide. Promega.com. Available online: https://www.promega.com/resources/pubhub/diamond-nucleic-acid-dye-is-a-safe-and-economical-alternative-to-ethidium-bromide/ (accessed on 28 October 2024).
- Kanokwongnuwut, P.; Kirkbride, P.; Linacre, A. Visualising latent DNA on swabs. Forensic Sci. Int. 2018, 291, 115–123. [Google Scholar] [CrossRef]
- Aljumaili, T.; Haines, A.M. An evaluation of the RapidHIT™ ID system for hair roots stained with Diamond™ Nucleic Acid Dye. Forensic Sci. Int. Genet. 2024, 69, 103003. [Google Scholar] [CrossRef]
- Haines, A.M.; Tobe, S.S.; Kobus, H.; Linacre, A. Successful direct STR amplification of hair follicles after nuclear staining. Forensic Sci. Int. Genet. Suppl. Ser. 2015, 5, e65–e66. [Google Scholar] [CrossRef]
- Haines, A.M.; Tobe, S.S.; Linacre, A. Optimization of diamond nucleic acid dye for quantitative PCR. BioTechniques 2016, 61, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Gupta, R.; Singh, R.; Jasuja, O.P. Effects of latent fingerprint development reagents on subsequent forensic DNA typing: A review. J. Forensic Leg. Med. 2015, 32, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Davys, J.R.K. The Use of Diamond Nucleic Acid Dye to Locate Both Finger Mark and Touch DNA on Non-Porous Items Obtained from Drug Seizures. Doctoral’s Dissertation, The University of Auckland, Auckland, New Zealand, 2021. [Google Scholar]
- Haines, A.M.; Tobe, S.S.; Kobus, H.; Linacre, A. Finding DNA: Using fluorescent in situ detection. Forensic Sci. Int. Genet. Suppl. Ser. 2015, 5, e501–e502. [Google Scholar] [CrossRef]
- Bourzac, K.M.; LaVine, L.J.; Rice, M.S. Analysis of DAPI and SYBR green I as alternatives to ethidium bromide for nucleic acid staining in agarose gel electrophoresis. J. Chem. Educ. 2003, 80, 1292. [Google Scholar] [CrossRef]
- Haines, A.M.; Linacre, A. A rapid screening method using DNA binding dyes to determine whether hair follicles have sufficient DNA for successful profiling. Forensic Sci. Int. 2016, 262, 190–195. [Google Scholar] [CrossRef]
- Hughes, D.A.; Szkuta, B.; van Oorschot, R.A.; Conlan, X.A. “Technical Note:” Optimisation of Diamond™ Nucleic Acid Dye preparation, application, and visualisation, for latent DNA detection. Forensic Sci. Int. 2022, 330, 111096. [Google Scholar] [CrossRef]
- Linacre, A.; Petcharoen, P. Detection of Latent DNA Using a DNA Binding Dye. In Forensic DNA Analysis: Methods and Protocols; Springer: New York, NY, USA, 2023; pp. 359–366. [Google Scholar]
- Cook, R.; Mitchell, N.; Henry, J. Assessment of Diamond™ Nucleic Acid Dye for the identification and targeted sampling of latent DNA in operational casework. Forensic Sci. Int. Genet. 2021, 55, 102579. [Google Scholar] [CrossRef]
- Petcharoen, P.; Kirkbride, K.P.; Linacre, A. Monitoring cell loss through repetitive deposition. J. Forensic Sci. 2022, 67, 2453–2457. [Google Scholar] [CrossRef]
- Champion, J.; Kanokwongnuwut, P.; van Oorschot, R.A.H.; Taylor, D.; Linacre, A. Evaluation of a fluorescent dye to visualize touch DNA on various substrates. J. Forensic Sci. 2021, 66, 1435–1442. [Google Scholar] [CrossRef]
- Nolan, M.; Handt, O.; Linacre, A. Persistence of cellular material after exposure to water. J. Forensic Sci. 2023, 68, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Kanokwongnuwut, P.; Kirkbride, K.P.; Linacre, A. An assessment of tape-lifts. Forensic Sci. Int. Genet. 2020, 47, 102292. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Mogensen, H.; Petersen, C.; Petersen, J.; Holmer, A.; Børsting, C.; Pereira, V. Optimization of the collection and analysis of touch DNA traces. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 98–99. [Google Scholar] [CrossRef]
- Michalski, S.; Shaler, R.; Dorman, F.L. The evaluation of fatty acid ratios in latent fingermarks by gas chromatography/mass spectrometry (GC/MS) analysis. J. Forensic Sci. 2013, 58, S215–S220. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.V.; Asano, K.; Bohanon, A. Chemical characterization of fingerprints from adults and children. In Forensic Evidence Analysis and Crime Scene Investigation; SPIE: Bellingham, WA, USA, 1997; Volume 2941, pp. 89–95. [Google Scholar]
- Cadd, S.; Islam, M.; Manson, P.; Bleay, S. Fingerprint composition and aging: A literature review. Sci. Justice 2015, 55, 219–238. [Google Scholar] [CrossRef]
- Champod, C.; Lennard, C.J.; Margot, P.; Stoilovic, M. Fingerprints and Other Ridge Skin Impressions; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Hamilton, P.B. Amino-acids on Hands. Nature 1965, 205, 284–285. [Google Scholar] [CrossRef]
- van Helmond, W.; van Herwijnen, A.W.; van Riemsdijk, J.J.; van Bochove, M.A.; de Poot, C.J.; de Puit, M. Chemical profiling of fingerprints using mass spectrometry. Forensic Chem. 2019, 16, 100183. [Google Scholar] [CrossRef]
- Croxton, R.S.; Baron, M.G.; Butler, D.; Kent, T.; Sears, V.G. Variation in amino acid and lipid composition of latent fingerprints. Forensic Sci. Int. 2010, 199, 93–102. [Google Scholar] [CrossRef]
- Norlin, S.; Nilsson, M.; Heden, P.; Allen, M. Evaluation of the impact of different visualization techniques on DNA in fingerprints. J. Forensic Identif. 2013, 63, 189–204. [Google Scholar]
- Wang, M.; Li, M.; Yu, A.; Zhu, Y.; Yang, M.; Mao, C. Fluorescent nanomaterials for the development of latent fingerprints in forensic sciences. Adv. Funct. Mater. 2017, 27, 1606243. [Google Scholar] [CrossRef]
- Andersen, J.; Bramble, S. The effects of fingermark enhancement light sources on subsequent PCR-str DNA analysis of fresh bloodstains. J. Forensic Sci. 1997, 42, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Haines, A.M.; Tobe, S.S.; Kobus, H.J.; Linacre, A. Properties of nucleic acid staining dyes used in gel electrophoresis. Electrophoresis 2015, 36, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schellenberg, H.; Walhorn, V.; Toensing, K.; Anselmetti, D. Binding mechanism of fluorescent dyes to DNA characterized by magnetic tweezers. Mater. Today Proc. 2017, 4, S218–S225. [Google Scholar] [CrossRef]


| Product | Purchased From | |
|---|---|---|
| Diamond™ Nucleic Acid Dye | Promega Corporation | Madison, WI, USA |
| Deoxyribonucleic acid from herring sperm | Sigma-Aldrich | Darmstadt, Germany |
| TBE buffer | Chempur | Silesia, Poland |
| Direct Tissue PCR Kit | EURx | Gdansk, Poland |
| Sample | Integrated Area |
|---|---|
| Reference DNA | 12.90 |
| Fingerprint DNA | 8.95 |
| Fingerprint DNA after PCR | 14.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnomska, M.; Lewkowicz, A.; Gruszczyńska, E.; Walczewska-Szewc, K.; Gryczyński, Z.; Bojarski, P.; Steinborn, S. Preliminary Investigation of a Potential Optical Biosensor Using the Diamond™ Nucleic Acid Dye Applied to DNA and Friction Ridge Analysis from Fingerprint Traces. Biosensors 2024, 14, 546. https://doi.org/10.3390/bios14110546
Czarnomska M, Lewkowicz A, Gruszczyńska E, Walczewska-Szewc K, Gryczyński Z, Bojarski P, Steinborn S. Preliminary Investigation of a Potential Optical Biosensor Using the Diamond™ Nucleic Acid Dye Applied to DNA and Friction Ridge Analysis from Fingerprint Traces. Biosensors. 2024; 14(11):546. https://doi.org/10.3390/bios14110546
Chicago/Turabian StyleCzarnomska, Martyna, Aneta Lewkowicz, Emilia Gruszczyńska, Katarzyna Walczewska-Szewc, Zygmunt Gryczyński, Piotr Bojarski, and Sławomir Steinborn. 2024. "Preliminary Investigation of a Potential Optical Biosensor Using the Diamond™ Nucleic Acid Dye Applied to DNA and Friction Ridge Analysis from Fingerprint Traces" Biosensors 14, no. 11: 546. https://doi.org/10.3390/bios14110546
APA StyleCzarnomska, M., Lewkowicz, A., Gruszczyńska, E., Walczewska-Szewc, K., Gryczyński, Z., Bojarski, P., & Steinborn, S. (2024). Preliminary Investigation of a Potential Optical Biosensor Using the Diamond™ Nucleic Acid Dye Applied to DNA and Friction Ridge Analysis from Fingerprint Traces. Biosensors, 14(11), 546. https://doi.org/10.3390/bios14110546







