ZnS and Reduced Graphene Oxide Nanocomposite-Based Non-Enzymatic Biosensor for the Photoelectrochemical Detection of Uric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the ZnS/RGO and Related ZnS/RGO/ITO Electrodes
2.3. Characterization of the ZnS/RGO Nanocomposites
2.4. Photoelectrochemical Test of the ZnS/RGO/ITO Working Electrodes and the Detection of Uric Acid
3. Results and Discussion
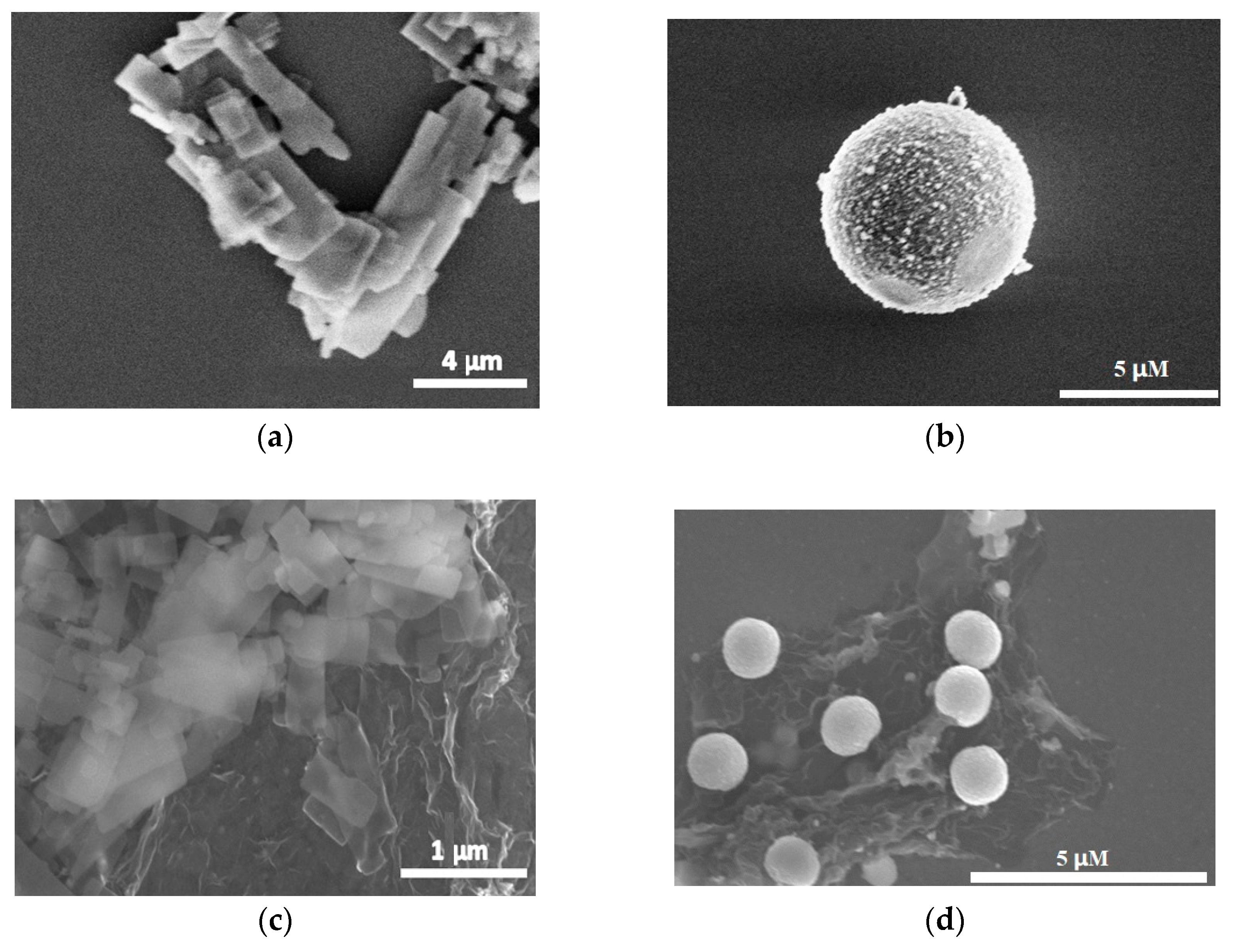
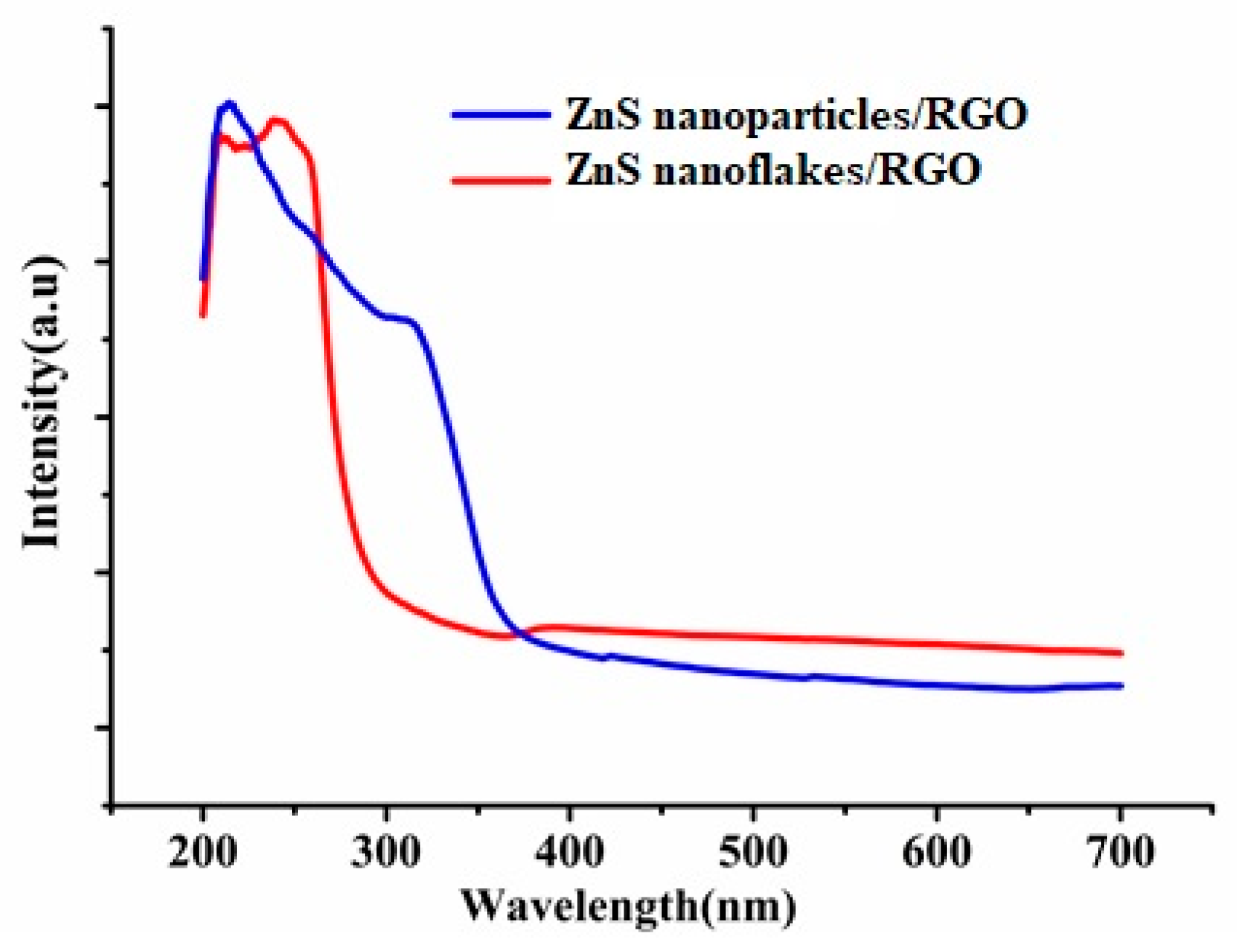
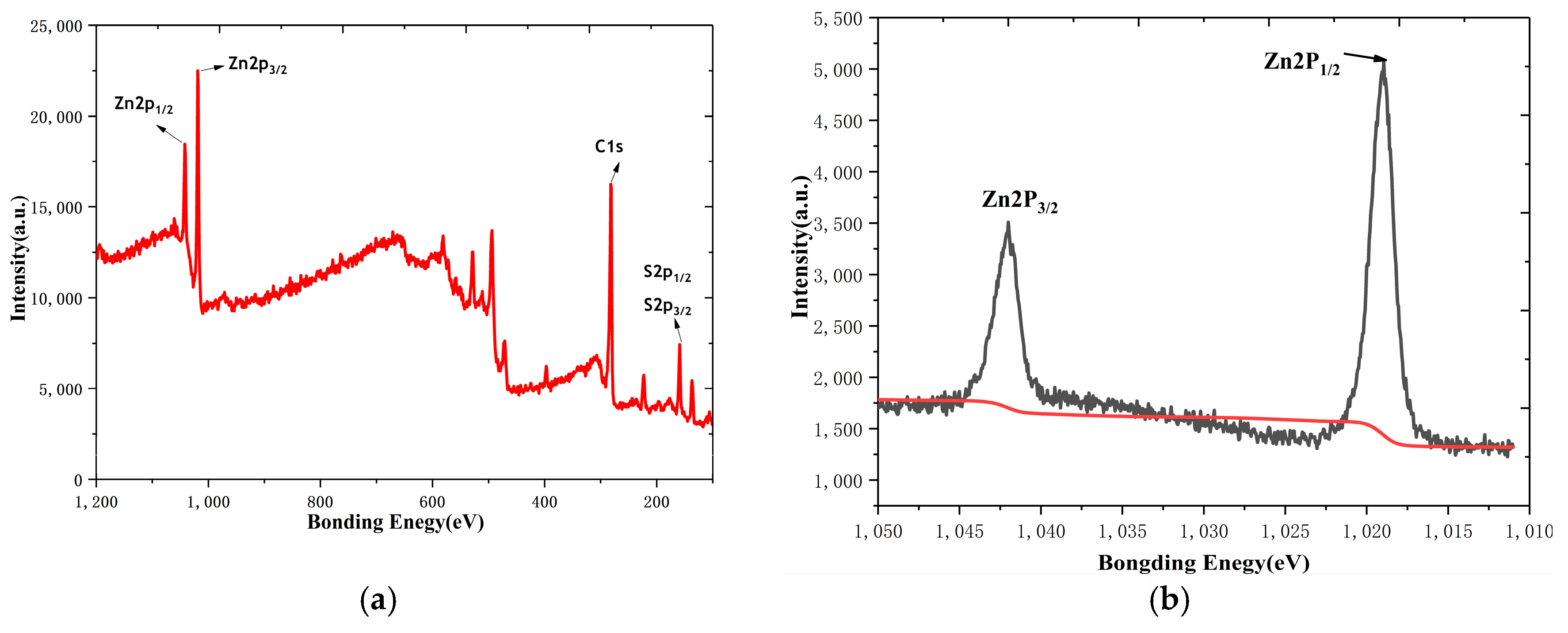
3.1. Characterization of ZnS/RGO Nanocomposite Electrode
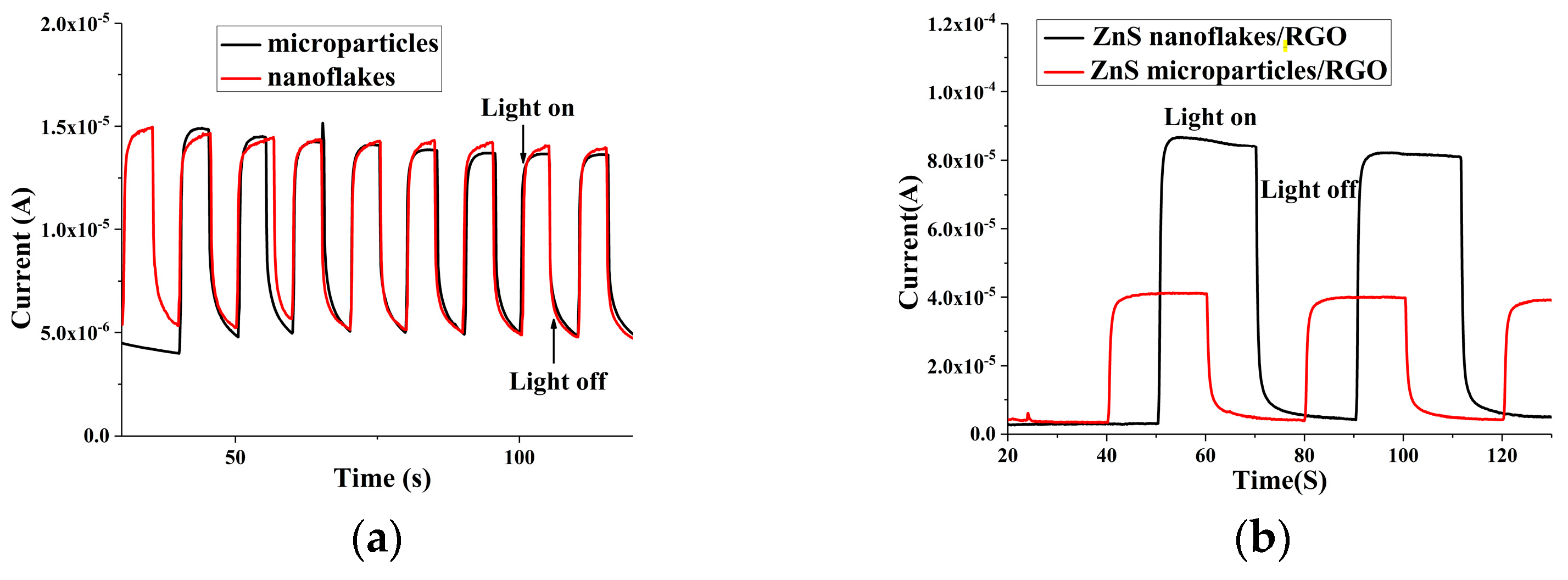
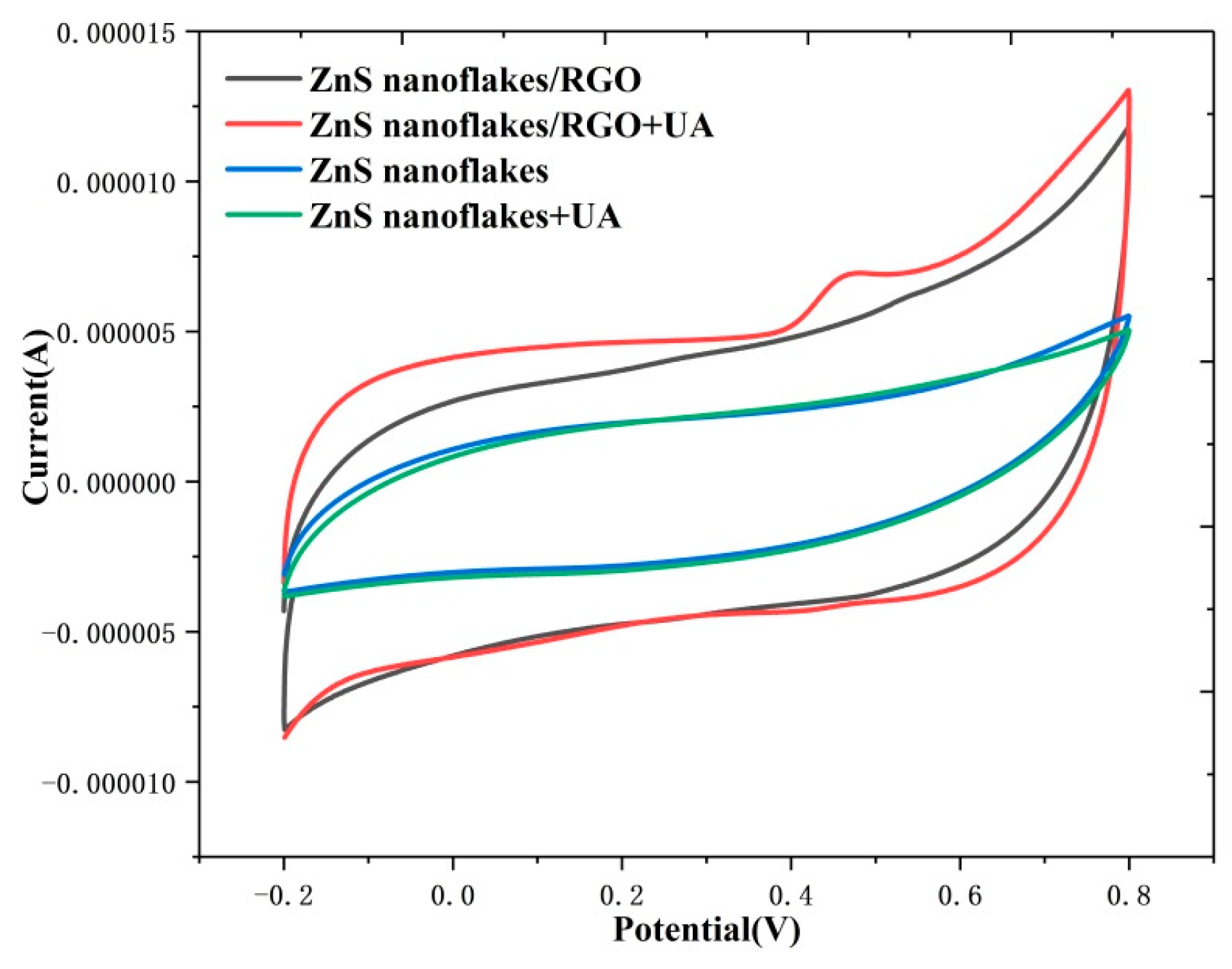
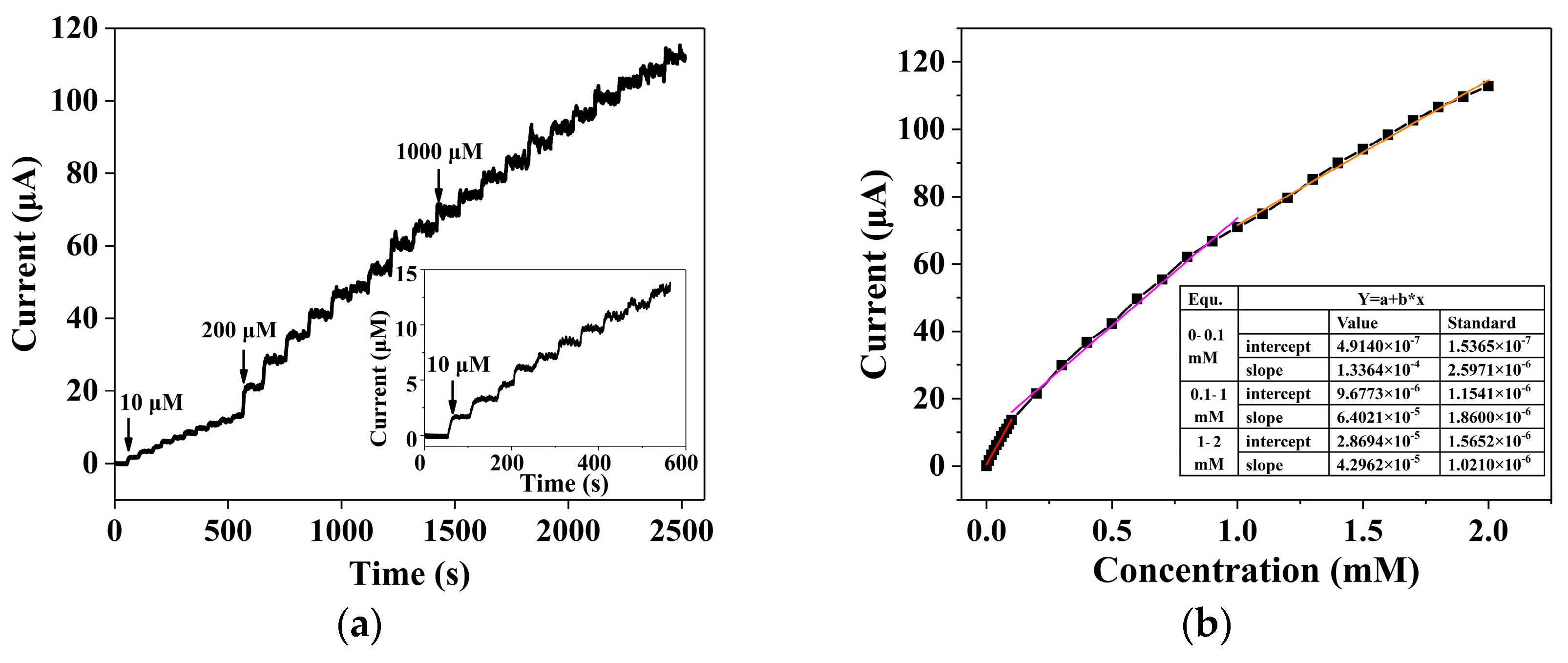
3.2. Electrochemical Properties and Photoelecrochemical Detection of Uric Acid by the ZnS Nanoflake/RGO Electrode
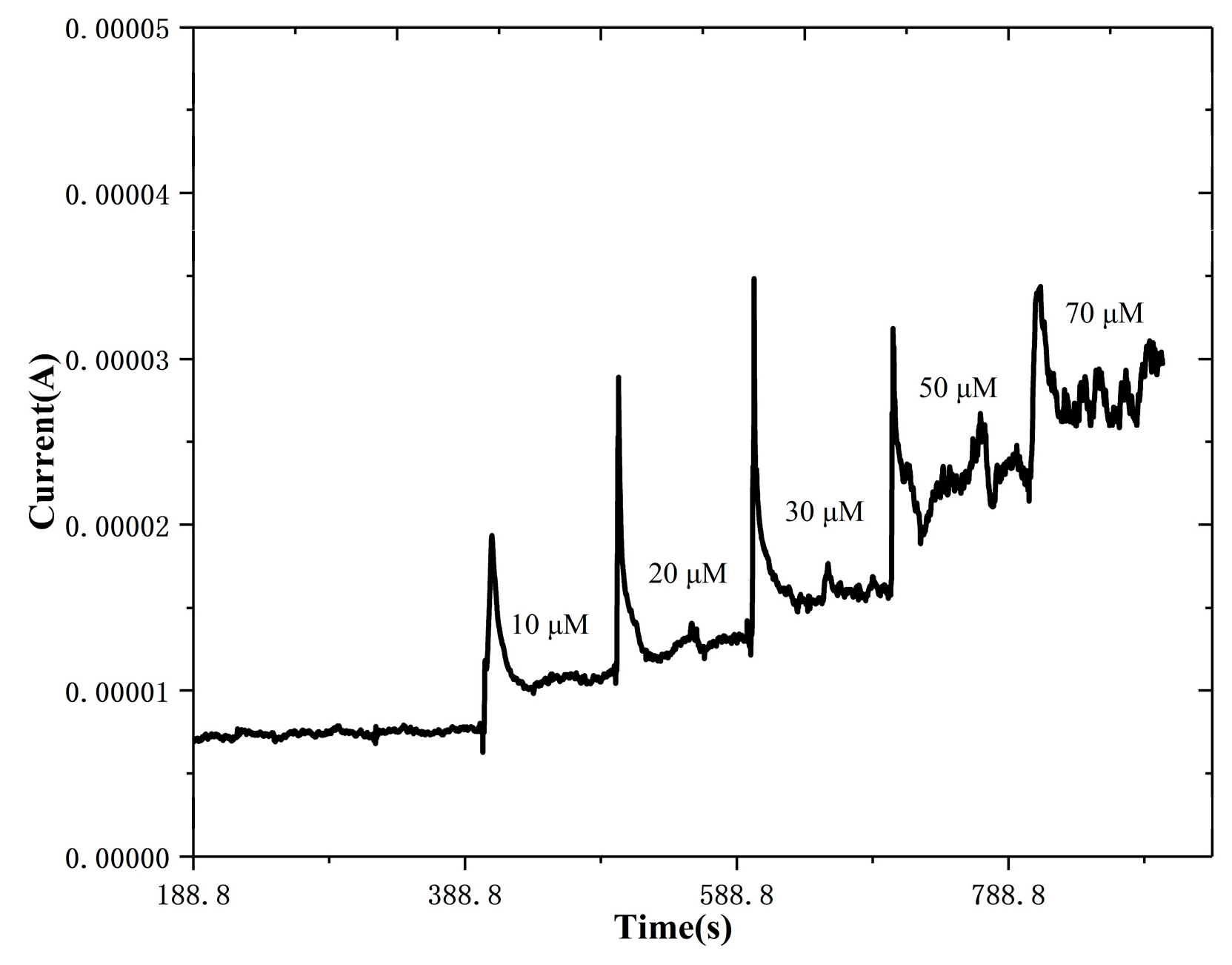
3.3. Anti-Interference Capability, Long-Term Stability and UA Detection in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alderman, M.H.; Cohen, H.; Madhavan, S.; Kivlighn, S. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension 1999, 34, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Akyilmaz, E.; Sezgintürk, M.K.; Dinçkaya, E. A biosensor based on urate oxidase–peroxidase coupled enzyme system for uric acid determination in urine. Talanta 2003, 61, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Shen, L.-Y.; Xu, H.; Wang, Z.-Y.; Yang, X.-J.; Huang, Y.-L.; Redshaw, C.; Zhang, Q.-L. A 1-Hydroxy-2,4-Diformylnaphthalene-Based Fluorescent Probe and Its Detection of Sulfites/Bisulfite. Molecules 2021, 26, 3064. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, R.; Biswas, S. A Pyrene-Functionalized Metal–Organic Framework for Nonenzymatic and Ratiometric Detection of Uric Acid in Biological Fluid via Conformational Change. Inorg. Chem. 2019, 58, 5654–5663. [Google Scholar] [CrossRef]
- Geng, L.-Y.; Zhao, Y.; Kamya, E.; Guo, J.-T.; Sun, B.; Feng, Y.-K.; Zhu, M.-F.; Ren, X.-K. Turn-off/on fluorescent sensors for Cu2+ and ATP in aqueous solution based on a tetraphenylethylene derivative. Mater. Chem. C 2019, 7, 2640–2645. [Google Scholar] [CrossRef]
- Huang, L.-X.; Guo, Q.; Chen, Y.; Verwilst, P.; Son, S.; Wu, J.-B.; Cao, Q.-Y.; Kim, J.S. Nanomolar detection of adenosine triphosphate (ATP) using a nanostructured fluorescent chemosensing ensemble. Chem. Commun. 2019, 55, 14135–14138. [Google Scholar] [CrossRef]
- Lobas, M.A.; Tao, R.; Nagai, J.; Kronschläger, M.T.; Borden, P.M.; Marvin, J.S.; Looger, L.L.; Khakh, B.S. A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP. Nat. Commun. 2019, 10, 711. [Google Scholar] [CrossRef]
- Sun, Z.; Liang, P. Determination of Cr(III) and total chromium in water samples by cloud point extraction and flame atomic absorption spectrometry. Microchim. Acta 2008, 162, 121–125. [Google Scholar] [CrossRef]
- Dey, N.; Bhattacharya, S. Nanomolar level detection of uric acid in blood serum and pest-infested grain samples by an amphiphilic probe. Anal. Chem. 2017, 89, 10376–10383. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, M.; Zhao, J.; Han, C.; Liu, X.; Xiao, Z.; Zhang, L.; Xu, B.; Guo, W.; Wang, R. Fluorescence turn-on detection of uric acid by a water-stable metal–organic nanotube with high selectivity and sensitivity. Mater. Chem. C 2017, 5, 601–606. [Google Scholar] [CrossRef]
- Gavrilenko, N.A.; Volgina, T.N.; Pugachev, E.V.; Gavrilenko, M.A. Visual determination of malachite green in sea fish samples. Food Chem. 2019, 274, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Westley, C.; Xu, Y.; Thilaganathan, B.; Carnell, A.J.; Turner, N.J.; Goodacre, R. Absolute quantification of uric acid in human urine using surface enhanced Raman scattering with the standard addition method. Anal. Chem. 2017, 89, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.-C.; Kuo, J.-S.; Chang, W.-H.; Juang, D.-J.; Shih, Y.; Lai, J.-S. Rapid and reliable high-performance liquid chromatographic method for analysing human plasma serotonin, 5-hydroxyindoleacetic acid, homovanillic acid and 3,4-dihydroxyphenylacetic acid. Chromatogr. B 1993, 617, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Fang, X.; Zhang, C.; Xu, R.; Xu, B. Determination of serum uric acid using high-performance liquid chromatography (HPLC)/isotope dilution mass spectrometry (ID-MS) as a candidate reference method. Chromatogr. B 2007, 857, 287–295. [Google Scholar] [CrossRef]
- Zhou, S.; Zuo, R.; Zhu, Z.; Wu, D.; Vasa, K.; Deng, Y.; Zuo, Y. An eco-friendly hydrophilic interaction HPLC method for the determination of renal function biomarkers, creatinine and uric acid, in human fluids. Anal. Methods 2013, 5, 1307–1311. [Google Scholar] [CrossRef]
- Guo, J. Uric acid monitoring with a smartphone as the electrochemical analyzer. Anal. Chem. 2016, 88, 11986–11989. [Google Scholar] [CrossRef]
- Su, C.-H.; Sun, C.-L.; Liao, Y.-C. Printed combinatorial sensors for simultaneous detection of ascorbic acid, uric acid, dopamine, and nitrite. ACS Omega 2017, 2, 4245–4252. [Google Scholar] [CrossRef]
- Cai, X.; Hu, A.; Feng, F. Synthesis of a sulfonated methylene blue-backboned polymer for biodetections. Dyes Pigm. 2022, 203, 110360. [Google Scholar] [CrossRef]
- Samoson, K.; Soleh, A.; Saisahas, K.; Promsuwan, K.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Chang, K.H.; Abdullah, A.F.L.; Tayayuth, K.; et al. Facile fabrication of a flexible laser induced gold nanoparticle/chitosan/porous graphene electrode for uric acid detection. Talanta 2022, 243, 123319. [Google Scholar] [CrossRef]
- Choi, D.Y.; Yang, J.C.; Hong, S.W.; Park, J. Molecularly imprinted polymer-based electrochemical impedimetric sensors on screen-printed carbon electrodes for the detection of trace cytokine IL-1β. Biosens. Bioelectron. 2022, 204, 114073. [Google Scholar] [CrossRef]
- Park, R.; Jeon, S.; Jeong, J.; Park, S.Y.; Han, D.W.; Hong, S.W. Recent advances of point-of-care devices integrated with molecularly imprinted polymers-based biosensors: From biomolecule sensing design to intraoral fluid testing. Biosensors 2022, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Ashraf, N.; Zohuri, G.H.; Arbab-Zavar, M.H. Water-compatible synthesis of core-shell polysilicate molecularly imprinted polymer on polyvinylpyrrolidone capped gold nanoparticles for electrochemical sensing of uric acid. Microchem. J. 2022, 177, 107312. [Google Scholar] [CrossRef]
- Hasseb, A.A.; Shehab, O.R.; El Nashar, R.M. Application of molecularly imprinted polymers for electrochemical detection of some important biomedical markers and pathogens. Curr. Opin. Electrochem. 2022, 31, 100848. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Lin, J.-H.; Hsu, D.-X.; Wang, S.-H.; Lin, S.-Y.; Hsueh, T.-J. Enhanced non-enzymatic glucose biosensor of ZnO nanowires via decorated Pt nanoparticles and illuminated with UV/green light emitting diodes. Sens. Actuators B Chem. 2017, 238, 150–159. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, N.; Wei, X.; Jiang, Z.; Kuo, W.C.H. Synthesis of ZnS urchin-like nanostructures for electrochemical determination of uric acid. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar]
- Fareza, A.R.; Nugroho FA, A.; Fauzia, V. One-Step Coating of a ZnS Nanoparticle/MoS2 Nanosheet Composite on Supported ZnO Nanorods as Anodes for Photoelectrochemical Water Splitting. ACS Appl. Nano Mater. 2022, 5, 16051–16060. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Y.; Tang, Y.; Gao, F.; Jing, W.; Du, Y.; Han, F. A novel flexible non-enzymatic electrochemical glucose sensor of excellent performance with ZnO nanorods modified on stainless steel wire sieve and stimulated via UV irradiation. Ceram. Int. 2022, 48, 14395–14405. [Google Scholar] [CrossRef]
- Han, Z.; Li, G.C.C.; Yu, Y.; Zhou, Y. Preparation of 1D cubic Cd0.8Zn0.2S solid-solution nanowires using levelling effect of TGA and improved photocatalytic H2-production activity. Mater. Chem. A 2015, 3, 1696–1702. [Google Scholar] [CrossRef]
- Du, J.; Yu, X.; Wu, Y.; Di, J. ZnS nanoparticels electrodeposited onto ITO electrode as a platform for fabrication of enzyme-based biosensors of glucose. Mater. Sci. Eng. C 2013, 33, 2031–2036. [Google Scholar] [CrossRef]
- Ertek, B.; Akgul, C.; Dilgin, Y. Photoelectrochemical glucose biosensor based on a dehydrogenase enzyme and NAD+/NADH redox couple using a quantum dot modified pencil graphite electrode. RSC Adv. 2016, 6, 20058–20066. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, R.; Zhang, C.; Chen, J.; Zheng, K.; Wang, C.; Zhang, Z.; Lin, S. Rational design an oval heterostructure and tight linking interface of ZnS@CdS(3) for sensitively photoelectrochemical bioanalysis of PSA. J. Alloys Compd. 2024, 972, 172797. [Google Scholar] [CrossRef]
- Van Bui, H.; Van Thai, D.; Dai Nguyen, T.; Tran, H.T.; Nui, N.D.; Hung, N.M. Mn-doped ZnS nanoparticle photoanodes: Synthesis, structural, optical, and photoelectrochemical characteristics. Mater. Chem. Phys. 2023, 307, 128081. [Google Scholar]
- Tang, X.; Wang, Y.; Liu, Z.; Fei, M.; Gao, R.; Xie, Y.; Hu, X.; Zhao, P.; Fei, J. ZnS-assembled CdIn2S4 Z-type heterojunction as a photoelectrochemical sensor for ultra-trace detection of m-nitrophenol in environmental water samples. Sens. Actuators B-Chem. 2024, 400, 34921. [Google Scholar] [CrossRef]
- Yang, J.; Duan, X.; Guo, W.; Li, D.; Zhang, H.; Zheng, W. Electrochemical performances investigation of NiS/rGO composite as electrode material for supercapacitors. Nano Energy 2014, 5, 74–81. [Google Scholar] [CrossRef]
- Mei, L.; Xu, C.; Yang, T.; Ma, J.; Chen, L.; Li, Q.; Wang, T. Superior electrochemical performance of ultrasmall SnS2 nanocrystals decorated on flexible RGO in lithium-ion batteries. J. Mater. Chem. A 2013, 1, 8658–8664. [Google Scholar] [CrossRef]
- Yin, L.; Chen, D.; Cui, X.; Ge, L.; Yang, J.; Yu, L.; Zhang, B.; Zhang, R.; Shao, G. Normal-pressure microwave rapid synthesis of hierarchical SnO2@rGO nanostructures with superhigh surface areas as high-quality gas-sensing and electrochemical active materials. Nanoscale 2014, 6, 13690–13700. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chang, C.-K.; Tsai, Y.-C.; Liao, C.-S. Electrochemically synthesized graphene/polypyrrole composites and their use in supercapacitor. Carbon 2012, 50, 2331–2336. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Palanisamy, S.; Chen, S.-M. A simple electrochemical approach to fabricate a glucose biosensor based on graphene–glucose oxidase biocomposite. Biosens. Bioelectron. 2013, 39, 70–75. [Google Scholar] [CrossRef]
- Sun, Z.; Fu, H.; Deng, L.; Wang, J. Redox-active thionine–graphene oxide hybrid nanosheet: One-pot, rapid synthesis, and application as a sensing platform for uric acid. Anal. Chim. Acta 2013, 761, 84–91. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, X.; Peng, N.; Wang, J.; Jiang, Z. Study of ZnS Nanostructures Based Electrochemical and Photoelectrochemical Biosensors for Uric Acid Detection. Sensors 2017, 17, 1235. [Google Scholar] [CrossRef]
- Yue, G.H.; Yan, P.X.; Yan, D.; Liu, J.Z.; Qu, D.M.; Yang, Q.; Fan, X.Y. Synthesis of two-dimensional micron-sized single-crystalline ZnS thin nanosheets and their photoluminescence properties. J. Cryst. Growth 2006, 293, 428–432. [Google Scholar] [CrossRef]
- Miao, F.; Guan, X.; Tao, B.; Zang, Y. Pd/ZnS/ZnO sensitive selective detection photoelectrochemical sensor for the detection of Cd2+. Vacuum 2023, 211, 111972. [Google Scholar] [CrossRef]
- Wang, C.; Du, J.; Wang, H.; Zou, C.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Safavi, A.; Maleki, N.; Moradlou, O.; Tajabadi, F. Simultaneous determination of dopamine, ascorbic acid, and uric acid using carbon ionic liquid electrode. Anal. Biochem. 2006, 359, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zheng, Y.; Wang, A.; Cai, W.; Deng, B.; Zhang, Z. An Electrochemical Sensor Based on Reduced Graphene Oxide and ZnO Nanorods-Modified Glassy Carbon Electrode for Uric Acid Detection. Arab. J. Sci. Eng. 2016, 41, 135–141. [Google Scholar] [CrossRef]
- Ghanbari, K.H.; Hajian, A. Electrochemical characterization of Au/ZnO/PPy/RGO nanocomposite and its application for simultaneous determination of ascorbic acid, epinephrine, and uric acid. J. Electroanal. Chem. 2017, 801, 466–479. [Google Scholar] [CrossRef]
- Quan, C.Y.; Yang, M.H.; Hou, Y. Electrochemical sensor using cobalt oxide-modified porous carbon for uric acid determination. Microchim. Acta 2023, 190, 401. [Google Scholar] [CrossRef]
- Putra, B.R.; Nisa, U.; Heryanto, R.; Khalil, M.; Khoerunnisa, F.; Ridhova, A.; Thaha, Y.N.; Marken, F.; Wahyuni, W.T. Selective non-enzymatic uric acid sensing in the presence of dopamine: Electropolymerized poly-pyrrole modified with a reduced graphene oxide/PEDOT:PSS composite. Analyst 2022, 147, 5334–5346. [Google Scholar] [CrossRef]
- Yue, H.Y.; Wu, P.F.; Huang, S.; Gao, X.; Wang, Z.; Wang, W.Q.; Zhang, H.J.; Song, S.S. Electrochemical determination of levodopa in the presence of uric acid using ZnO nanoflowers-reduced graphene oxide. J. Mater. Sci.-Mater. Electron. 2019, 30, 3984–3993. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, J.; Tang, Y.; Song, X.; Zhou, W.; Li, Y.; Gao, F. Enhanced sensing performance of flexible non-enzymatic electrochemical glucose sensors using hollow Fe3O4 nanospheres of controllable morphologies. Ceram. Int. 2024, 50, 38009–38021. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Zhang, S.; Huang, Y.; Wei, Y.; Cai, H.; Jia, Z.; Su, X. Au-wrapped CuS-Interlaced Chain Structure for Ultrafast Response and High Sensitive Non-Enzymatic Glucose Sensor. J. Electrochem. Soc. 2023, 170, 057509. [Google Scholar] [CrossRef]
- Zhu, S.; Li, H.; Niu, W.; Xu, G. Simultaneous electrochemical determination of uric acid, dopamine, and ascorbic acid at single-walled carbon nanohorn modified glassy carbon electrode. Biosens. Bioelectron. 2009, 25, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Song, H.; Zhang, X.; Cheng, X.; Xu, Y.; Zhao, H.; Gao, S.; Huo, L. Enhanced non-enzyme nitrite electrochemical sensing property based on stir bar-shaped ZnO nanorods decorated with nitrogen-doped reduced graphene oxide. Sens. Actuators B-Chem. 2022, 355, 131313. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, J.; Tang, Y.; Liu, S.; Du, Y.; Jing, W. Investigation on the surface morphologies of reduced graphene oxide coating on the interfacial characteristics and electro-catalytic capacity of enzymatic glucose sensors. Nanotechnology 2023, 34, 015501. [Google Scholar] [CrossRef] [PubMed]







| Materials | Method | Sensitivity | LOD | Linear Range | Reference |
|---|---|---|---|---|---|
| Nafion/uricase/ZnS nanoparticles/ITO | Enzymatic electrochemistry | 43.18 | 1.79 | 0.01–1.5 | [40] |
| Nafion/uricase/ZnS urchin-like nanostructures/ITO | Enzymatic electrochemistry | 76.12 | 0.70 | 0.01–1.7 | [40] |
| Nafion/uricase/ZnS nanoflakes/ITO | Enzymatic electrochemistry | 34.28 | 1.51 | 0.01–2.0 | [40] |
| Nafion/uricase/ZnO–RGO/ITO | Enzymatic photoelectrochemistry | 427.8 | 0.039 | 0.01–0.2 | [45] |
| Nafon/uricase/ZnO–RGO-Au/ITO | Enzymatic electrochemistry | 768.6 | 0.29 | 0.2–2 | [46] |
| RGO/PEDOT:PSS/GCE | Non-enzymatic electrochemistry | 0.05 | 0.01–0.1 | [47] | |
| Co-N/C@MWCNTs | Non-enzymatic electrochemistry | 0.09 | 0.001–0.04 | [48] | |
| ZnO film/RGO/ITO | Non-enzymatic electrochemistry | 150.7 | 0.45 | 1–40 | [49] |
| ZnS nanoflakes–RGO/ITO | Non-enzymatic photoelectrochemistry | 534.56 | 0.048 | 0.01–2 | This work |
| Sample | Added (μM) | Found (μM) | Recovery (%) |
|---|---|---|---|
| 1 | 10 | 10.50 | 105 |
| 2 | 10 | 8.51 | 85.1 |
| 3 | 10 | 10.10 | 101.0 |
| 4 | 20 | 17.32 | 85.16 |
| 5 | 20 | 17.56 | 87.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Peng, N.; Gao, W.; Hu, F.; Zhang, C.; Wei, X. ZnS and Reduced Graphene Oxide Nanocomposite-Based Non-Enzymatic Biosensor for the Photoelectrochemical Detection of Uric Acid. Biosensors 2024, 14, 488. https://doi.org/10.3390/bios14100488
Zhao Y, Peng N, Gao W, Hu F, Zhang C, Wei X. ZnS and Reduced Graphene Oxide Nanocomposite-Based Non-Enzymatic Biosensor for the Photoelectrochemical Detection of Uric Acid. Biosensors. 2024; 14(10):488. https://doi.org/10.3390/bios14100488
Chicago/Turabian StyleZhao, Yao, Niancai Peng, Weizhuo Gao, Fei Hu, Chuanyu Zhang, and Xueyong Wei. 2024. "ZnS and Reduced Graphene Oxide Nanocomposite-Based Non-Enzymatic Biosensor for the Photoelectrochemical Detection of Uric Acid" Biosensors 14, no. 10: 488. https://doi.org/10.3390/bios14100488
APA StyleZhao, Y., Peng, N., Gao, W., Hu, F., Zhang, C., & Wei, X. (2024). ZnS and Reduced Graphene Oxide Nanocomposite-Based Non-Enzymatic Biosensor for the Photoelectrochemical Detection of Uric Acid. Biosensors, 14(10), 488. https://doi.org/10.3390/bios14100488







