A Low-Cost, Portable, Multi-Cancer Screening Device Based on a Ratio Fluorometry and Signal Correlation Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. ARPA-CS System Design
- As shown in Figure 1, the process starts with the MCU producing the modulation signal. This signal comprises a train of square pulses (Walsh function) produced utilizing the MCU’s integrated 12-bit DAC.
- The LED driver, providing amplitude modulation (AM), receives the modulation signal at its input, switching the high-power ultraviolet (UV) LED in an ON/OFF pattern (Walsh modulator).
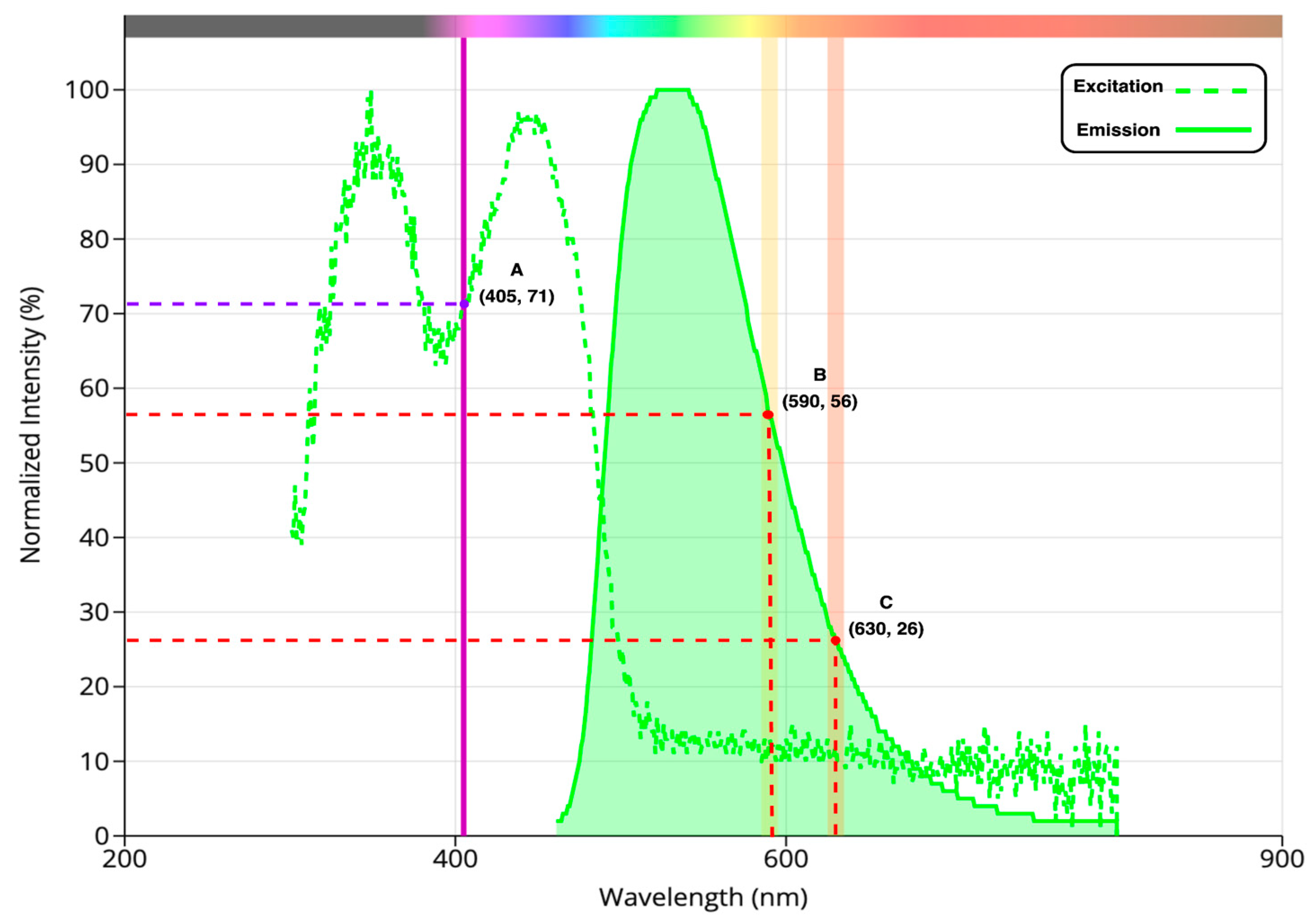
- Following the modulation, the light beam at 405 nm is directed through a collimator and then focused onto the sample within a cuvette placed in the sample chamber using two plano-convex lenses, exciting the sample, as shown in Figure 1.
- As a result, the sample emits yellow and red lights (corresponding to 590 and 630 nm, respectively), pulsating in intensity at the same modulation frequency, with the intensity level reflecting porphyrin concentrations.
- Two photodetectors capture the emitted light. As shown in Figure 1, each photodetector consists of a photodiode operated in photovoltaic mode, two plano-convex lenses, and a narrow bandpass filter. One detector’s filter is centered at 590 nm, while the other detector’s filter is centered at 630 nm. Thus, this configuration optimizes the system’s optical efficiency by maximally capturing the emitted light from the sample and directing it towards the photodiode.
- Coupled with each photodiode on a PCB, a TIA converts the sample’s faint optical signal to a usable voltage range, and the signal is fed back to the microcontroller’s integrated 16-bit ADC for processing. To prevent potential damage from exceeding the ADC’s input voltage limit (3.3 V), a dedicated ADC protection circuit is employed to clip any incoming voltage that surpasses this threshold.
- After detection, the microcontroller-based synchronous detector using signal processing techniques (covered in the following section) extracts the signal amplitude (of each photodetector) and rejects common noise that may arise from (1) internal electrical noise from the device, (2) electromagnetic interference from nearby sources, or (3) stray light leaking into the optical system.
- Consequently, this signal processing enables the system to perform ratio fluorometry based on the ratio of the signal amplitudes at 630 nm and 590 nm. This ratio is indicative of porphyrin levels within the sample, thereby serving as a biomarker for cancer screening.
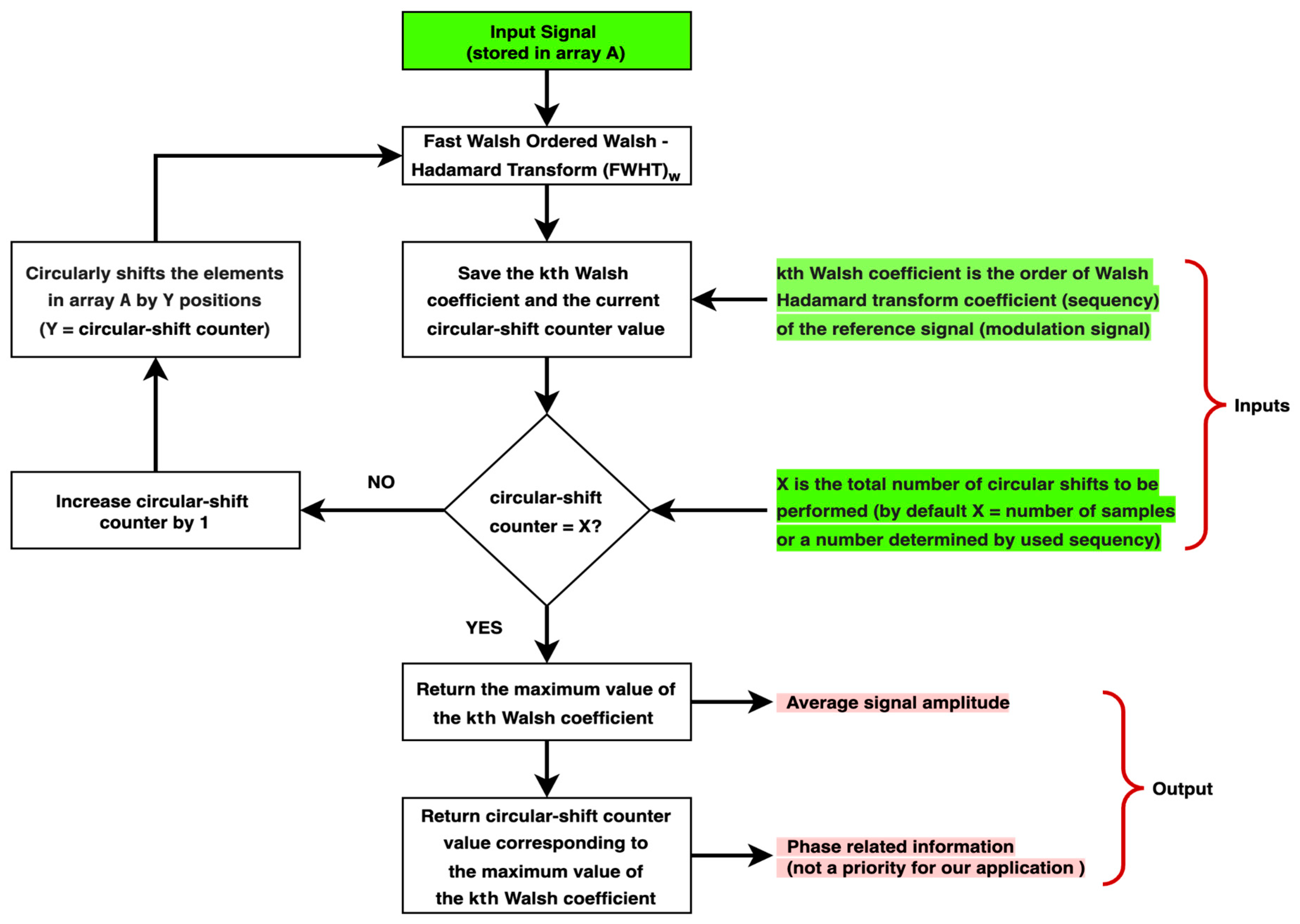
2.2. Synchronous Detector Based on (WHT)w and Cyclic Shift
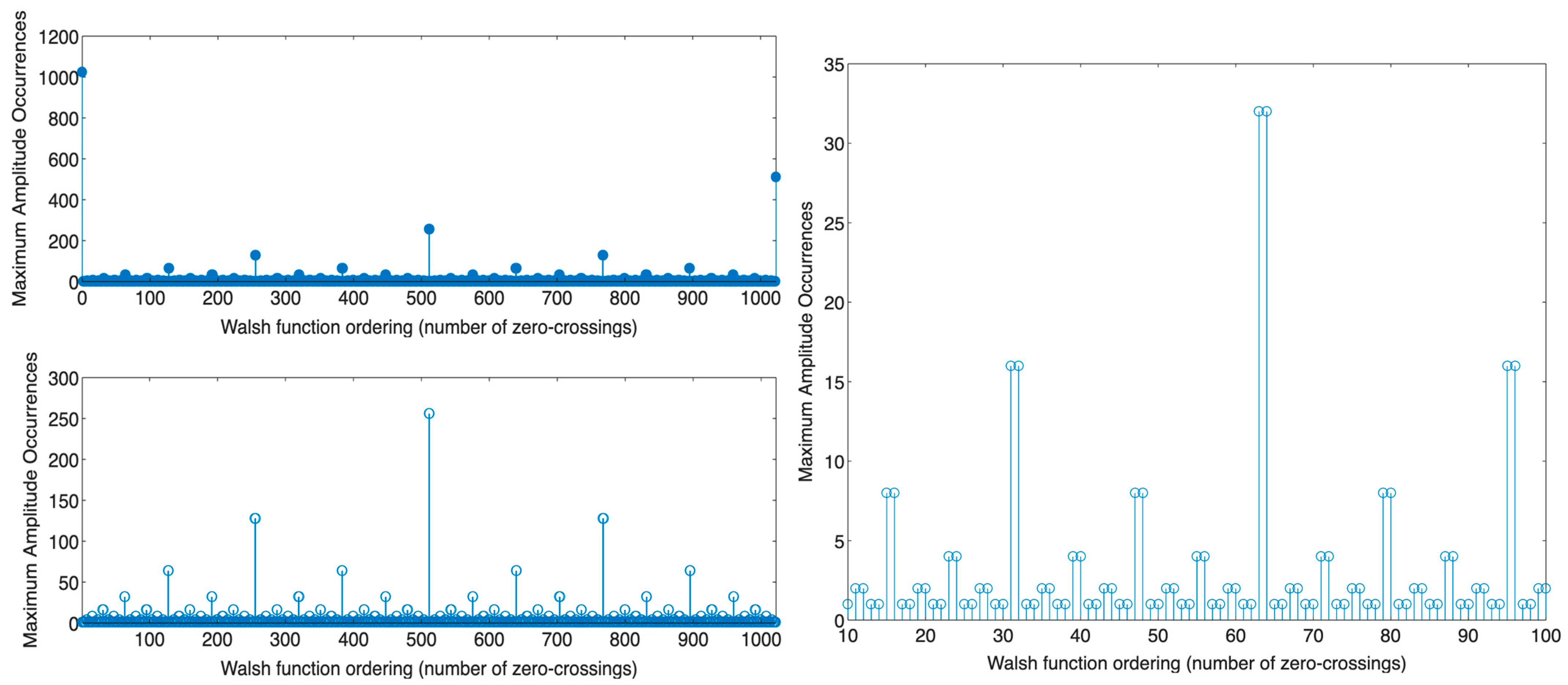
- For Walsh functions of odd sequency (when or is an odd number, where the number of zero-crossings () is odd or even, respectively [60]), the total number of times the function attains maximum amplitude in the sequency domain is one.
- For Walsh functions of even sequency (when or is an even number, where the number of zero-crossings () is odd or even, respectively [60]), the total number of times the function attains maximum amplitude in the sequency domain is more than one, defined as follows:
- For the Walsh function of order, where is its length, the total number of times the function attains maximum amplitude in the sequency domain is after cyclically shifting the signal along its entire length.
- For the Walsh function of order , the number of times the function attains maximum amplitude in the sequency domain is after cyclically shifting the signal along its entire length.
- For the remaining even Walsh functions, the total number of times the function attains maximum amplitude in the sequency domain may be 2, 4, 8, 16, 32, 64, or 128, determined by the function’s order.
2.3. Device Fabrication
2.4. Modulation Frequency Selection
2.5. ARPA-CS Signal Processing
2.6. Software Implementation and Graphical User Interface (GUI)
2.7. Device Calibration
2.8. Evaluation Method
2.8.1. Device Performance Specifications
2.8.2. Ratio Fluorometry
2.8.3. Simulant Test
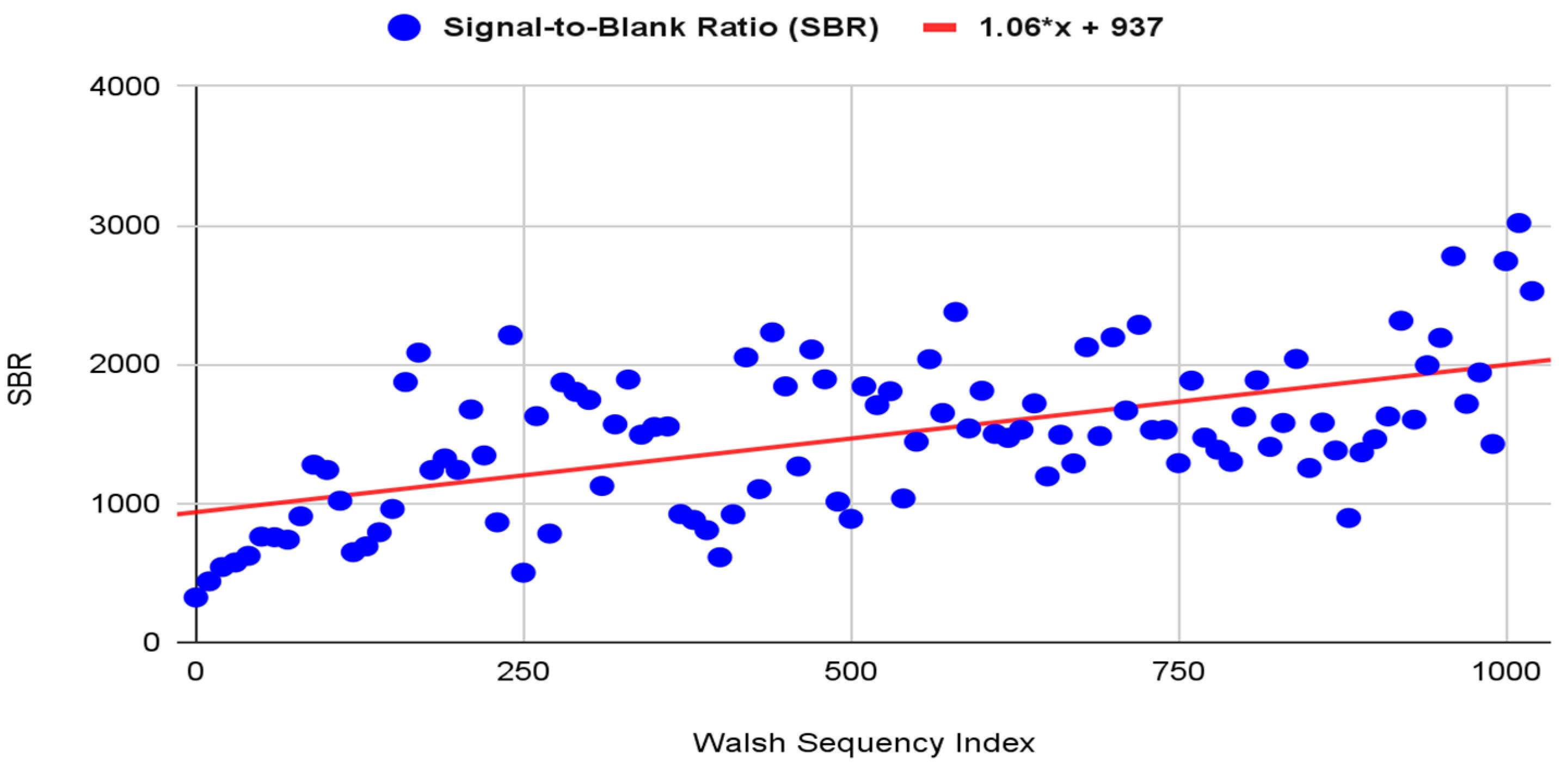
2.8.4. Synchronous Detection Efficiency
- (a)
- A stock solution of 0.3 mg of riboflavin dissolved in 100 mL distilled water, making the concentration 3 μg/mL or 3 ppm.
- (b)
- A porphyrin solution was prepared by treating crushed brown eggshells with a 2 M HCl–ethyl acetate mixture in a flask. After stirring at approximately 22 °C for 10–20 min, the organic layer was isolated via filtration [65].
3. Results
3.1. Device Performance Specifications
3.2. Ratio Fluorometry
3.3. Simulant Test
3.4. Synchronous Detection Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization (PAHO). Available online: https://www.paho.org/en/campaigns/world-cancer-day-2023-close-care-gap (accessed on 28 July 2024).
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Oeffinger, K. The Importance of Cancer Screening. Med. Clin. N. Am. 2020, 104, 919–938. [Google Scholar] [CrossRef]
- Philipson, T.; Durie, T.; Cong, Z.; Fendrick, A. The aggregate value of cancer screenings in the United States: Full potential value and value considering adherence. BMC Health Serv. Res. 2023, 23, 829. [Google Scholar] [CrossRef]
- Latos, W.; Aebisher, D.; Latos, M.; Krupka-Olek, M.; Dynarowicz, K.; Chodurek, E.; Cieślar, G.; Kawczyk-Krupka, A. Colonoscopy: Preparation and Potential Complications. Diagnostics (Basel, Switzerland). Diagnostics 2022, 12, 747. [Google Scholar] [CrossRef] [PubMed]
- Huffstetler, A.; Fraiman, J.; Brownlee, S.; Stoto, M.; Lin, K. An Estimate of Severe Harms Due to Screening Colonoscopy: A Systematic Review. JABFM 2023, 36, 493–500. [Google Scholar] [CrossRef]
- Nelson, D.; England, A.; Cheptoo, M.; Mercer, C. A comparative study of pain experienced during successive mammography examinations in patients with a family history of breast cancer and those who have had breast cancer surgery. Radiography 2020, 26, 76–81. [Google Scholar] [CrossRef]
- Sidek, M.; Amajid, K.; Loh, Y.; Rosli, M.; Hashim, I.; Suffian, N.; Abdullah, N.; Midin, M. The prevalent factors of anxiety in women undergoing mammography. Front. Psychiatry 2023, 14, 1085115. [Google Scholar] [CrossRef]
- Medical University of South Carolina. Available online: https://muschealth.org/medical-services/ddc/patients/gi-procedures/upper-endoscopy (accessed on 28 July 2024).
- Johns Hopkins Medicine. Available online: https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/upper-gi-endoscopy (accessed on 28 July 2024).
- Kindt, I.; Martiny, F.; Gram, E.; Bie, A.; Jauernik, C.; Rahbek, O.; Nielsen, S.; Siersma, V.; Bang, C.; Brodersen, J. The risk of bleeding and perforation from sigmoidoscopy or colonoscopy in colorectal cancer screening: A systematic review and meta-analyses. PLoS ONE 2023, 18, e0292797. [Google Scholar] [CrossRef]
- Shi, H.; Sun, Z.; Ju, F. Understanding the harm of low-dose computed tomography radiation to the body (Review). Exp. Ther. Med. 2022, 24, 534. [Google Scholar] [CrossRef]
- Meulepas, J.; Ronckers, C.; Smets, A.; Nievelstein, R.; Gradowska, P.; Lee, C.; Jahnen, A.; Straten, M.; Wit, M.; Zonnenberg, B.; et al. Radiation exposure from pediatric CT scans and subsequent cancer risk in the Netherlands. J. Natl. Cancer Inst. 2019, 111, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S.; Park, J.; Lee, S.; Kim, H.; Lee, W.; Cha, E.; Kim, K.; Lee, W.; Lee, J.; et al. Risk of hematologic malignant neoplasms from abdominopelvic computed tomographic radiation in patients who underwent appendectomy. JAMA Surg. 2021, 156, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Law, M.; Huang, B.; Ng, S.; Li, Z.; Meng, Q.; Khong, P. Radiation dose and cancer risk from pediatric CT examinations on 64-slice CT: A phantom study. Eur. J. Radiol. 2010, 76, e19–e23. [Google Scholar] [CrossRef] [PubMed]
- Griffey, R.; Sodickson, A. Cumulative radiation exposure and cancer risk estimates in emergency department patients undergoing repeat or multiple CT. AJR Am. J. Roentgenol. 2009, 192, 887–892. [Google Scholar] [CrossRef]
- Najib, F.; Hashemi, M.; Shiravani, Z.; Poordast, T.; Sharifi, S.; Askary, E. Diagnostic Accuracy of Cervical Pap Smear and Colposcopy in Detecting Premalignant and Malignant Lesions of Cervix. Indian. J. Surg. Oncol. 2020, 11, 453–458. [Google Scholar] [CrossRef]
- Merriel, S.; Pocock, L.; Gilbert, E.; Creavin, S.; Walter, F.; Spencer, A.; Hamilton, W. Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients. BMC Med. 2022, 20, 54. [Google Scholar] [CrossRef]
- Ratushnyak, S.; Hoogendoorm, M. Cost-Effectiveness of Cancer Screening: Health and Costs in Life Years Gained. AJPM 2019, 57, 792–799. [Google Scholar] [CrossRef]
- Getaneh, A.; Heijnsdijk, E.; Roobol, M.; de Koning, H. Assessment of harms, benefits, and cost-effectiveness of prostate cancer screening: A micro-simulation study of 230 scenarios. Cancer Med. 2020, 9, 7742–7750. [Google Scholar] [CrossRef]
- Frija, G.; Blažić, I.; Frush, D.; Hierath, M.; Kawooya, M.; Donoso-Bach, L.; Brkljačić, B. How to improve access to medical imaging in low- and middle-income countries ? eClinicalMedicine 2021, 38, 101034. [Google Scholar] [CrossRef]
- Shrirao, A.; Schloss, R.; Fritz, Z.; Shrirao, M.; Rosen, R.; Yarmush, M. Autofluorescence of blood and its application in biomedical and clinical research. Biotechnol. Bioeng. 2021, 118, 4550–4576. [Google Scholar] [CrossRef]
- Masilamani, V.; Alzahrani, K.; AlSalhi, M.; Aldiab, A. Cancer diagnosis by autofluorescence of blood components. J. Lumin. 2004, 109, 143–154. [Google Scholar] [CrossRef]
- Lualdi, M.; Colombo, A.; Leo, E.; Morelli, D.; Vannelli, A.; Battaglia, L.; Poiasina, E.; Marchesini, R. Natural fluorescence spectroscopy of human blood plasma in the diagnosis of colorectal cancer: Feasibility study and preliminary results. Tumori 2007, 93, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Devanesan, S.; Masilamani, V.; AlSalhi, M.; Prasad, S.; Alshebly, M.; Alqahtany, F.; Aljaafreh, M. Cancer screening by the fluorescence spectra of blood and urine—A double blind study. J. King Saud. Univ.-Sci. 2021, 33, 101456. [Google Scholar] [CrossRef]
- Madhuri, S.; Aruna, P.; Bibi, M.; Gowri, V.; Koteeswaran, D.; Ganesan, S. Ultraviolet fluorescence spectroscopy of blood plasma in the discrimination of cancer from normal. In Proceedings of the Optical Diagnostics of Biological Fluids and Advanced Techniques in Analytical Cytology, San Jose, CA, USA, 2 May 1997. [Google Scholar] [CrossRef]
- Masilamani, V.; Trinka, V.; Al Salhi, M.; Elangovan, M.; Raghavan, V.; Al Diab, A.; Hajjar, W.; Ainia, M.; Al-Mustafa, A.; Al-Nachawati, H. A new lung cancer biomarker--a preliminary report. Photomedicine and laser surgery. Photomed. Laser Surg. 2011, 29, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lualdi, M.; Battaglia, L.; Colombo, A.; Leo, E.; Morelli, D.; Poiasina, E.; Vannelli, A.; Marchesini, R. Colorectal cancer detection by means of optical fluoroscopy. A study on 494 subjects. FBE 2010, 2, 694–700. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalaivani, R.; Masilamani, V.; AlSalhi, M.; Devanesan, S.; Ramamurthy, P.; Palled, S.; Ganesh, K. Cervical cancer detection by time-resolved spectra of blood components. J. Biomed. Opt. 2014, 19, 057011. [Google Scholar] [CrossRef]
- Kalaivani, R.; Masilamani, V.; Sivaji, K.; Elangovan, M.; Selvaraj, V.; Balamurugan, S.; Al-Salhi, M. Fluorescence spectra of blood components for breast cancer diagnosis. Photomed. Laser Surg. 2008, 26, 251–256. [Google Scholar] [CrossRef]
- Masilamani, V.; Alsalhi, M.; Vijmasi, T.; Govindarajan, K.; Rai, R.; Atif, M.; Prasad, S.; Aldwayyan, A. Fluorescence spectra of blood and urine for cervical cancer detection. J. Biomed. Opt. 2012, 17, 0980011–0980016. [Google Scholar] [CrossRef]
- Silva, F.; Bellini, M.; Tristão, V.; Schor, N.; Vieira, N.; Courrol, L. Intrinsic Fluorescence of Protoporphyrin IX from Blood Samples Can Yield Information on the Growth of Prostate Tumours. J. Fluoresc. 2010, 20, 1159–1165. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Connal, S.; Cameron, J.; Sala, A.; Brennan, P.; Palmer, D.; Palmer, J.; Perlow, H.; Baker, M. Liquid biopsies: The future of cancer early detection. J. Transl. Med. 2023, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Bolandi, N.; Hemmati, A.; Eyvazi, S.; Ghasemzadeh, S.; Baradaran, B.; Oroojalian, F.; Majidi, M.; Guardia, M.; Mokhtarzadeh, A. State-of-the-art cancer biomarker detection by portable (Bio) sensing technology: A critical review. Microchem. J. 2022, 177, 107248. [Google Scholar] [CrossRef]
- Jiang, Y.; Sima, Y.; Liu, L.; Zhou, C.; Shi, S.; Wan, K.; Chen, A.; Tang, N.; He, Q.; Liu, J. Research progress on portable electrochemical sensors for detection of mycotoxins in food and environmental samples. Chem. Eng. J. 2024, 485, 149860. [Google Scholar] [CrossRef]
- Gallay, C.; Girardet, A.; Viviano, M.; Catarino, R.; Benski, A.; Tran, P.; Ecabert, C.; Thiran, J.; Vassilakos, P.; Petignat, P. Cervical cancer screening in low-resourcesettings: A smartphone image application as analternative to colposcopy. Int. J. Womens Health 2017, 22, 455–461. [Google Scholar] [CrossRef]
- Tran, P.; Benski, C.; Viviano, M.; Petignat, P.; Combescure, C.; Jinoro, J.; Herinianasolo, J.; Vassilakos, P. Performance of smartphone-based digital images for cervical cancer screening in a low-resource context. Int. J. Technol. Assess. Health Care 2018, 34, 337–342. [Google Scholar] [CrossRef]
- Catarino, R.; Vassilakos, P.; Scaringella, S.; Malinverno, M.; Hamme, U.; Gauthier, D.; Matute, J.; Petignat, P. Smartphone Use for Cervical Cancer Screening in Low-Resource Countries: A Pilot Study Conducted in Madagascar. PLoS ONE 2015, 10, e0134309. [Google Scholar] [CrossRef]
- Asgary, R.; Staderini, N.; Hleta, S.; Saavedra, P.; Abrego, L.; Rusch, B.; Luce, T.; Pasipamire, L.; Ndlangamandla, M.; Beideck, E.; et al. Evaluating smartphone strategies for reliability, reproducibility, and quality of VIA for cervical cancer screening in the Shiselweni region of Eswatini: A cohort study. PloS Med. 2020, 17, e1003378. [Google Scholar] [CrossRef]
- Sami, J.; Makajio, S.; Jeannot, E.; Kenfack, B.; Vinals, R.; Vassilakos, P.; Petignat, P. Smartphone-Based Visual Inspection with Acetic Acid: An Innovative Tool to Improve Cervical Cancer Screening in Low-Resource Setting. Healthcare 2022, 10, 391. [Google Scholar] [CrossRef]
- Uthoff, R.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Spires, O.; Anbarani, A.; Smith, P.; et al. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS ONE 2018, 13, e0207493. [Google Scholar] [CrossRef]
- Kalwa, U.; Legner, C.; Kong, T.; Pandey, S. Skin Cancer Diagnostics with an All-Inclusive Smartphone Application. Symmetry 2019, 11, 790. [Google Scholar] [CrossRef]
- Ray, P.; Dash, D.; De, D. A Systematic Review of Wearable Systems for Cancer Detection: Current State and Challenges. J. Med. Syst. 2017, 41, 180. [Google Scholar] [CrossRef] [PubMed]
- Pascua, J.; Prado, A.; Solis, B.; Cid-Andres, A.; Cambiador, C. Trends in fabrication, data gathering, validation, and application of molecular fluorometer and spectrofluorometer. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2019, 220, 116837. [Google Scholar] [CrossRef]
- Ibsen Photonics. Available online: https://ibsen.com/technologies/fluorescence-spectroscopy/fluorescence-instrumentation/ (accessed on 30 July 2024).
- Singseeta, W.; Wongsing, B.; Naksen, P.; Jarujamrus, P.; Pencharee, S. Portable Multispectral Fluorometer for Determination of Formalin in Food Samples Using Nitrogen-Doped Carbon Dots as the Fluorescence Probe. Talanta Open 2023, 7, 100199. [Google Scholar] [CrossRef]
- English, C.; Kitzhaber, Z.; Williams, J.; Humphries, A.; Myrick, M. Challenges of Spectrofluorometry Part 1: Collect Data Right the First Time. Spectroscopy 2021, 36, 44–46. [Google Scholar] [CrossRef]
- English, C.; Kitzhaber, Z.; Williams, J.; Humphries, A.; Myrick, M. Challenges of Spectrofluorometry Part 2: Instrument-Specific Concerns. Spectroscopy 2021, 36, 41–45. [Google Scholar] [CrossRef]
- English, C.; Kitzhaber, Z.; Williams, J.; Humphries, A.; Myrick, M. Challenges of Spectrofluorometry Part 3: Sample-Specific Concerns. Spectroscopy 2021, 36, 30–35. [Google Scholar] [CrossRef]
- Kleinfeld, A.; Vodkin, P. Method and Apparatus for Ratio Fluorometry. U.S. Patent 6,999,173, 25 September 2003. [Google Scholar]
- Yoshihiro, T.; Hiroya, S. A Walsh Waoofonn Analyzer and Its Applications to Filtering of Pulse Signals. Mem. Sch. Eng. Okayama Univ. 1979, 13, 163–180. [Google Scholar]
- Degani, I. Signal Processing Techniques Applied to Biomedical Diagnostics. Doctoral Dissertation, Massachusetts Institute of Technology, Cambridge, MA, USA, 2022. [Google Scholar]
- Graeme, J. Photodiode Amplifiers: Op Amp Solutions, 1st ed.; McGraw-Hill: New York, NY, USA, 1995; pp. 129–163. [Google Scholar]
- Meade, M. Lock-in Amplifiers: Principles and Applications; Peter Peregrinus: London, UK, 1983; pp. 16–35. [Google Scholar]
- Ashrafi, A. Walsh–Hadamard Transforms: A Review. Adv. Imaging Electron. Phys. 2017, 201, 1–55. [Google Scholar]
- O’Haver, T. A Pragmatic Introduction to Signal Processing: With Applications in Scientific Measurement; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2016; pp. 113–115. [Google Scholar]
- Klette, R. Concise Computer Vision—An Introduction into Theory and Algorithms, 1st ed.; Springer: London, UK, 2014; pp. 14–16. [Google Scholar] [CrossRef]
- Ahemd, N.; Rao, R. Orthogonal Transforms for Digital Signal Processing, 1st ed.; Springer: Heidelberg, Germany, 1979; pp. 85–93. [Google Scholar] [CrossRef]
- Irshid, M. A Simple Recursive Definition for Walsh Functions. IEEE Trans. Electromagn. Compat. 1986, 28, 276–279. [Google Scholar] [CrossRef]
- Johnson, M. Photodetection and Measurement: Maximizing Performance in Optical Systems, 1st ed.; McGraw-Hill: New York, NY, USA, 2003; pp. 45–77. [Google Scholar]
- Borman, P.; Elder, D. Q2(R1) Validation of Analytical Procedures: Text and Methodology. In ICH Quality Guidelines, 1st ed.; Teasdale, A., Elder, A., Nims, R., Eds.; WILEY Online Library: Hoboken, NJ, USA, 2017; pp. 127–158. [Google Scholar] [CrossRef]
- AAT Bioquest. Available online: https://www.aatbio.com/fluorescence-excitation-emission-spectrum-graph-viewer/ribofla-vin (accessed on 6 October 2024).
- Overway, K.; Studtman, C.; Varner, T. Extraction of PPIX from Brown Eggshells for Use in Dye-Sensitized Solar Cells. Chem. Fac. Scholarsh. 2018, 9. [Google Scholar]
- Dean, M.; Miller, T.; Bruckner, C. Egg-Citing! Isolation of Protoporphyrin IX from Brown Eggshells and Its Detection by Optical Spectroscopy and Chemiluminescence. J. Chem. Educ. 2011, 88, 788–792. [Google Scholar] [CrossRef]
- Samiullah, S.; Robers, J.; Chousalkar, K. Eggshell color in brown-egg laying hens—A review. Poult. Sci. 2015, 94, 2566–2575. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Floris, F.; Rensing, M.; Andersson-Engels, S. Fluorescence Spectroscopy Study of Protoporphyrin IX in Optical Tissue Simulating Liquid Phantoms. Materials 2020, 13, 2105. [Google Scholar] [CrossRef] [PubMed]
- Medscape. Available online: https://emedicine.medscape.com/article/2088605-overview?form=fpf (accessed on 3 August 2024).
- Agilent Technologies. Available online: https://www.agilent.com/en-us/agilent404?s=www.agilent.com/cs/library/technicaloverviews/Public/5991-3851EN.pdf (accessed on 3 August 2024).
- HORIBA Scientific. Available online: https://static.horiba.com/fileadmin/Horiba/Products/Scientific/Molecular_and_Microanalysis/FluoroMax/FluoroMax_Series.pdf (accessed on 3 August 2024).







| Specification | Description | Method |
|---|---|---|
| LOD | Minimum detectable concentration of a specific molecule. |
|
| Dynamic Range | The interval between the upper and lower concentrations of the analyte (including these concentrations) for which a suitable level of linearity has been demonstrated. | Analyze visually from the calibration curve. |
| Linearity | The ability to produce a proportional relationship between the concentration of the fluorescent molecule and the measured fluorescence intensity. | in regression analysis is the measure of linearity strength between concentration and fluorescence intensity. |
| Repeatability | The ability to produce consistent fluorescence readings for the same sample under identical conditions. is used to express repeatability. |
|
| Stability | Consistency in producing the same values over time for the same sample. is used to express stability. |
|
| Specification | Value | Comment |
|---|---|---|
| LOD | 0.296 µg/mL or 296 ppb | = 459 |
| Dynamic Range | More than four orders of magnitude | — |
| Linearity | 0.992 | — |
| Repeatability | 0.21% | — |
| Stability | 0.60% | — |
| Volume | — | |
| Weight | 740 g | — |
| Concentration (µg/mL) or (ppm) | Fluorescence Intensity (590 nm) | Calibrated Readings | Modulation Sequence |
|---|---|---|---|
| 3 | 14,745.287 | 14,727.813 | 1023 |
| 1.5 | 8432.916 | 8415.169 | |
| 0.75 | 3393.295 | 3375.548 | |
| 0.375 | 1153.053 | 1135.306 | |
| 0.187 | 386.782 | 369.035 | |
| 0.093 | 109.650 | 91.903 | |
| 0.046 | 38.566 | 20.819 | |
| 0.023 | 20.458 | 2.711 | |
| 0.011 | 18.757 | 1.01 | |
| Blank solution | 17.474 | 0 |
| Analyte | Concentration (µg/mL) | Calibrated Fluorescence Intensity (590 nm) | Calibrated Fluorescence Intensity (630 nm) | Ratio (I630nm/I590nm) |
|---|---|---|---|---|
| Riboflavin | 3 | 14,727.813 | 6274.461 | 0.426 |
| 1.5 | 8415.169 | 3559.262 | 0.422 | |
| 0.75 | 3375.548 | 1358.86 | 0.402 |
| Analyte | Calibrated Fluorescence Intensity (590 nm) | Calibrated Fluorescence Intensity (630 nm) | Ratio (I630nm/I590nm) |
|---|---|---|---|
| Porphyrin | 96.455 | 4805.743 | 49.82 |
| Reading No. | Fluorescence Intensity at 590 nm (Riboflavin Sample) | Modulation Sequency |
|---|---|---|
| 1 | 21,161.072 | 1023 |
| 2 | 21,114.001 | |
| 3 | 21,167.485 | |
| 4 | 21,207.688 | |
| 5 | 21,118.311 | |
| 6 | 21,228.713 | |
| 7 | 21,160.219 | |
| 8 | 21,241.393 | |
| 9 | 21,195.318 | |
| 10 | 21,222.090 |
| Reading No. | Fluorescence Intensity at 590 nm (Riboflavin Sample) | Modulation Sequency |
|---|---|---|
| 1 | 21,740.362 | 1023 |
| 2 | 21,823.546 | |
| 3 | 21,987.590 | |
| 4 | 21,966.765 | |
| 5 | 21,982.763 | |
| 6 | 21,998.738 | |
| 7 | 21,916.649 | |
| 8 | 22,119.437 | |
| 9 | 22,147.506 | |
| 10 | 22,134.718 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, A.S.; Aldhaheri, R.W. A Low-Cost, Portable, Multi-Cancer Screening Device Based on a Ratio Fluorometry and Signal Correlation Technique. Biosensors 2024, 14, 482. https://doi.org/10.3390/bios14100482
Alghamdi AS, Aldhaheri RW. A Low-Cost, Portable, Multi-Cancer Screening Device Based on a Ratio Fluorometry and Signal Correlation Technique. Biosensors. 2024; 14(10):482. https://doi.org/10.3390/bios14100482
Chicago/Turabian StyleAlghamdi, Abdulaziz S., and Rabah W. Aldhaheri. 2024. "A Low-Cost, Portable, Multi-Cancer Screening Device Based on a Ratio Fluorometry and Signal Correlation Technique" Biosensors 14, no. 10: 482. https://doi.org/10.3390/bios14100482
APA StyleAlghamdi, A. S., & Aldhaheri, R. W. (2024). A Low-Cost, Portable, Multi-Cancer Screening Device Based on a Ratio Fluorometry and Signal Correlation Technique. Biosensors, 14(10), 482. https://doi.org/10.3390/bios14100482





