Advanced Electrochemical Monitoring of Carbendazim Fungicide in Foods Using Interfacial Superassembly of NRPC/NiMn Frameworks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the NRPC/NiMn Hierarchical Structure
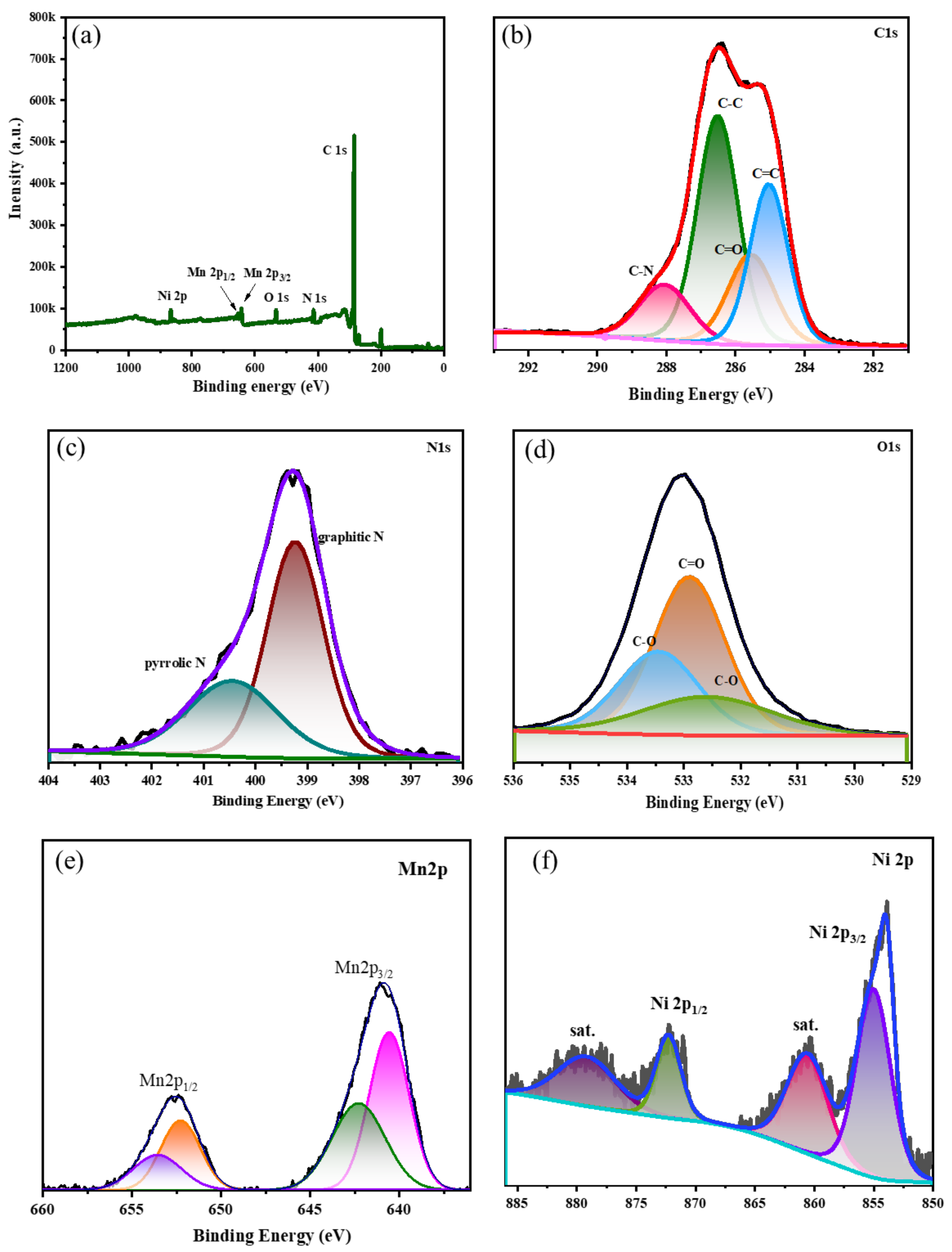
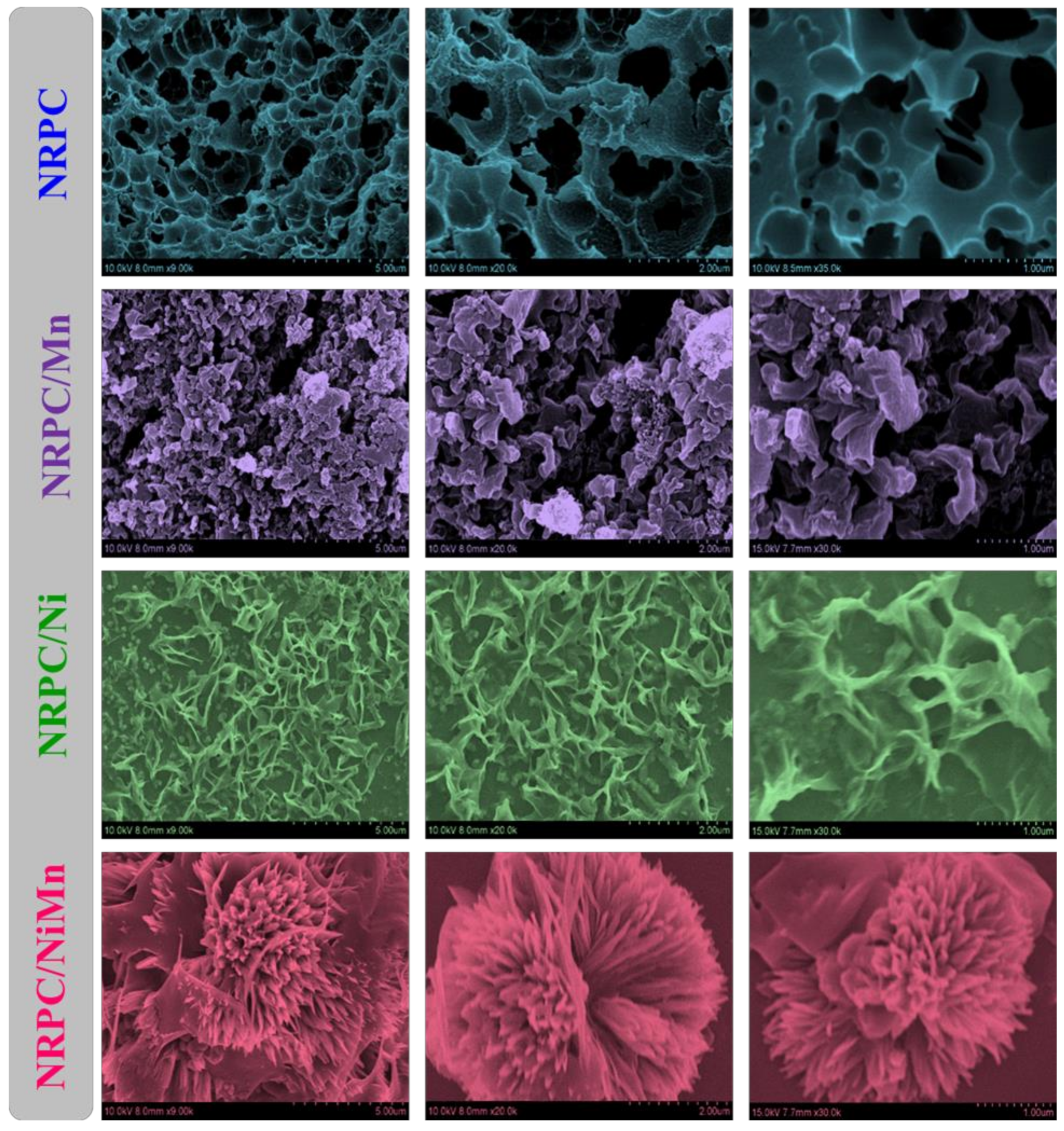
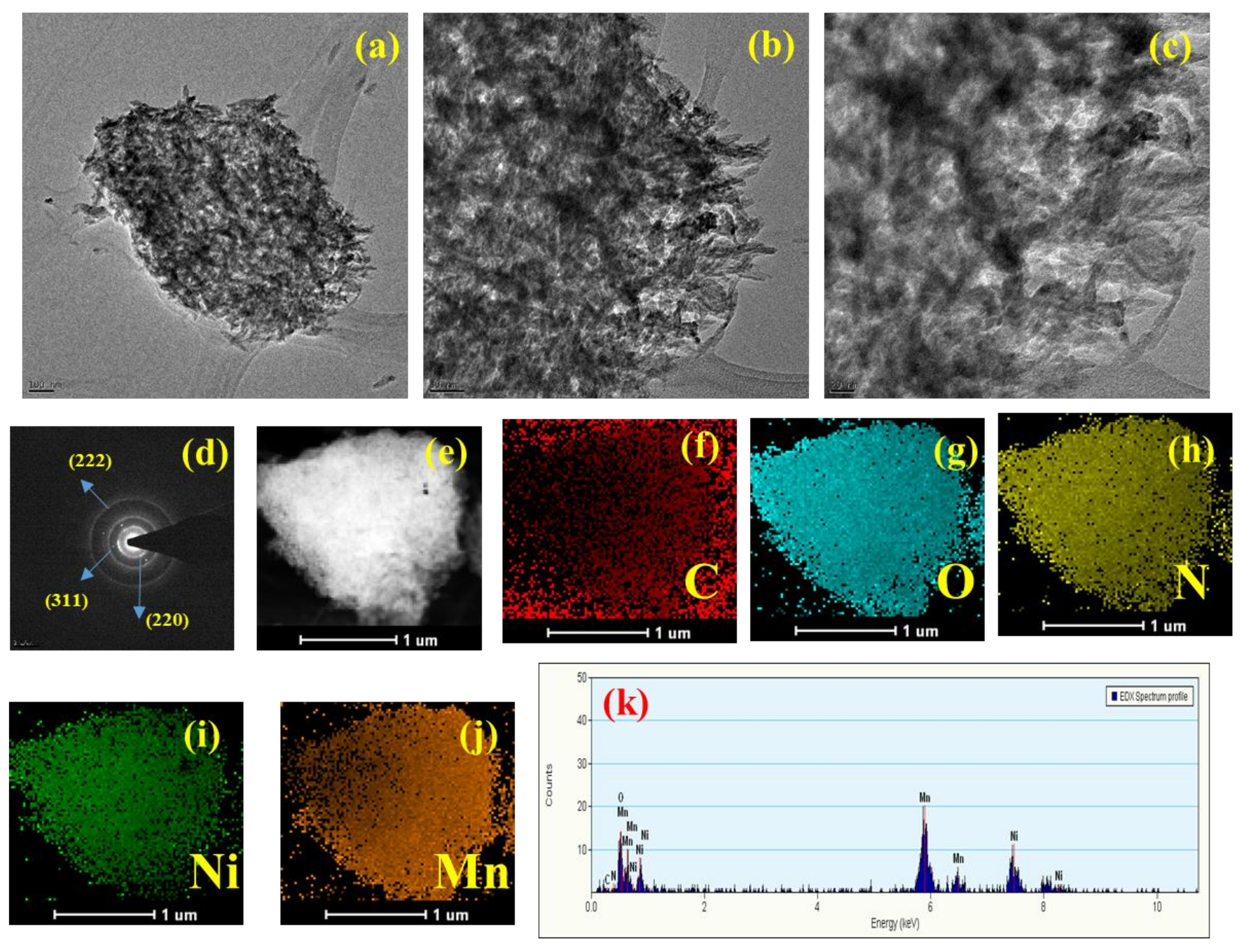
3. Results and Discussions
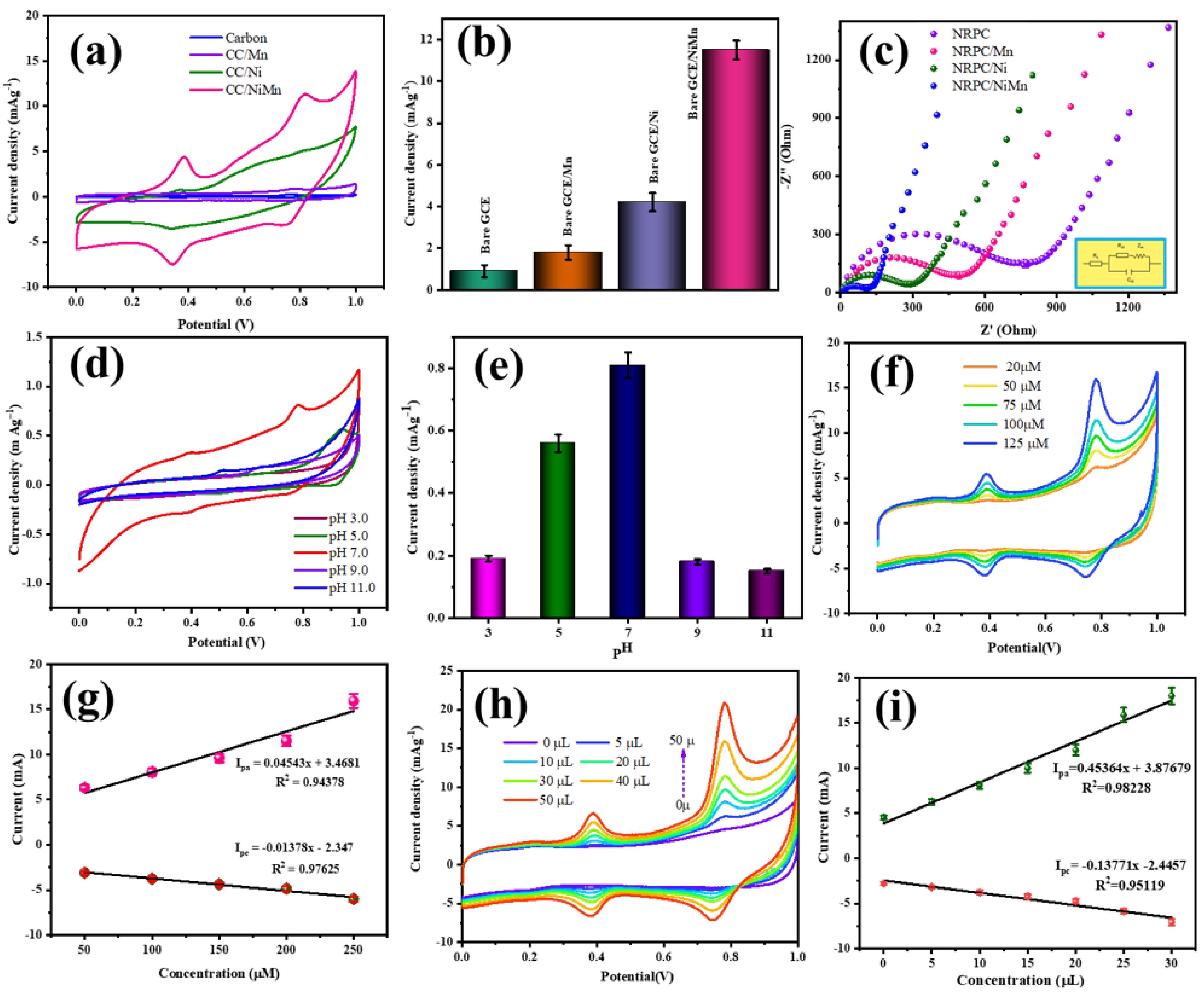
4. Electrochemical Characterization
4.1. Electrochemical Behavior of the Materials towards CBZ
4.2. Impedance Analysis
4.3. Effect of pH
4.4. Effect of Material Concentration (NRPC/NiMn)
4.5. Effect of CBZ Concentration
4.6. Effect of Scan Rate
4.7. Repeatability, Reproducibility, Stability and Anti-Interfering Ability of NRPC/NiMn@GCE Sensor
4.8. CBZ Detection in Real Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yukird, J.; Insin, N.; Chanajaree, R.; Rodthongkum, N. Fe3O4 Nanoparticle/Graphene Oxide Composites as Selective Probes and Self-Matrixes for Pesticide Detection by Electrochemistry and Laser Desorption/Ionization Mass Spectrometry. ACS Appl. Nano Mater. 2023, 6, 11912–11924. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, T.; Zhao, H.; Zhu, G.; Li, F.; Guo, M.; Ran, Q.; Komarneni, S. An Electrochemical Sensor Modified with Novel Nanohybrid of Super-P Carbon Black@zeolitic-Imidazolate-Framework-8 for Sensitive Detection of Carbendazim. Ceram. Int. 2023, 49, 23775–23787. [Google Scholar] [CrossRef]
- Lambraki, I.A.; Chadag, M.V.; Cousins, M.; Graells, T.; Leger, A.; Henriksson, P.J.G.; Troell, M.F.; Harbarth, S.; Wernli, D.; Jorgensen, P.S.; et al. Factors Impacting Antimicrobial Resistance in the South East Asian Food System and Potential Places to Intervene: A Participatory, One Health Study. Front. Microbiol. 2023, 13, 992507. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Z.; Lan, X.; Chen, Y.; Zhang, L.; Ji, R.; Wang, L. Determination of Carbendazim and Metiram Pesticides Residues in Reapeseed and Peanut Oils by Fluorescence Spectrophotometry. Meas. J. Int. Meas. Confed. 2015, 73, 313–317. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. Selection and Characterization of DNA Aptamers for Electrochemical Biosensing of Carbendazim. Anal. Chem. 2017, 89, 3138–3145. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Cabrera, L.C.; Pastor, P.M. The 2020 European Union report on pesticide residues in food. EFSA J. 2022, 20, 7215. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.W.; Pu, H.; Wei, Q. A Rapid Dual-Channel Readout Approach for Sensing Carbendazim with 4-Aminobenzenethiol-Functionalized Core-Shell Au@Ag Nanoparticles. Analyst 2020, 145, 1801–1809. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Kumar, V.; Datta, S.; Wani, A.B.; Singh, D.; Singh, K.; Singh, J. Toxicity, Monitoring and Biodegradation of the Fungicide Carbendazim. Environ. Chem. Lett. 2016, 14, 317–329. [Google Scholar] [CrossRef]
- Huan, Z.; Luo, J.; Xu, Z.; Xie, D. Acute Toxicity and Genotoxicity of Carbendazim, Main Impurities and Metabolite to Earthworms (Eisenia foetida). Bull. Environ. Contam. Toxicol. 2016, 96, 62–69. [Google Scholar] [CrossRef]
- Özcan, A.; Hamid, F.; Özcan, A.A. Synthesizing of a Nanocomposite Based on the Formation of Silver Nanoparticles on Fumed Silica to Develop an Electrochemical Sensor for Carbendazim Detection. Talanta 2021, 222, 121591. [Google Scholar] [CrossRef]
- Zhao, H.; Ran, Q.; Li, Y.; Li, B.; Liu, B.; Ma, H.; Zhang, M.; Komarneni, S. Highly Sensitive Detection of Gallic Acid Based on 3D Interconnected Porous Carbon Nanotubes/Carbon Nanosheets Modified Glassy Carbon Electrode. J. Mater. Res. Technol. 2020, 9, 9422–9433. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, S.; Li, J.; Wang, E.; Dong, S. Cyclodextrin-Graphene Hybrid Nanosheets as Enhanced Sensing Platform for Ultrasensitive Determination of Carbendazim. Talanta 2011, 84, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, G.; Wu, X.; Xu, P. Comparative Proteomic Analyses Provide Novel Insights into the Effects of Grafting Wound and Hetero-Grafting per Se on Bottle Gourd. Sci. Hortic. 2016, 200, 1–6. [Google Scholar] [CrossRef]
- Radisic, M.; Grujic, S.; Vasiljevic, T.; Lausevic, M. Determination of Selected Pesticides in Fruit Juices by Matrix Solid-Phase Dispersion and Liquid Chromatography-Tandem Mass Spectrometry. Food Chem. 2009, 113, 712–719. [Google Scholar] [CrossRef]
- Pourreza, N.; Rastegarzadeh, S.; Larki, A. Determination of Fungicide Carbendazim in Water and Soil Samples Using Dispersive Liquid-Liquid Microextraction and Microvolume UV-Vis Spectrophotometry. Talanta 2015, 134, 24–29. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Chen, Z.; Li, L.; You, T. An Ultra-Sensitive Aptasensor Based on Carbon Nanohorns/Gold Nanoparticles Composites for Impedimetric Detection of Carbendazim at Picogram Levels. J. Colloid Interface Sci. 2019, 546, 92–100. [Google Scholar] [CrossRef]
- Gao, N.; He, C.; Ma, M.; Cai, Z.; Zhou, Y.; Chang, G.; Wang, X.; He, Y. Electrochemical Co-Deposition Synthesis of Au-ZrO2-Graphene Nanocomposite for a Nonenzymatic Methyl Parathion Sensor. Anal. Chim. Acta 2019, 1072, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Anshori, I.; Nuraviana Rizalputri, L.; Rona Althof, R.; Sean Surjadi, S.; Harimurti, S.; Gumilar, G.; Yuliarto, B.; Handayani, M. Functionalized Multi-Walled Carbon Nanotube/Silver Nanoparticle (f-MWCNT/AgNP) Nanocomposites as Non-Enzymatic Electrochemical Biosensors for Dopamine Detection. Nanocomposites 2021, 7, 97–108. [Google Scholar] [CrossRef]
- Fu, S.; Ma, X.; Wang, S.; Zha, Q.; Wen, W.; Hu, B. Surfactant-Assisted Carbon Black for the Electrochemical Detection of Endocrine Disruptors. Surf. Interfaces 2021, 24, 101128. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, F.; Dubovyk, V.; Guo, M.; Zhu, G.; Ran, Q.; Zhao, H. Electrochemical Sensing Platform Based on Graphitized and Carboxylated Multi-Walled Carbon Nanotubes Decorated with Cerium Oxide Nanoparticles for Sensitive Detection of Methyl Parathion. J. Mater. Res. Technol. 2022, 19, 3738–3748. [Google Scholar] [CrossRef]
- Ji, J.; Zhou, Z.; Zhao, X.; Sun, J.; Sun, X. Electrochemical Sensor Based on Molecularly Imprinted Film at Au Nanoparticles-Carbon Nanotubes Modified Electrode for Determination of Cholesterol. Biosens. Bioelectron. 2015, 66, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, Y.; Lv, X.; Lei, W.; Ding, Y.; Chen, C.; Lv, J.; Feng, S.; Chen, S.M.; Hao, Q. A Sensitive Sensing Platform for Acetaminophen Based on Palladium and Multi-Walled Carbon Nanotube Composites and Electrochemical Detection Mechanism. Mater. Chem. Phys. 2020, 239, 121977. [Google Scholar] [CrossRef]
- Gao, X.; Gao, Y.; Bian, C.; Ma, H.; Liu, H. Electroactive Nanoporous Gold Driven Electrochemical Sensor for the Simultaneous Detection of Carbendazim and Methyl Parathion. Electrochim. Acta 2019, 310, 78–85. [Google Scholar] [CrossRef]
- Li, D.; Zhao, H.; Wang, G.; Rinklebe, J.; Lam, S.S.; Liu, R.; Bai, L. Ultrasensitive Determination of Diquat Using a Novel Nanohybrid Sensor Based on Super-P Nanoparticles Dispersed Palygorskite Nanofibers. Sens. Actuators B Chem. 2022, 367, 132142. [Google Scholar] [CrossRef]
- Li, K.; Kang, J.; Zhan, T.; Cao, W.; Liu, X.; Gao, H.; Si, C.; She, X. Electrochemical Sensing Platform for Naphthol Isomers Based on in Situ Growth of ZIF-8 on Reduced Graphene Oxide by a Reaction-Diffusion Technique. J. Colloid Interface Sci. 2021, 581, 576–585. [Google Scholar] [CrossRef]
- Zhang, W.; Zong, L.; Liu, S.; Pei, S.; Zhang, Y.; Ding, X.; Jiang, B.; Zhang, Y. An Electrochemical Sensor Based on Electro-Polymerization of Caffeic Acid and Zn/Ni-ZIF-8–800 on Glassy Carbon Electrode for the Sensitive Detection of Acetaminophen. Biosens. Bioelectron. 2019, 131, 200–206. [Google Scholar] [CrossRef]
- Nie, M.; Lu, S.; Lei, D.; Yang, C.; Zhao, Z. Rapid Synthesis of ZIF-8 Nanocrystals for Electrochemical Detection of Dopamine. J. Electrochem. Soc. 2017, 164, H952–H957. [Google Scholar] [CrossRef]
- Li, R.; Ren, X.; Feng, X.; Li, X.; Hu, C.; Wang, B. A Highly Stable Metal- and Nitrogen-Doped Nanocomposite Derived from Zn/Ni-ZIF-8 Capable of CO2 Capture and Separation. Chem. Commun. 2014, 50, 6894–6897. [Google Scholar] [CrossRef]
- Kim, J.; Kang, I.; Kim, S.; Kang, J. Facile synthesis of partially oxidized Mn3O4-functionalized carbon cathodes for rechargeable Li–O2 batteries. RSC Adv. 2018, 8, 22226–22232. [Google Scholar] [CrossRef]
- Yao, L.; Zhou, C.; Hu, N.; Hu, J.; Hong, M.; Zhang, L.; Zhang, Y. Flexible Graphene/Carbon Nanotube Hybrid Papers Chemical-Reduction-Tailored by Gallic Acid for High-Performance Electrochemical Capacitive Energy Storages. Appl. Surf. Sci. 2018, 435, 699–707. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, L.; Zhang, L. Simultaneous Electrochemical Detection of Benzimidazole Fungicides Carbendazim and Thiabendazole Using a Novel Nanohybrid Material-Modified Electrode. J. Agric. Food Chem. 2017, 65, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.R.; Shipway, P.H.; Zhu, Y.; Weston, D.P. Effective Dispersal of CNTs in the Fabrication of Electrodeposited Nanocomposites. Surf. Coat. Technol. 2011, 205, 4832–4837. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Chen, S.M. Design and Construction of the Gadolinium Oxide Nanorod-Embedded Graphene Aerogel: A Potential Application for Electrochemical Detection of Postharvest Fungicide. ACS Appl. Mater. Interfaces 2020, 12, 16216–16226. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yi, Y.; Sun, H.; Wang, K.; Han, Z.; Wu, X. Cyclodextrins functionalized hollow carbon nanospheres by introducing nanogold for enhanced electrochemical sensing of o-dihydroxybenzene and p-dihydroxybenzene. J. Mater. Chem. B 2015, 3, 45–52. [Google Scholar] [CrossRef]
- Han, H.S.; You, J.M.; Seol, H.; Jeong, H.; Jeon, S. Electrochemical Sensor for Hydroquinone and Catechol Based on Electrochemically Reduced GO-Terthiophene-CNT. Sens. Actuators B Chem. 2014, 194, 460–469. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Xu, S.; Liu, A.; Da, L.; Lin, D.; Jiang, C. Photoinduced Electron Transfer-Triggered g-C3N4/Rhodamine B Sensing System for the Ratiometric Fluorescence Quantitation of Carbendazim. Anal. Chem. 2023, 95, 4536–4542. [Google Scholar] [CrossRef]



| Real Samples | CBZ Spiked (µM) | CBZ Found (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Apple | 10 | 4.86 | 97.29 | 2.01 |
| 20 | 10.27 | 102.69 | 1.88 | |
| 30 | 14.86 | 99.09 | 0.66 | |
| Carrot | 10 | 4.99 | 99.83 | 0.14 |
| 20 | 10.02 | 100.16 | 0.14 | |
| 30 | 14.99 | 99.94 | 0.05 | |
| Broccoli | 10 | 4.85 | 96.94 | 2.15 |
| 20 | 10.30 | 103.05 | 2.09 | |
| 30 | 14.85 | 98.98 | 0.71 | |
| Grapes | 10 | 5.18 | 103.6 | 2.5 |
| 20 | 9.64 | 96.40 | 2.59 | |
| 30 | 15.18 | 101.2 | 0.84 | |
| Blueberry | 10 | 5.25 | 104.98 | 3.45 |
| 20 | 9.83 | 101.29 | 1.21 | |
| 30 | 14.38 | 95.87 | 2.98 | |
| Tap water | 10 | 5.06 | 101.19 | 0.84 |
| 20 | 9.88 | 98.81 | 0.85 | |
| 30 | 15.05 | 100.39 | 0.25 |
| Sensor Type | Electrode Material | Detection Method | Limit of Detection (LOD) | Linear Range | Reference |
|---|---|---|---|---|---|
| Au-ZrO2-GNs/GCE | Gold and zirconia nanocomposites on graphene sheets | Electrochemical detection | 0.001 µM | - | [5] |
| f-MWCNT/AgNPs | Functionalized multi-walled carbon nanotubes/silver | Electrochemical detection | 0.2778 mM | 0–8 mM | [6] |
| Pd-MWCNTs | Palladium-modified multi-walled carbon nanotubes | Electrochemical detection | 0.13 µM | 0.5–100 µM | [17] |
| NPG/GC | Nano-porous gold on glassy carbon | Electrochemical detection | 0.24 mM | - | [20] |
| NRPC/NiMn@GCE | Nitrogen-rich porous carbon (NRPC) with Ni & Mn | Electrochemical detection | 25.64 µM | 5–50 µM | [this work] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asrafali, S.P.; Periyasamy, T.; Kim, S.C.; Lee, J. Advanced Electrochemical Monitoring of Carbendazim Fungicide in Foods Using Interfacial Superassembly of NRPC/NiMn Frameworks. Biosensors 2024, 14, 474. https://doi.org/10.3390/bios14100474
Asrafali SP, Periyasamy T, Kim SC, Lee J. Advanced Electrochemical Monitoring of Carbendazim Fungicide in Foods Using Interfacial Superassembly of NRPC/NiMn Frameworks. Biosensors. 2024; 14(10):474. https://doi.org/10.3390/bios14100474
Chicago/Turabian StyleAsrafali, Shakila Parveen, Thirukumaran Periyasamy, Seong Cheol Kim, and Jaewoong Lee. 2024. "Advanced Electrochemical Monitoring of Carbendazim Fungicide in Foods Using Interfacial Superassembly of NRPC/NiMn Frameworks" Biosensors 14, no. 10: 474. https://doi.org/10.3390/bios14100474
APA StyleAsrafali, S. P., Periyasamy, T., Kim, S. C., & Lee, J. (2024). Advanced Electrochemical Monitoring of Carbendazim Fungicide in Foods Using Interfacial Superassembly of NRPC/NiMn Frameworks. Biosensors, 14(10), 474. https://doi.org/10.3390/bios14100474







