Faradaic Impedimetric Immunosensor for Label-Free Point-of-Care Detection of COVID-19 Antibodies Using Gold-Interdigitated Electrode Array
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. SARS-CoV-2 Spike Protein and Serum Samples
2.3. Instruments, Electrical Contact Pad, and PDMS Mask
2.4. IDA Fabrication and Functionalization
2.5. Faradaic EIS Drift Signal Stabilization Protocol
2.6. Spike Protein Immobilization
2.7. Faradaic EIS Immunoassay of SARS-CoV-2 Antibodies
3. Results and Discussion
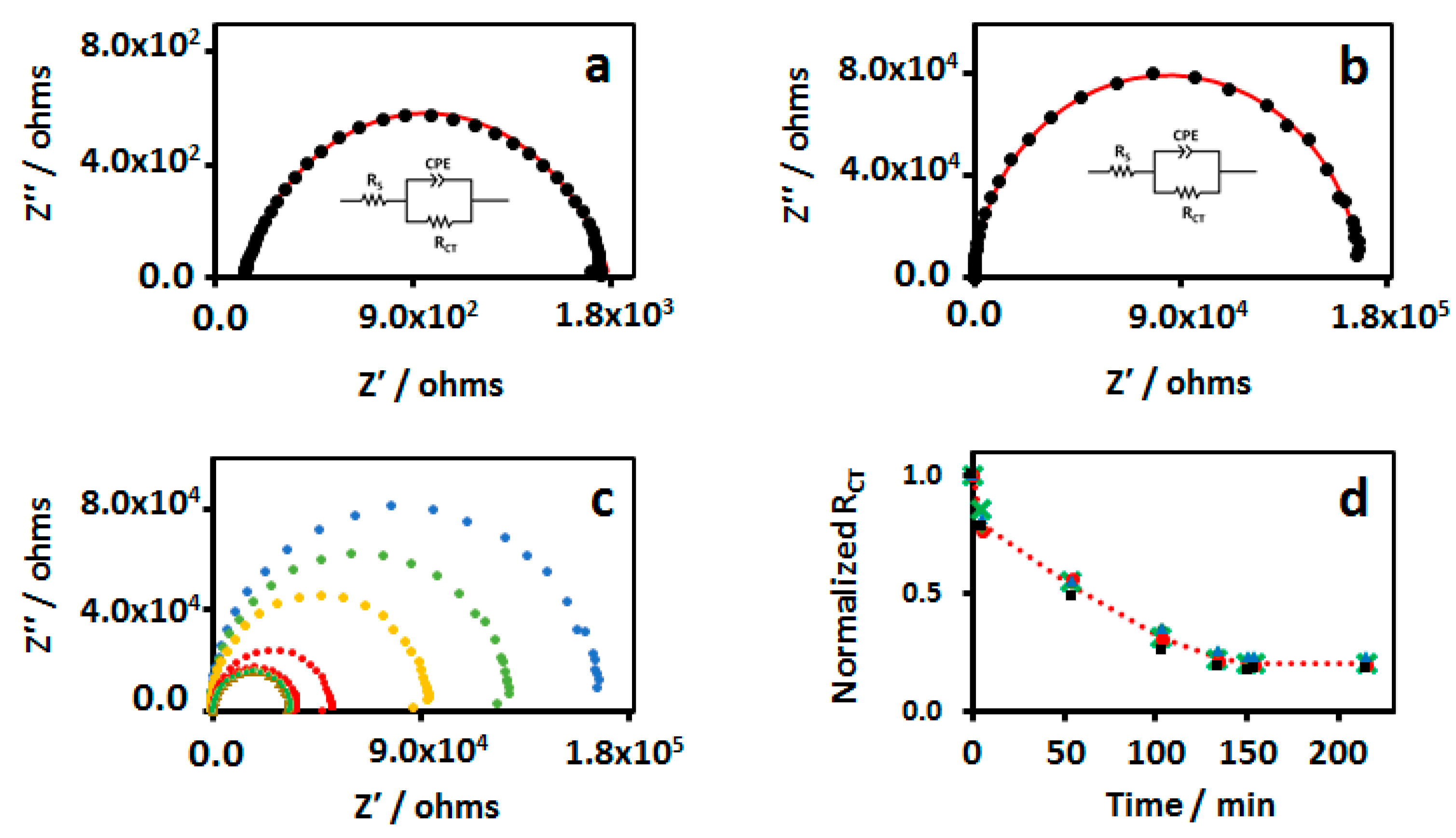
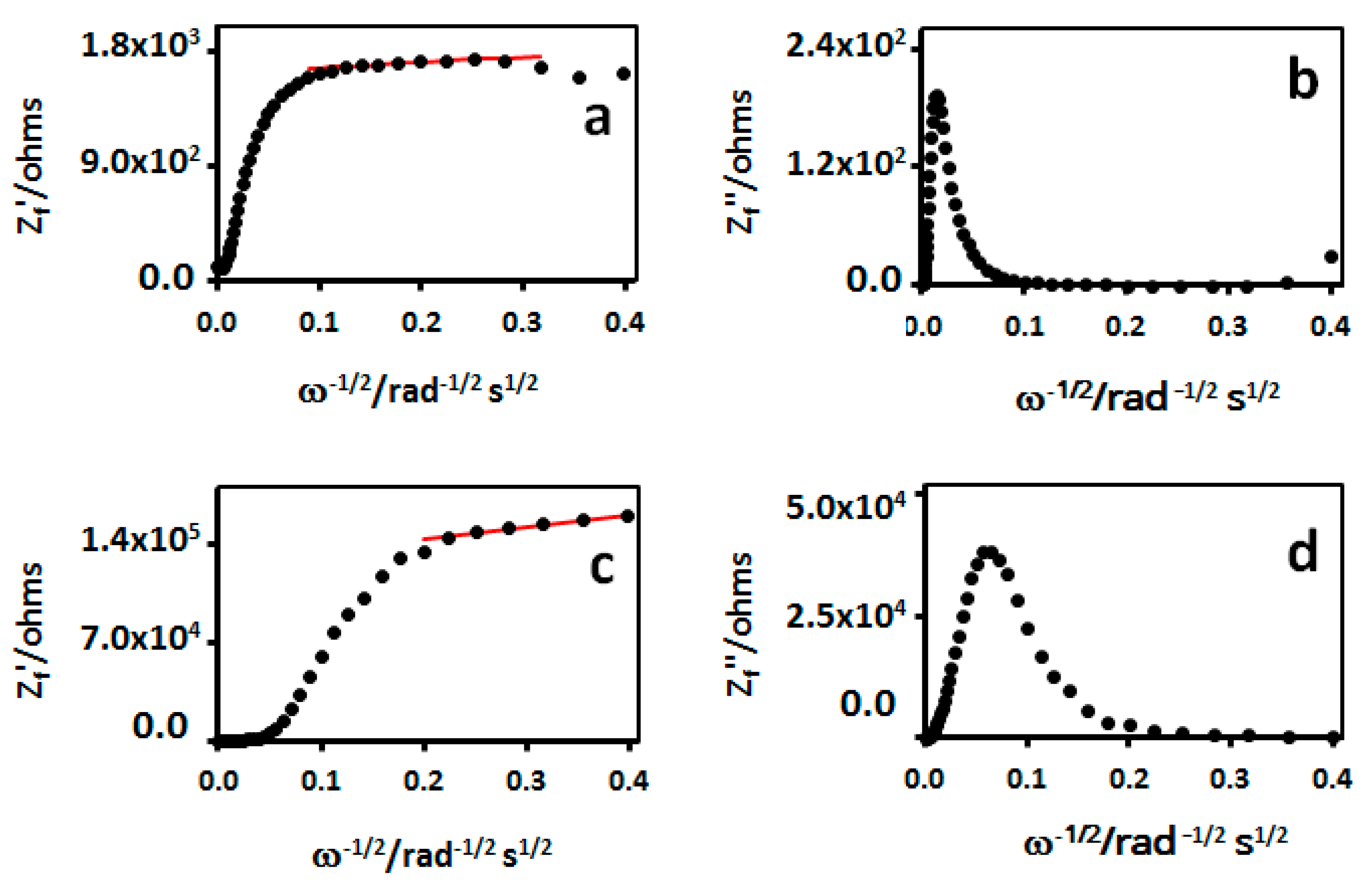
3.1. Interfacial Charge-Transfer and Dynamics in Freshly Prepared MHA Au-IDA
3.2. Impedance Characterization of Defects in MHA Au-IDA
3.3. Standard Curve for Anti-SARS-CoV-2 Antibody Test Using MHA Au-IDA Biosensor
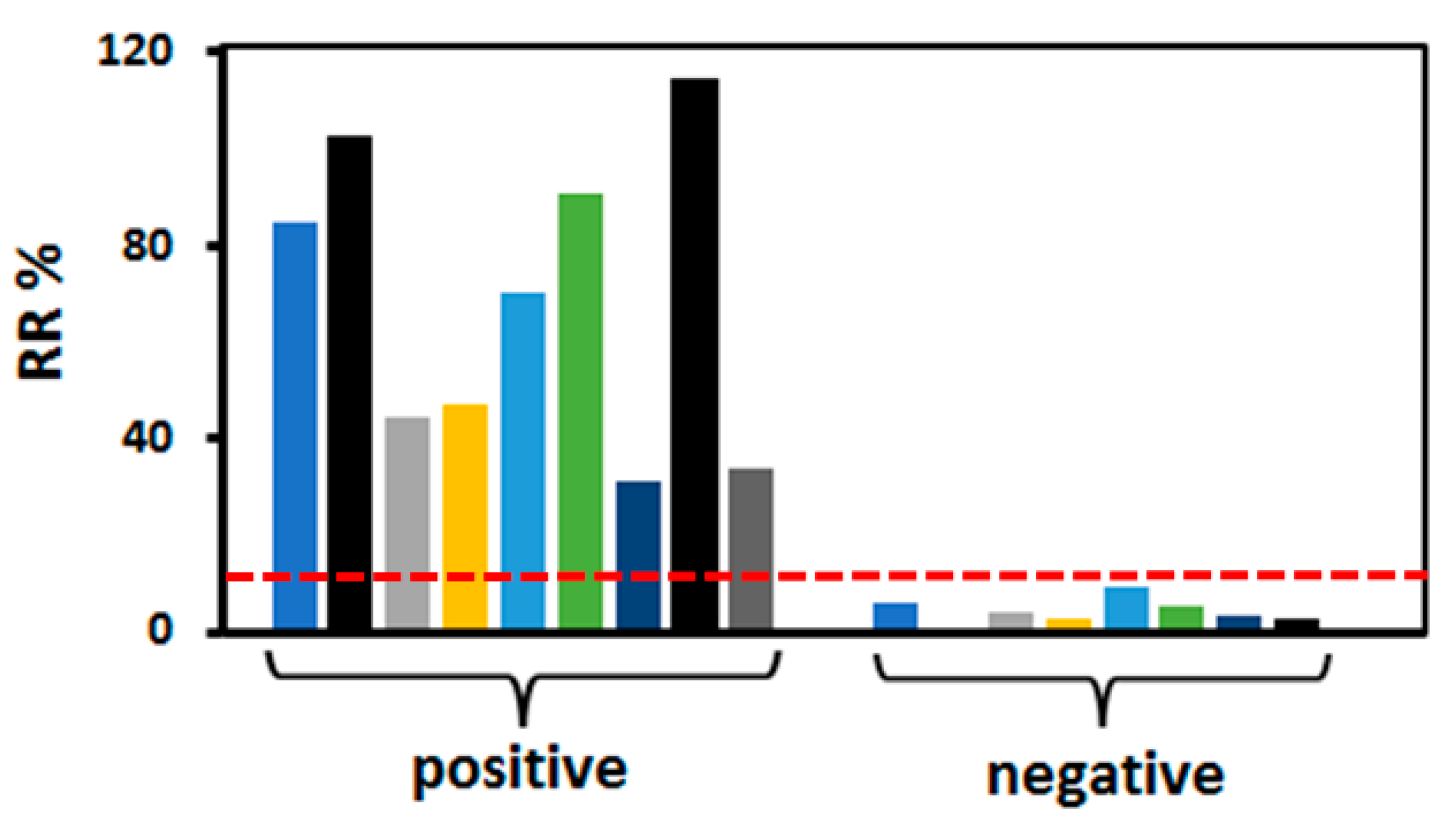
3.4. Performance of MHA Au-IDA Biosensor to Detect Anti-SARS-CoV-2 Antibodies in Human Serum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization Declares COVID-19 a “Pandemic”. Available online: https://time.com/5791661/who-coronavirus-pandemic-declaration/ (accessed on 11 March 2020).
- Yu, F.; Xiang, R.; Deng, X.; Wang, L.; Yu, Z.; Tian, S.; Liang, R.; Li, Y.; Ying, T.; Jiang, S. Receptor-binding domain-specific human neutralizing monoclonal antibodies against SARS-CoV and SARS-CoV-2. Signal Transduct. Target. Ther. 2020, 5, 212. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, L.; Rocha, C.; Sidarovich, A.; Kempf, A.; Schulz, S.; Cossmann, A.; Manger, B.; Baier, E.; Tampe, B.; et al. Comparable neutralisation evasion of SARS-CoV-2 omicron subvariants BA.1, BA.2, and BA.3. Lancet 2020, 22, 766. [Google Scholar] [CrossRef]
- da Silva, S.J.R.; Koh, A.; Pena, L.; Pardee, K. Recent insights into SARS-CoV-2 omicron variant. Rev. Med. Virol. 2023, 33, e2373. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Davis, J.J. Point of Care Sensors for Infectious Pathogens. Anal. Chem. 2021, 93, 184–197. [Google Scholar] [CrossRef]
- Song, Q.; Sun, X.; Dai, Z.; Gao, Y.; Gong, X.; Zhou, B.; Wu, J.; Wen, W. Point-of-care testing detection methods for COVID-19. Lab. A Chip 2021, 21, 1634. [Google Scholar] [CrossRef]
- Rasmi, Y.; Li, X.; Khan, J.; Ozer, T.; Choi, J.R. Emerging point-of-care biosensors for rapid diagnosis of COVID-19: Current progress, challenges, and future prospects. Anal. Bioanal. Chem. 2021, 413, 4137. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Martín, J.; Tena, N.; Asuero, A.G. Current state of diagnostic, screening and surveillance testing methods for COVID-19 from an analytical chemistry point of view. Microchem. J. 2021, 167, 106305. [Google Scholar] [CrossRef] [PubMed]
- Inaba, M.; Higashimoto, Y.; Toyama, Y.; Horiguchi, T.; Hibino, M.; Iwata, M.; Imaizumi, K.; Doi, Y. Diagnostic accuracy of LAMP versus PCR over the course of SARS-CoV-2 infection. Int. J. Infect. Dis. 2021, 107, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Mallett, S.; Allen, A.J.; Graziadio, S.; Taylor, S.A.; Sakai, N.S.; Green, K.; Suklan, J.; Hyde, C.; Shinkins, B.; Zhelev, Z.; et al. At what times during infection is SARS- CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med. 2020, 18, 346. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Gattuso, G.; Tsatsakis, A.; Spandidos, D.A.; Libra, M. Current and innovative methods for the diagnosis of COVID-19 infection (Review). Int. J. Mol. Med. 2021, 47, 100. [Google Scholar] [CrossRef] [PubMed]
- Barlev-Gross, M.; Weiss, S.; Ben-Shmuel, A.; Sittner, A.; Eden, K.; Mazuz, N.; Glinert, I.; Bar-David, E.; Puni, R.; Amit, S.; et al. Spike vs nucleocapsid SARS-CoV-2 antigen detection: Application in nasopharyngeal swab specimens. Anal. Bioanal. Chem. 2021, 413, 3501–3510. [Google Scholar] [CrossRef] [PubMed]
- Kyosei, Y.; Yamura, S.; Namba, M.; Yoshimura, T.; Watabe, S.; Ito, E. Antigen tests for COVID-19. Biophys. Physicobiol. 2021, 18, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Mak, G.C.; Cheng, P.K.; Lau, S.S.; Wong, K.K.; Lau, C.S.; Lam, E.T.; Chan, R.C.; Tsang, D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020, 129, 104500. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Sakai-tagawa, Y.; Koga, M.; Akasaka, O. Comparison of Rapid Antigen Tests for COVID-19. Viruses 2020, 12, 1420. [Google Scholar] [CrossRef]
- Liu, G.; Rusling, J.F. COVID-19 Antibody Tests and Their Limitations. ACS Sens. 2021, 6, 593–612. [Google Scholar] [CrossRef]
- Bastos, M.L.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic accuracy of serological tests for covid-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516. [Google Scholar] [CrossRef]
- Lee, C.Y.P.; Lin, R.T.P.; Renia, L.; Ng, L.F.P. Serological Approaches for COVID-19: Epidemiologic Perspective on Surveillance and Control. Front. Immunol. 2020, 11, 879. [Google Scholar] [CrossRef]
- Fu, Z.; Rais, Y.; Dara, D.; Jackson, D.; Drabovich, A.P. Rational Design and Development of SARS-CoV-2 Serological Diagnostics by Immunoprecipitation-Targeted Proteomics. Anal. Chem. 2022, 94, 12990–12999. [Google Scholar] [CrossRef]
- Patel, E.U.; Bloch, E.M.; Clarke, W.; Hsieh, Y.H.; Boon, D.; Eby, Y.; Fernandez, R.E.; Baker, O.R.; Keruly, M.; Kirby, C.S.; et al. Comparative Performance of Five Commercially Available Serologic Assays to Detect Antibodies to SARS-CoV-2 and Identify Individuals with High Neutralizing Titers. J. Clin. Microbiol. 2021, 59, e02257-20. [Google Scholar] [CrossRef]
- Spearman, P. Diagnostic testing for SARS-CoV-2/COVID-19. Curr. Opin. Pediatr. 2021, 33, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Charlton, C.L.; Kanji, J.N.; Johal, K.; Bailey, A.; Plitt, S.S.; MacDonald, C.; Kunst, A.; Buss, E.; Burnes, L.E.; Fonseca, K.; et al. Evaluation of Six Commercial Mid- to High-Volume Antibody and Six Point-of-Care Lateral Flow Assays for Detection of SARS-CoV-2 Antibodies. J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Budd, J.; Miller, B.S.; Weckman, N.E.; Cherkaoui, D.; Huang, D.; Decruz, A.T.; Fongwen, N.; Han, G.-R.; Broto, M.; Estcourt, C.S.; et al. Lateral flow test engineering and lessons learned from COVID-19. Nat. Rev. Bioeng. 2023, 1, 13–31. [Google Scholar] [CrossRef]
- Pei, F.; Feng, S.; Hu, W.; Liu, B.; Mu, X.; Hao, Q.; Cao, Y.; Lei, W.; Tong, Z. Sandwich mode lateral flow assay for point-of-care detecting SARS-CoV-2. Talanta 2023, 253, 124051. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Ding, L.; Huang, X.; Xiong, Y. Point-of-careCOVID-19diagnosticspoweredbylateral flowassay. Trends Anal. Chem. 2021, 145, 116452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis. ACS Nano 2021, 15, 3593–3611. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Furst, A.L.; Francis, M.B. Impedance-Based Detection of Bacteria. Chem. Rev. 2019, 119, 700–726. [Google Scholar] [CrossRef]
- Online, V.A.; Mao, S.; Fu, L.; Yin, C.; Liu, X.; Karimi, H. The role of electrochemical biosensors in SARS- and review. RSC Adv. 2022, 12, 22592–22607. [Google Scholar]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Min, J.; Sempionatto, J.R.; Teymourian, H.; Wang, J.; Gao, W. Wearable electrochemical biosensors in North America. Biosens. Bioelectron. 2021, 172, 112750. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Fan, R.; Andrew, T.L. Perspective–Challenges in Developing Wearable Electrochemical Sensors for Longitudinal Health Monitoring. J. Electrochem. Soc. 2020, 167, 037542. [Google Scholar] [CrossRef]
- Barnes, E.O.; Lewis, G.E.M.; Dale, S.E.C.; Compton, R.G. Generator-collector double electrode systems: A review. Analyst 2012, 137, 1068–1081. [Google Scholar] [CrossRef]
- Niwa, O.; Morita, M.; Tabei, H. Electrochemical Behavior of Reversible Redox Species at Interdigitated Array Electrodes with Different Geometries: Consideration of Redox Cycling and Collection Efficiency. Anal. Cham. 1990, 62, 447–452. [Google Scholar] [CrossRef]
- Min, J.; Baeumner, A.J. Characterization and optimization of interdigitated ultramicroelectrode arrays as electrochemical biosensor transducers. Electroanalysis 2004, 16, 724–729. [Google Scholar] [CrossRef]
- Heo, J.I.; Shim, D.S.; Teixidor, G.T.; Oh, S.; Madou, M.J. Carbon Interdigitated Array Nanoelectrodes for Electrochemical. J. Electrochem. Soc. 2011, 158, 76–80. [Google Scholar] [CrossRef]
- Barnes, E.O.; Fernández-La-Villa, A.; Pozo-Ayuso, D.F.; Castaño-Alvarez, M.; Lewis, G.E.; Dale, S.E.; Marken, F.; Compton, R.G. Interdigitated ring electrodes: Theory and experiment. J. Electroanal. Chem. 2013, 709, 57–64. [Google Scholar] [CrossRef][Green Version]
- Sarkar, S.; Lai, S.C.S.; Lemay, S.G. Unconventional Electrochemistry in Micro-/Nanofluidic Systems. Micromachines 2016, 7, 81. [Google Scholar] [CrossRef]
- Sawhney, M.A.; Azzopardi, E.A.; Teixeira, S.R.; Francis, L.W.; Conlan, R.S.; Gazze, S.A. Measuring the impact on impedance spectroscopy of pseudo-reference electrode accumulations. Electrochem. Commun. 2019, 105, 106508. [Google Scholar] [CrossRef]
- Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A.; Selvaganapathy, P.R.; Deen, M.J.; Landheer, D. Microfabricated Reference Electrodes and their Biosensing Applications. Sensors 2010, 10, 1679–1715. [Google Scholar] [CrossRef]
- Shoute, L.C.T.; Abdelrasoul, G.N.; Ma, Y.; Duarte, P.A.; Edwards, C.; Zhuo, R.; Zeng, J.; Feng, Y.; Charlton, C.L.; Kanji, J.N.; et al. Label-free impedimetric immunosensor for point-of-care detection of COVID-19 antibodies. Microsyst. Nanoeng. 2023, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Zukauskas, S.; Rucinskiene, A.; Ratautaite, V.; Ramanaviciene, A.; Pilvenyte, G.; Bechelany, M.; Ramanavicius, A. Electrochemical Biosensor for the Determination of Specific Antibodies against SARS-CoV-2 Spike Protein. Int. J. Mol. Sci. 2023, 24, 718. [Google Scholar] [CrossRef]
- Liustrovaite, V.; Drobysh, M.; Rucinskiene, A.; Baradoke, A.; Ramanaviciene, A.; Plikusiene, I.; Samukaite-Bubniene, U.; Viter, R.; Chen, C.F.; Ramanavicius, A. Towards an Electrochemical Immunosensor for the Detection of Antibodies against SARS-CoV-2 Spike Protein Towards an Electrochemical Immunosensor for the Detection of Antibodies against SARS-CoV-2 Spike Protein. J. Electrochem. Soc. 2022, 169, 37523. [Google Scholar] [CrossRef]
- Garrote, B.L.; Santos, A.; Bueno, P.R. Perspectives on and Precautions for the Uses of Electric Spectroscopic Methods in Label-free Biosensing Applications. ACS Sens. 2019, 4, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Liv, L.; Kayabay, H. An Electrochemical Biosensing Platform for the SARS-CoV-2 Spike Antibody Detection Based on the Functionalised SARS-CoV-2 Spike Antigen Modified Electrode. Chem. Select. 2022, 7, e202200256. [Google Scholar] [CrossRef]
- Sadique, M.A.; Yadav, S.; Ranjan, P.; Khan, R.; Khan, F.; Kumar, A.; Biswas, D. Highly Sensitive Electrochemical Immunosensor Platforms for Dual Detection of SARS-CoV-2 Antigen and Antibody based on Gold Nanoparticle Functionalized Graphene Oxide Nanocomposites. ACS Appl. Bio Mater. 2022, 5, 2421–2430. [Google Scholar] [CrossRef]
- Ali, A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Gao, S.J.; Panat, R. Sensing of COVID-19 Antibodies in Seconds via Aerosol Jet Nanoprinted Reduced-Graphene-Oxide-Coated 3D Electrodes. Adv. Mater. 2021, 33, 2006647. [Google Scholar] [CrossRef]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N. Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Frasco, M.F.; Margarida, A.; Mateus, D.; Sebasti, A.I.; Cruz, T.; Sebastião, A.I.; Matos, A.M.; Carmo, A.; Cruz, T.; et al. Materials Today Bio An ultra-sensitive electrochemical biosensor using the Spike protein for capturing antibodies against SARS-CoV-2 in point-of-care. Mater. Today Bio 2022, 16, 100354. [Google Scholar]
- Nunez, F.A.; Castro, A.C.H.; Oliveira, V.L.; De Lima, A.C.; Oliveira, J.R.; Medeiros, G.X.; De Freddy, A.; Nunez, A.C.H.; Castro, V.L.; de Oliveira, A.C.; et al. Electrochemical Immunosensors Based on Zinc Oxide Nanorods for Detection of Antibodies Against SARS-CoV-2 Spike Protein in Convalescent and Vaccinated Individuals. ACS Biomater. Sci. Eng. 2023, 9, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.C.H.; Bezerra, I.R.S.; Pascon, A.M.; da Silva, G.H.; Philot, E.A.; de Oliveira, V.L.; Mancini, R.S.N.; Schleder, G.R.; Castro, C.E.; de Carvalho, L.R.S.; et al. Modular Label-Free Electrochemical Biosensor. ACS Nano 2022, 16, 14239–14253. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.C.B.; Santos, A.; Martins, D.C.; Góes, M.S.; Bueno, P.R. Biosensors and Bioelectronics Comparing label free electrochemical impedimetric and capacitive biosensing architectures. Biosens. Bioelectronic. 2014, 57, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Dijksma, M.; Boukamp, B.A.; Kamp, B.; Van Bennekom, W.P. Effect of Hexacyanoferrate (II/III) on Self-Assembled Monolayers of Thioctic Acid and 11-Mercaptoundecanoic Acid on Gold. Langmuir 2002, 18, 3105–3112. [Google Scholar] [CrossRef]
- Vogt, S.; Su, Q. Critical View on Electrochemical Impedance Spectroscopy Using the Ferri/Ferrocyanide Redox Couple at Gold Electrodes. Anal. Chem. 2016, 88, 4383–4390. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Xia, H.; Long, Y. Revisiting a classical redox process on a gold electrode by operando ToF-SIMS: Where does the gold go? Chem. Sci. 2019, 10, 6215–6219. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Lee, S.; Chung, M. Enhanced electrochemical biosensing on gold electrodes with a ferri/ferrocyanide redox couple. Analyst 2021, 146, 5236. [Google Scholar]
- Widrig, A.; Porter, M.D. The electrochemical desorption of n-alkanethiol from polycrystalline Au and Ag electrodes monolayers. J. Electroanal. Chem. Interfacial Electrochem. 1991, 310, 335–359. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef]
- Cortés, E.; Rubert, A.A.; Benitez, G.; Carro, P.; Vela, M.E.; Salvarezza, R.C. Enhanced Stability of Thiolate Self-Assembled Monolayers (SAMs) on Nanostructured Gold Substrates. Langmuir 2009, 25, 7280–7285. [Google Scholar] [CrossRef]
- Schlenoff, J.B.; Ly, H. Stability and Self-Exchange in Alkanethiol Monolayers. J. Am. Chem. Soc. 1995, 117, 12528–12536. [Google Scholar] [CrossRef]
- Prathima, N.; Harini, M.; Rai, N.; Chandrashekara, R.H.; Ayappa, K.G.; Sampath, S.; Biswas, S.K. Thermal Study of Accumulation of Conformational Disorders in the Self-Assembled Monolayers of C8 and C18 Alkanethiols on the Au (111) Surface. Langmuir 2005, 21, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.; Huang, P.J.; Dave, N.; Liu, J. Dissociation and Degradation of Thiol-Modified DNA on Gold Nanoparticles in Aqueous and Organic Solvents. Langmuir 2011, 27, 6132–6137. [Google Scholar] [CrossRef]
- Chandekar, A.; Sengupta, S.K.; Whitten, J.E. Thermal stability of thiol and silane monolayers: A comparative study. Appl. Surf. Sci. 2010, 256, 2742–2749. [Google Scholar] [CrossRef]
- Hoang, T.K.N.; Deriemaeker, L.; La, V.B.; Finsy, R. Monitoring the simultaneous Ostwald ripening and solubilization of emulsions. Langmuir 2004, 20, 8966–8969. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, I.; Slyozov, V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- González, M.C.R.; Carro, P.; Pensa, E.; Vericat, C.; Salvarezza, R.; Creus, A.H. The Role of a Double Molecular Anchor on the Mobility and Self-Assembly of Thiols on Au(111): The Case of Mercaptobenzoic Acid. Chem. Phys. Chem. 2017, 18, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Vericat, C.; Vela, M.E.; Salvarezza, R.C. Self-assembled monolayers of alkanethiols on Au(111): Surface structures, defects and dynamics. Phys. Chem. Chem. Phys. 2005, 7, 3258–3268. [Google Scholar] [CrossRef]
- Kanyong, P.; Davis, J.J. Homogeneous functional self-assembled monolayers: Faradaic impedance baseline signal drift suppression for high-sensitivity immunosensing of C-reactive protein. J. Electroanal. Chem. 2020, 856, 113675. [Google Scholar] [CrossRef]
- Zeng, J.; Duarte, P.A.; Ma, Y.; Savchenko, O.; Shoute, L.; Khaniani, Y.; Babiuk, S.; Zhuo, R.; Abdelrasoul, G.N.; Charlton, C.; et al. An impedimetric biosensor for COVID-19 serology test and modification of sensor performance via dielectrophoresis force. Biosens. Bioelectron. 2022, 213, 114476. [Google Scholar] [CrossRef]
- Ma, Y.; To, D.; Zeng, J.; Shoute, L.C.; Wu, M.; Babiuk, S.; Zhuo, R.; Charlton, C.; Kanji, J.N.; Babiuk, L.; et al. Improving immunoassay detection accuracy of anti-SARS-CoV-2 antibodies through dual modality validation. Biosens. Bioelectron. X 2022, 11, 100176. [Google Scholar] [CrossRef] [PubMed]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Savéant, J.M.; Tessier, D. Charge Transfer at Partially Blocked Surfaces A Model for The Case of Microscopic Active and Inactive Sites. J. Electroanal. Chem. 1983, 147, 39–51. [Google Scholar] [CrossRef]
- Tokuda, K.; Gueshi, T.; Matsuda, H. Voltammetry at Partially Covered Electrodes Part III. Faradaic Impedance Measurements at Model Electrodes Model. Electrodes. J. Electroanal. Chem. 1979, 102, 41–48. [Google Scholar]
- García-Raya, D.; Madueño, R.; Sevilla, J.M.; Blázquez, M.; Pineda, T. Electrochimica Acta Electrochemical characterization of a 1,8-octanedithiol self-assembled monolayer (ODT-SAM) on a Au(111) single crystal electrode. Electrochim. Acta 2008, 53, 8026–8033. [Google Scholar] [CrossRef]
- Lu, X.; Yuan, H.; Zuo, G.; Yang, J. Study of the size and separation of pinholes in the self-assembled thiol-porphyrin monolayers on gold electrodes. Thin Solid. Films 2008, 516, 6476–6482. [Google Scholar] [CrossRef]
- Sabatani, E.; Rubinstein, I.; Maoz, R.; Sagiv, J. Organized self-assembling monolayers on electrodes: Part, I. Octadecyl derivatives on gold. J. Electroanal. Chem. 1987, 219, 365–371. [Google Scholar] [CrossRef]
- Finklea, H.O.; Snider, D.A.; Fedyk, J.; Sabatani, E.; Gafni, Y.; Rubinstein, I. Characterization of octadecanethiol-coated gold electrodes as microarray electrodes by cyclic voltammetry and ac impedance spectroscopy. Langmuir 1993, 9, 3660–3667. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Zhang, Y. Relationship between Electronic Tunneling Coefficient and Electrode Potential Investigated Using Self-Assembled Alkanethiol Monolayers on Gold Electrodes. J. Phys. Chem. 1993, 97, 11497–11500. [Google Scholar] [CrossRef]
- Janek, R.P.; Fawcett, W.R.; Ulman, A. Impedance Spectroscopy of Self-Assembled Monolayers on Au(111): Sodium Ferrocyanide Charge Transfer at Modified Electrodes. Langmuir 1998, 14, 3011–3018. [Google Scholar] [CrossRef]
- Diao, P.; Jiang, D.; Cui, X.; Gu, D.; Tong, R.; Zhong, B. Studies of structural disorder of self-assembled thiol monolayers on gold by cyclic voltammetry and ac impedance. J. Electroanal. Chem. 1999, 464, 61–67. [Google Scholar] [CrossRef]
- Diao, P.; Guo, M.; Tong, R. Characterization of defects in the formation process of self-assembled thiol monolayers by electrochemical impedance spectroscopy. J. Electroanal. Chem. 2001, 495, 98–105. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Montemayor, C.; Fatás, E.; Pingarrón, J.M. Characterization of alkanethiol-self-assembled monolayers-modified gold electrodes by electrochemical impedance spectroscopy. J. Electroanal. Chem. 2006, 586, 112–121. [Google Scholar] [CrossRef]
- Strutwolf, J.; Sullivan, K.O. Microstructures by Selective Desorption of Self-Assembled Monolayer from Polycrystalline Gold Electrodes. Electroanalysis 2007, 19, 1467–1475. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef]
- Harris, A.R.; Molino, P.J.; Kapsa, R.M.; Clark, G.M.; Paolini, A.G.; Wallace, G.G. Correlation of the impedance and effective electrode area of doped PEDOT modified electrodes for brain–machine interfaces. Analyst 2015, 140, 3164–3174. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.-H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2020, 4250, 1058. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, F.; Zhang, J.; Chen, Q.; Zheng, Z.; Zhou, Q.; Chen, D.; Li, J.; Chen, J. Vaccine design based on 16 epitopes of SARS-CoV-2 spike protein. J. Med. Virol. 2021, 93, 2115–2131. [Google Scholar] [CrossRef] [PubMed]
- Jaago, M.; Rähni, A.; Pupina, N.; Pihlak, A.; Sadam, H. Differential patterns of cross—Reactive antibody response against SARS-CoV-2 spike protein detected for chronically ill and healthy COVID-19 naïve individuals. Sci. Rep. 2022, 12, 16817. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; von Bülow, S.; Blanc, F.E.C.; Gecht, M.; Covino, R.; Hummer, G. Computational epitope map of SARS-CoV-2 spike protein. PLoS Comput. Biol. Comput. Biol. 2021, 17, e1008790. [Google Scholar] [CrossRef] [PubMed]
- Bio-Red Laboratories. Calibration Factors Using WHO International Standard (NIBSC 20/136) for Anti–SARS-CoV-2 Immunoglobulins; Bio-Red Laboratories: Redmond, WA, USA, 2020. [Google Scholar]
- Kanji, J.N.; Pabbaraju, K.; Wong, A.; Beitku, C.; Deo, A. Effect of not vortexing nasopharyngeal and throat swabs on SARS-CoV-2 nucleic acid detection: A pilot study. J. Virol. Methods 2022, 301, 114468. [Google Scholar] [CrossRef] [PubMed]
- Pabbaraju, K.; Zelyas, N.; Wong, A.; Croxen, M.A.; Lynch, T. Evolving strategy for an evolving virus: Development of real-time PCR assays for detecting all SARS-CoV-2 variants of concern. J. Virol. Methods 2022, 307, 114553. [Google Scholar] [CrossRef]
- Zelyas, N.; Pabbaraju, K.; Croxen, M.A.; Lynch, T.; Buss, E.; Murphy, S.A. Precision Response to the Rise of the SARS-CoV-2 B.1.1.7 Variant of Concern by Combining Novel PCR Assays and Genome Sequencing for Rapid Variant Detection and Surveillance. Microbiol. Spectr. 2021, 1, e00315-21. [Google Scholar] [CrossRef]
- Kanji, J.N.; Zelyas, N.; MacDonald, C.; Pabbaraju, K.; Khan, M.N.; Prasad, A.; Hu, J.; Diggle, M.; Berenger, B.M.; Tipples, G. False negative rate of COVID-19 PCR testing: A discordant testing analysis. Virol. J. 2021, 18, 1–6. [Google Scholar] [CrossRef]
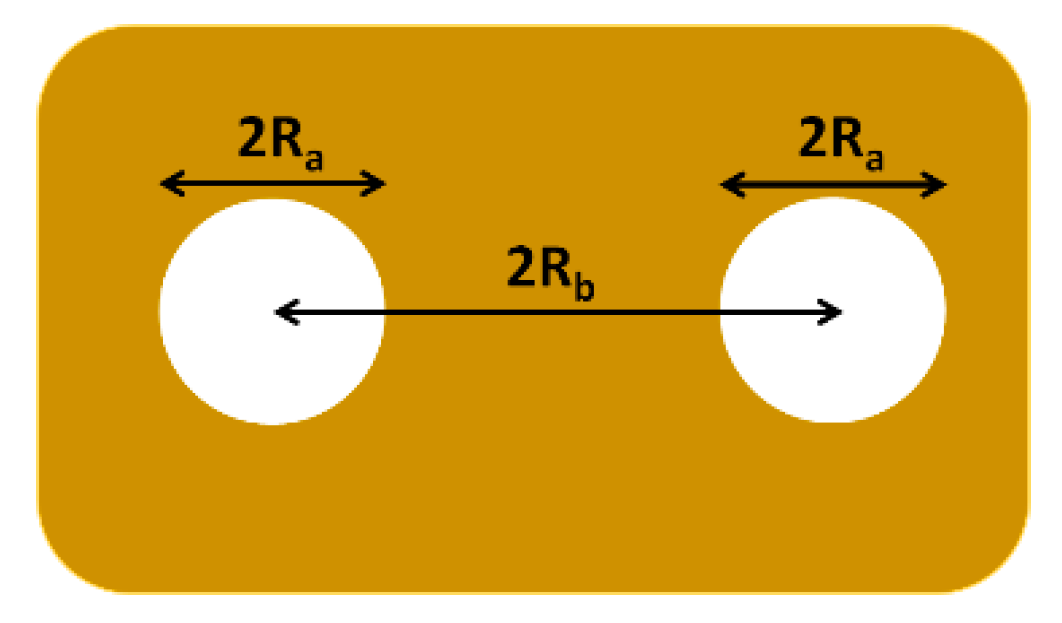
 ) Ra versus θ and (
) Ra versus θ and ( ) Rb versus θ plots. (b) (
) Rb versus θ plots. (b) ( ) Rct versus Ra and (
) Rct versus Ra and ( ) Rct versus Rb plots. The solid lines are linear regression of the experimental data.
) Rct versus Rb plots. The solid lines are linear regression of the experimental data.
 ) Ra versus θ and (
) Ra versus θ and ( ) Rb versus θ plots. (b) (
) Rb versus θ plots. (b) ( ) Rct versus Ra and (
) Rct versus Ra and ( ) Rct versus Rb plots. The solid lines are linear regression of the experimental data.
) Rct versus Rb plots. The solid lines are linear regression of the experimental data.
 ) [Fe(CN)6]−4, (
) [Fe(CN)6]−4, ( ) [Fe(CN)6]−3, (
) [Fe(CN)6]−3, ( ) spike, and (
) spike, and ( ) anit-SARS-CoV-2 antibody. Dashed arrow shows diffusion of [Fe(CN)6]−4 in the pinhole to approach the bare gold surface for interfacial electron transfer.
) anit-SARS-CoV-2 antibody. Dashed arrow shows diffusion of [Fe(CN)6]−4 in the pinhole to approach the bare gold surface for interfacial electron transfer.
 ) [Fe(CN)6]−4, (
) [Fe(CN)6]−4, ( ) [Fe(CN)6]−3, (
) [Fe(CN)6]−3, ( ) spike, and (
) spike, and ( ) anit-SARS-CoV-2 antibody. Dashed arrow shows diffusion of [Fe(CN)6]−4 in the pinhole to approach the bare gold surface for interfacial electron transfer.
) anit-SARS-CoV-2 antibody. Dashed arrow shows diffusion of [Fe(CN)6]−4 in the pinhole to approach the bare gold surface for interfacial electron transfer.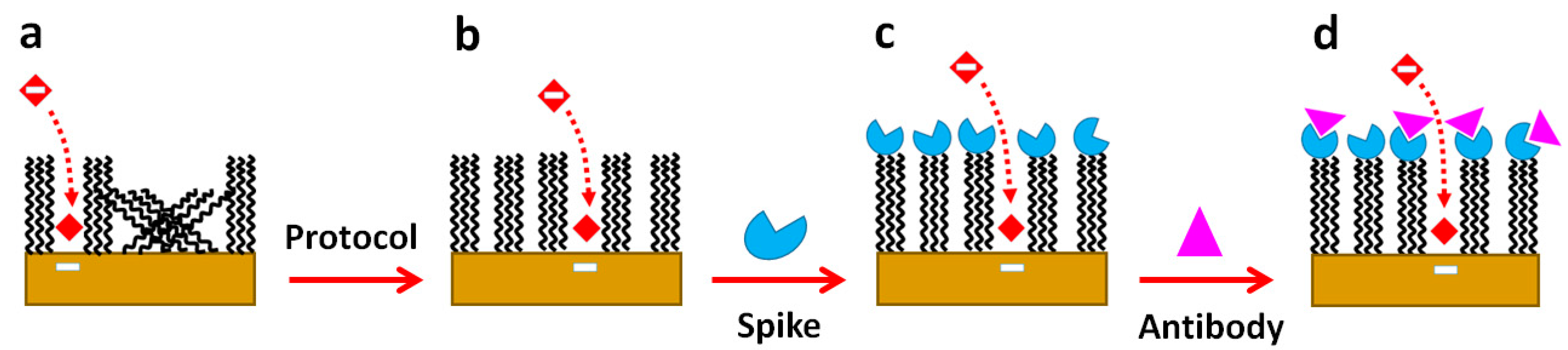
 ) 1% milk solution containinng 1x PBST at pH 7.4 and then in (
) 1% milk solution containinng 1x PBST at pH 7.4 and then in ( ) different concentrations of IgG1 in 0.1% milk solution containing 1x PBST at pH 7.4. IgG1 concentrations were (a) 50 µg/mL, (b) 5 µg/mL, (c) 0.5 µg/mL, and (d) 0.05 µg/mL. EIS spectra were recorded in in 5 mM [Fe(CN)6]−3/4 1x Tri-buffered solution at pH 7.4.
) different concentrations of IgG1 in 0.1% milk solution containing 1x PBST at pH 7.4. IgG1 concentrations were (a) 50 µg/mL, (b) 5 µg/mL, (c) 0.5 µg/mL, and (d) 0.05 µg/mL. EIS spectra were recorded in in 5 mM [Fe(CN)6]−3/4 1x Tri-buffered solution at pH 7.4.
 ) 1% milk solution containinng 1x PBST at pH 7.4 and then in (
) 1% milk solution containinng 1x PBST at pH 7.4 and then in ( ) different concentrations of IgG1 in 0.1% milk solution containing 1x PBST at pH 7.4. IgG1 concentrations were (a) 50 µg/mL, (b) 5 µg/mL, (c) 0.5 µg/mL, and (d) 0.05 µg/mL. EIS spectra were recorded in in 5 mM [Fe(CN)6]−3/4 1x Tri-buffered solution at pH 7.4.
) different concentrations of IgG1 in 0.1% milk solution containing 1x PBST at pH 7.4. IgG1 concentrations were (a) 50 µg/mL, (b) 5 µg/mL, (c) 0.5 µg/mL, and (d) 0.05 µg/mL. EIS spectra were recorded in in 5 mM [Fe(CN)6]−3/4 1x Tri-buffered solution at pH 7.4.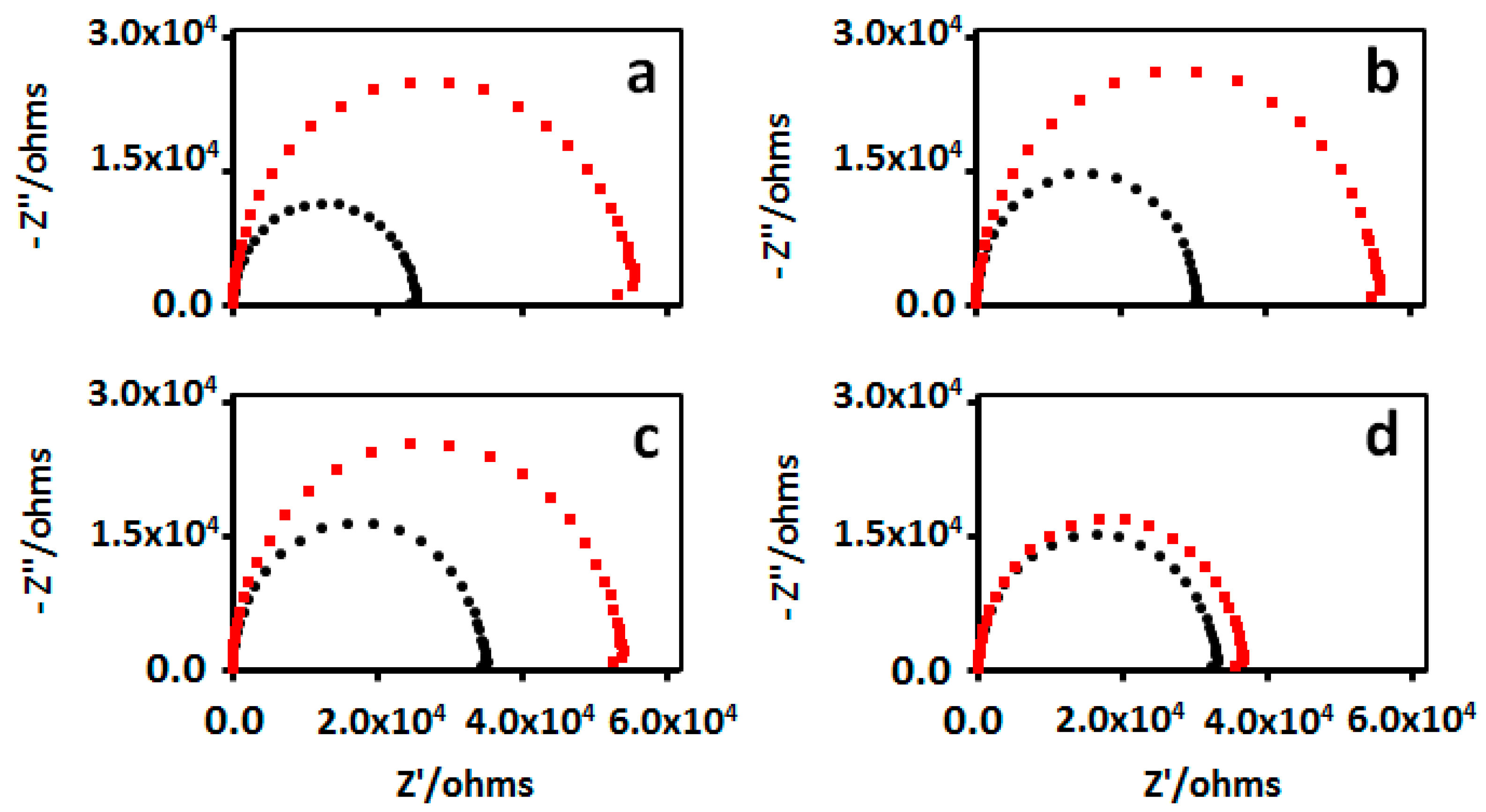
 ) Experimental data, (
) Experimental data, ( )% relative standard deviation (n = 3), and linear regression (
)% relative standard deviation (n = 3), and linear regression ( ) to the experimental data points yielded RR% = 35.43 log [IgG] − 46.98, R2 = 0.995.
) to the experimental data points yielded RR% = 35.43 log [IgG] − 46.98, R2 = 0.995.
 ) Experimental data, (
) Experimental data, ( )% relative standard deviation (n = 3), and linear regression (
)% relative standard deviation (n = 3), and linear regression ( ) to the experimental data points yielded RR% = 35.43 log [IgG] − 46.98, R2 = 0.995.
) to the experimental data points yielded RR% = 35.43 log [IgG] − 46.98, R2 = 0.995.
| Time/Min | Rct/kohm | θ | Cs, µF/cm2 | Ra, µm | Rb, µm |
|---|---|---|---|---|---|
| 0 | 168.0 | 0.996 | 1.34 | 2.14 | 36.33 |
| 5 | 129.6 | 0.995 | 1.38 | 1.90 | 29.95 |
| 54 | 94.0 | 0.994 | 1.51 | 1.70 | 23.48 |
| 103 | 51.4 | 0.992 | 1.38 | 1.20 | 15.58 |
| 134 | 37.2 | 0.959 | 1.53 | 1.07 | 5.26 |
| 150 | 33.9 | 0.965 | 1.33 | 0.95 | 4.10 |
| 154 | 34.5 | 0.957 | 1.30 | 0.95 | 4.58 |
| 214 | 34.1 | 0.968 | 1.32 | 0.95 | 5.36 |
| Electrode | Electrode Modification | Technique | Recognition Element | Analyte | LOD | References |
|---|---|---|---|---|---|---|
| Au-AuNC | MUA-MHOH/EDC/NHS | CV | Spike | IgG | 51.0 ng/mL | Zukauskas et al. [44] |
| DPV | Spike | IgG | 91.5 ng/mL | |||
| PPA | Spike | IgG | 105.0 ng/mL | |||
| Au | MUA-MHOH/EDC/NHS | CV | Spike | IgG | 379.5 ng/mL | Liustrovaite et al. [45] |
| EIS | Spike | IgG | 298.5 ng/mL | |||
| GCE/Au | CysNH2/EDC/NHS | SWV | Spike | IgG | 9.3 ag/mL | Liv et al. [47] |
| GCE-GO−Au | GO-COOH/EDC/NHS | DPV | N-protein | IgG | 1.0 fg/mL | Sadique et al. [48] |
| Au/rGO | rGO-COOH/EDC/NHS | EIS | S1 spike | IgG | 0.42 pg/mL | Ali et al. [49] |
| EIS | RBD | IgG | 2.54 pg/mL | |||
| ePAD-GO | GO-COOH/EDC/NHS | SWV | RBD | IgG | 0.96 ng/mL | Yakoh et al. [50] |
| SPE-SWCNT | CNT-COOH/EDC/NHS | EIS | Spike | IgG | 0.7 pg/mL | Cardoso et al. [51] |
| FTO-ZnONR | Physisorbed/Electrostatic | EIS | Spike | IgG | 19.34 ng/mL | Nunez et al. [52] |
| GCE-AuNP | Physisorbed | EIS/SWV | Spike | IgG | 0.2 μg/mL | Castro et al. [53] |
| Au IDA | MHA/EDC/NHS | EIS | Spike | IgG | 21 ng/mL | This work |
| Au IDA | MUA/EDC/NHS | Non-Faradaic | Spike | IgG | 148.1 ng/mL | Shoute et al. [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoute, L.C.T.; Charlton, C.L.; Kanji, J.N.; Babiuk, S.; Babiuk, L.; Chen, J. Faradaic Impedimetric Immunosensor for Label-Free Point-of-Care Detection of COVID-19 Antibodies Using Gold-Interdigitated Electrode Array. Biosensors 2024, 14, 6. https://doi.org/10.3390/bios14010006
Shoute LCT, Charlton CL, Kanji JN, Babiuk S, Babiuk L, Chen J. Faradaic Impedimetric Immunosensor for Label-Free Point-of-Care Detection of COVID-19 Antibodies Using Gold-Interdigitated Electrode Array. Biosensors. 2024; 14(1):6. https://doi.org/10.3390/bios14010006
Chicago/Turabian StyleShoute, Lian C. T., Carmen L. Charlton, Jamil N. Kanji, Shawn Babiuk, Lorne Babiuk, and Jie Chen. 2024. "Faradaic Impedimetric Immunosensor for Label-Free Point-of-Care Detection of COVID-19 Antibodies Using Gold-Interdigitated Electrode Array" Biosensors 14, no. 1: 6. https://doi.org/10.3390/bios14010006
APA StyleShoute, L. C. T., Charlton, C. L., Kanji, J. N., Babiuk, S., Babiuk, L., & Chen, J. (2024). Faradaic Impedimetric Immunosensor for Label-Free Point-of-Care Detection of COVID-19 Antibodies Using Gold-Interdigitated Electrode Array. Biosensors, 14(1), 6. https://doi.org/10.3390/bios14010006






