Screen-Printed Textile-Based Electrochemical Biosensor for Noninvasive Monitoring of Glucose in Sweat
Abstract
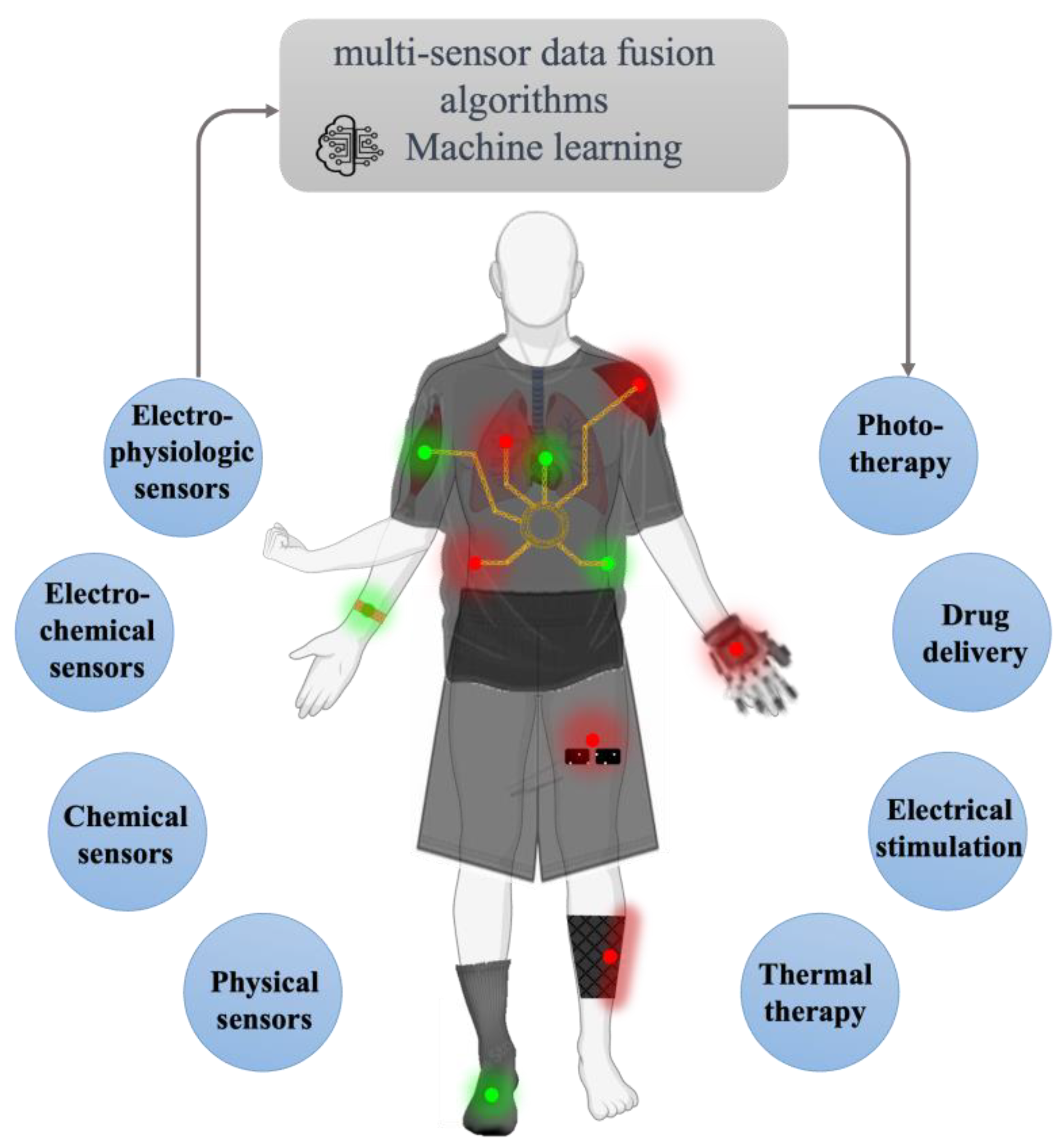
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
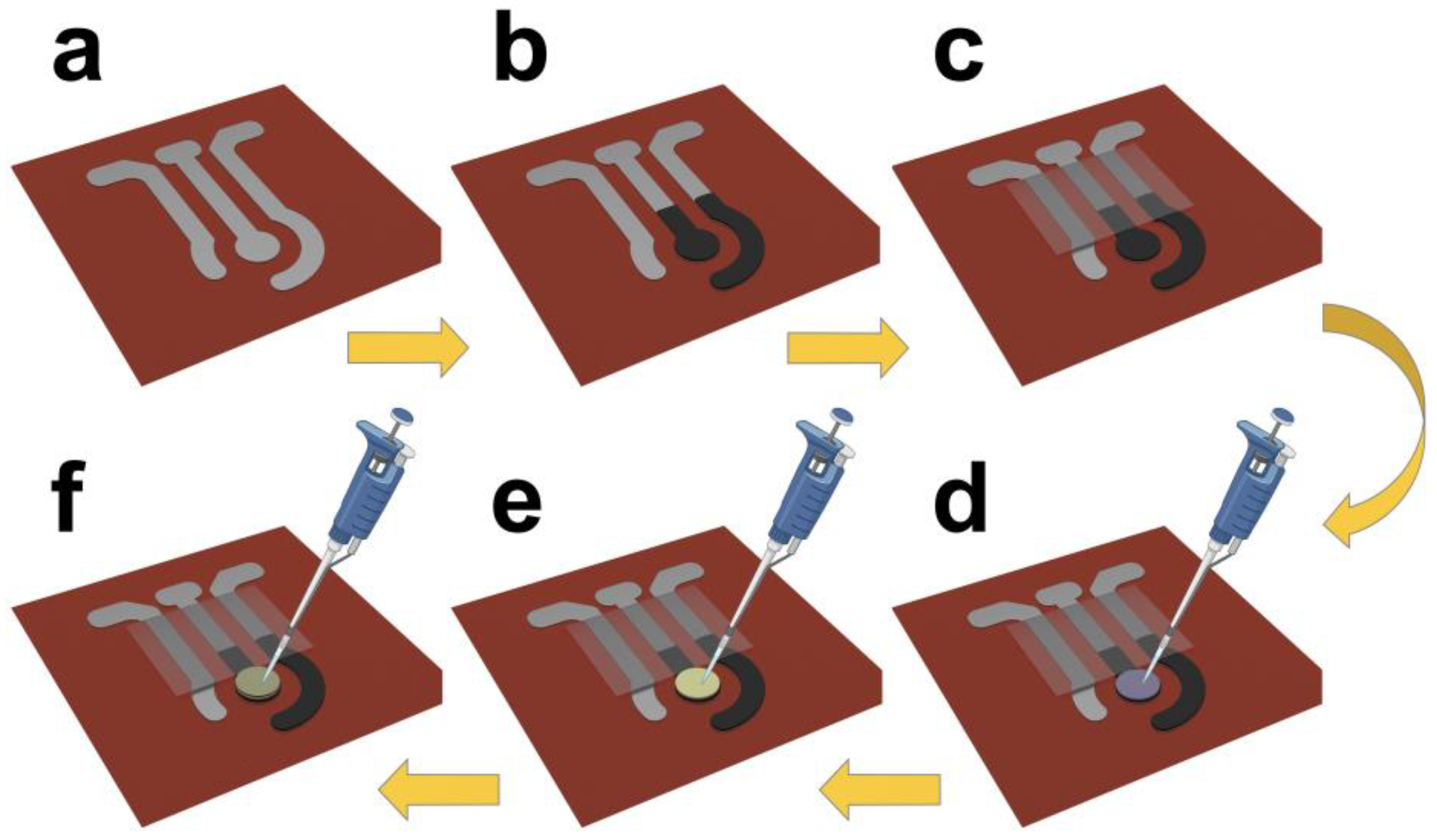
2.2. Fabrication of Working Electrode
2.3. GOx Immobilization
2.4. Characterization
2.5. On-Body Validation of Textile-Based Glucose Sensor
3. Results and Discussion
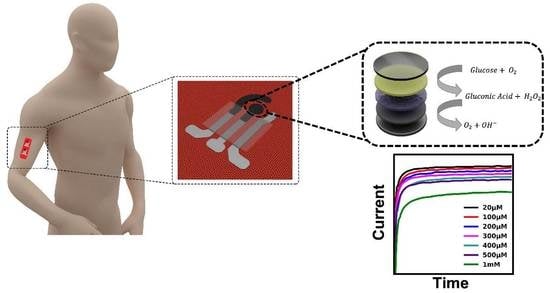
3.1. System Design of Wearable Textile-Based Glucose Sensor
3.2. CV Characterization of PB-Carbon Paste
3.3. CV Characterization of the Glucose Sensor in the Presence and Absence of GOx
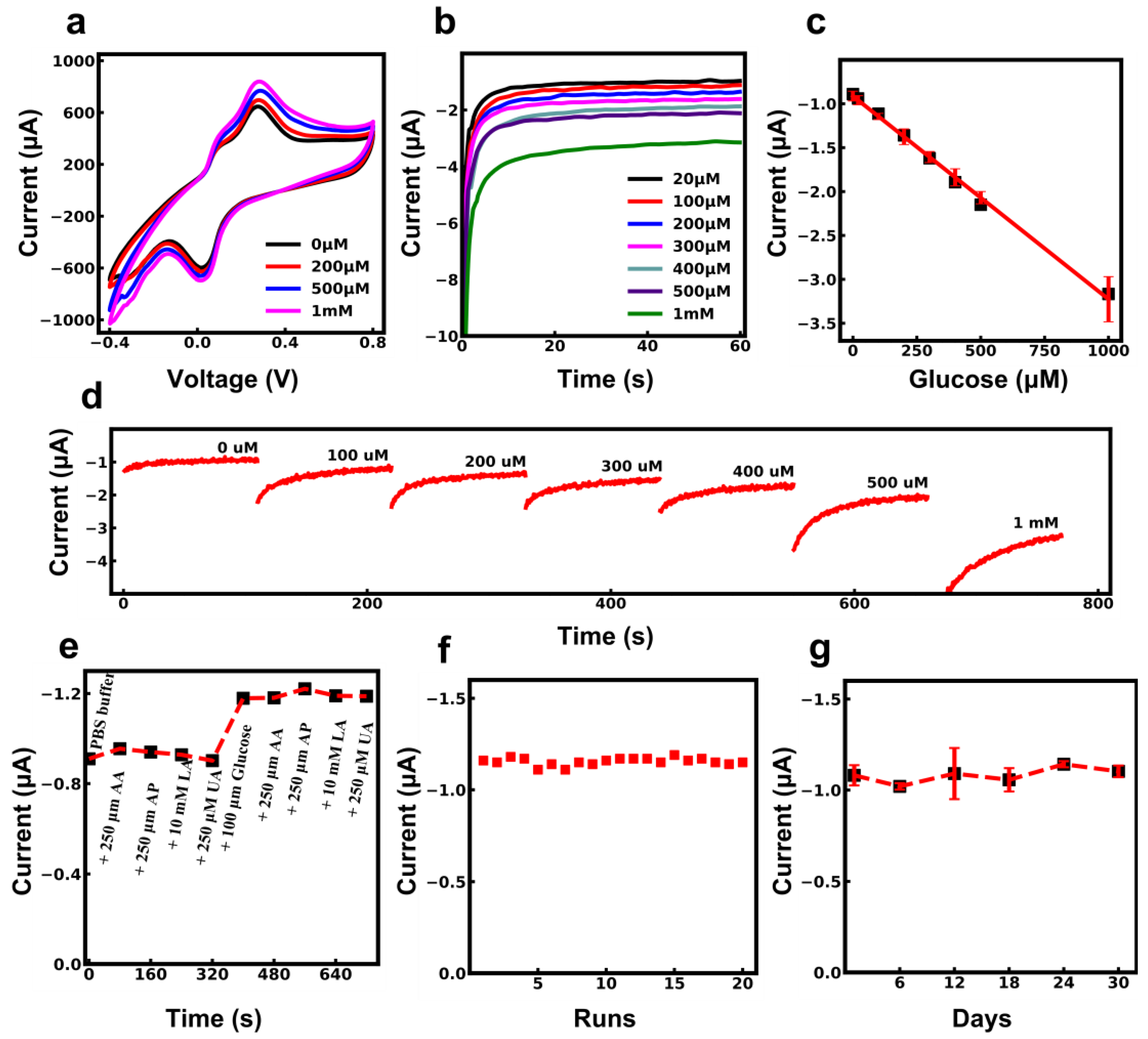
3.4. Electrochemical Characterization of Textile-Based Glucose Sensor
3.5. Stability Evaluation
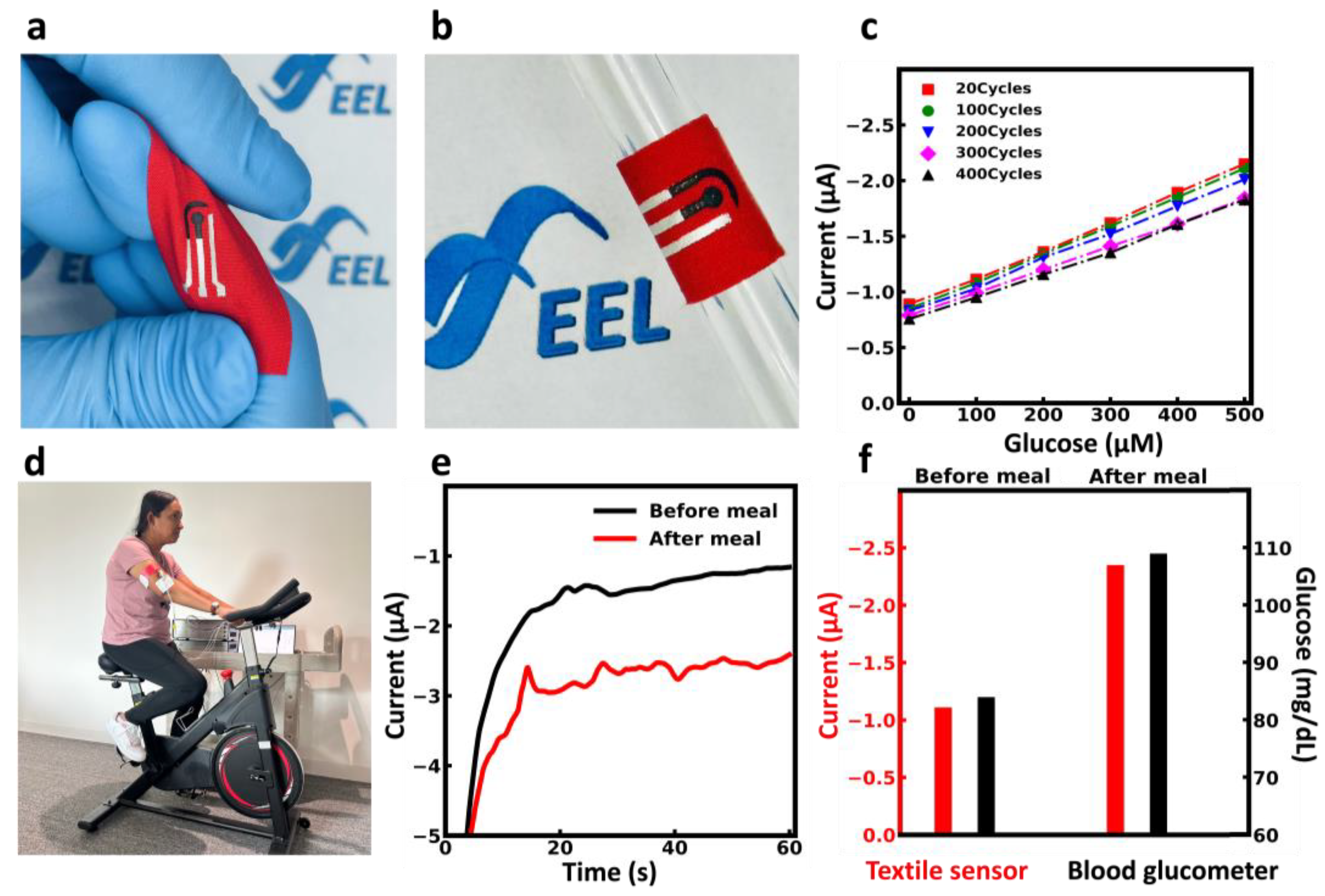
3.6. Flexibility Evaluation
3.7. On-Body Measurement
4. Current Challenges and Future Perspectives for the Wearable Textile Biosensing Platform
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Hartel, M.C.; Yu, N.; Garrido, P.R.; Kim, S.; Lee, J.; Bandaru, P.; Guan, S.; Lin, H.; Emaminejad, S.; et al. Epidermis-Inspired Wearable Piezoresistive Pressure Sensors Using Reduced Graphene Oxide Self-Wrapped Copper Nanowire Networks. Small Methods 2021, 6, 2100900. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, X.; Hu, H.; Wang, X.; Yue, W.; Mu, J.; Lou, Z.; Zhang, R.; Shi, K.; Chen, X.; et al. A photoacoustic patch for three-dimensional imaging of hemoglobin and core temperature. Nat. Commun. 2022, 13, 7757. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Haghniaz, R.; Hartel, M.C.; Guan, S.; Bahari, J.; Li, Z.; Baidya, A.; Cao, K.; Gao, X.; Li, J.; et al. A Breathable, Passive-Cooling, Non-Inflammatory, and Biodegradable Aerogel Electronics for Wearable Physical-Electrophysiological-Chemical Analysis. Adv. Mater. 2023, 35, 2209300. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Abidian, M.R.; Ahn, J.-H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology Roadmap for Flexible Sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef]
- Goud, K.Y.; Sandhu, S.S.; Teymourian, H.; Yin, L.; Tostado, N.; Raushel, F.M.; Harvey, S.P.; Moores, L.C.; Wang, J. Textile-based wearable solid-contact flexible fluoride sensor: Toward biodetection of G-type nerve agents. Biosens. Bioelectron. 2021, 182, 113172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015; Chapter 1; p. 12. [Google Scholar]
- Makaram, P.; Owens, D.; Aceros, J. Trends in Nanomaterial-Based Non-Invasive Diabetes Sensing Technologies. Diagnostics 2014, 4, 27–46. [Google Scholar] [CrossRef]
- Coster, S.; Gulliford, M.C.; Seed, P.T.; Powrie, J.K.; Swaminathan, R. Monitoring blood glucose control in diabetes mellitus: A systematic review. Health Technol. Assess. 2000, 4, 1. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2012, 3, 4473–4491. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Kim, J.; Sempionatto, J.R.; Imani, S.; Hartel, M.C.; Barfidokht, A.; Tang, G.; Campbell, A.S.; Mercier, P.P.; Wang, J. Simultaneous Monitoring of Sweat and Interstitial Fluid Using a Single Wearable Biosensor Platform. Adv. Sci. 2018, 5, 1800880. [Google Scholar] [CrossRef]
- Yokus, M.A.; Songkakul, T.; Pozdin, V.A.; Bozkurt, A.; Daniele, M.A. Wearable multiplexed biosensor system toward continuous monitoring of metabolites. Biosens. Bioelectron. 2020, 153, 112038. [Google Scholar] [CrossRef] [PubMed]
- Abellán-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallón, E. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Kim, J.; Li, S.; Zhao, Y.; Bahari, J.; Eliahoo, P.; Li, G.; Kawakita, S.; Haghniaz, R.; et al. Skin-interfaced electronics: A promising and intelligent paradigm for personalized healthcare. Biomaterials 2023, 296, 122075. [Google Scholar] [CrossRef]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-Based Noninvasive Glucose Monitoring: A Proof-of-Concept Study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef]
- Zhu, Y.; Haghniaz, R.; Hartel, M.C.; Mou, L.; Tian, X.; Garrido, P.R.; Wu, Z.; Hao, T.; Guan, S.; Ahadian, S.; et al. Recent Advances in Bioinspired Hydrogels: Materials, Devices, and Biosignal Computing. ACS Biomater. Sci. Eng. 2021, 9, 2048–2069. [Google Scholar] [CrossRef] [PubMed]
- De Pascali, C.; Francioso, L.; Giampetruzzi, L.; Rescio, G.; Signore, M.A.; Leone, A.; Siciliano, P. Modeling, Fabrication and Integration of Wearable Smart Sensors in a Monitoring Platform for Diabetic Patients. Sensors 2021, 21, 1847. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Moon, J.-M.; Wang, J. Touch-Based Fingertip Blood-Free Reliable Glucose Monitoring: Personalized Data Processing for Predicting Blood Glucose Concentrations. ACS Sensors 2021, 6, 1875–1883. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Li, Z.; Lu, T.J.; Lin, H.; Zhu, Y.; Ahadian, S.; Emaminejad, S.; Dokmeci, M.R.; Xu, F.; et al. Harnessing the wide-range strain sensitivity of bilayered PEDOT:PSS films for wearable health monitoring. Matter 2021, 4, 2886–2901. [Google Scholar] [CrossRef]
- Malon, R.S.P.; Chua, K.Y.; Wicaksono, D.H.B.; Córcoles, E.P. Cotton fabric-based electrochemical device for lactate measurement in saliva. Analyst 2014, 139, 3009–3016. [Google Scholar] [CrossRef]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef]
- Chen, X.; Gao, X.; Nomoto, A.; Shi, K.; Lin, M.; Hu, H.; Gu, Y.; Zhu, Y.; Wu, Z.; Chen, X.; et al. Fabric-substrated capacitive biopotential sensors enhanced by dielectric nanoparticles. Nano Res. 2021, 14, 3248–3252. [Google Scholar] [CrossRef]
- Luo, C.; Gil, I.; Fernandez-Garcia, R. Textile UHF-RFID antenna sensor for measurements of sucrose solutions in different levels of concentration. Meas. Sci. Technol. 2021, 32, 105112. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.W.; Hong, S.Y.; Lee, G.; Lee, H.; Song, C.; Keum, K.; Jeong, Y.R.; Jin, S.W.; Kim, D.S.; et al. Dynamically Stretchable Supercapacitor for Powering an Integrated Biosensor in an All-in-One Textile System. ACS Nano 2019, 13, 10469–10480. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Stavrakis, A.K.; Stojanović, G.M. Textile-based electrochemical sensors and their applications. Talanta 2022, 244, 123425. [Google Scholar] [CrossRef]
- Piper, A.; Månsson, I.; Khaliliazar, S.; Landin, R.; Hamedi, M.M. A disposable, wearable, flexible, stitched textile electrochemical biosensing platform. Biosens. Bioelectron. 2021, 194, 113604. [Google Scholar] [CrossRef]
- Liu, X.; Lillehoj, P.B. Embroidered electrochemical sensors for biomolecular detection. Lab A Chip 2016, 16, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhai, Q.; Dong, D.; An, T.; Gong, S.; Shi, Q.; Cheng, W. Highly Stretchable and Strain-Insensitive Fiber-Based Wearable Electrochemical Biosensor to Monitor Glucose in the Sweat. Anal. Chem. 2019, 91, 6569–6576. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhai, Q.; An, T.; Gong, S.; Cheng, W. Stretchable gold fiber-based wearable textile electrochemical biosensor for lactate monitoring in sweat. Talanta 2020, 222, 121484. [Google Scholar] [CrossRef]
- Liu, X.; Lillehoj, P.B. Embroidered electrochemical sensors on gauze for rapid quantification of wound biomarkers. Biosens. Bioelectron. 2017, 98, 189–194. [Google Scholar] [CrossRef]
- Mo, L.; Guo, Z.; Yang, L.; Zhang, Q.; Fang, Y.; Xin, Z.; Chen, Z.; Hu, K.; Han, L.; Li, L. Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics. Int. J. Mol. Sci. 2019, 20, 2124. [Google Scholar] [CrossRef]
- Bacalzo, N.P., Jr.; Go, L.P.; Querebillo, C.J.; Hildebrandt, P.; Limpoco, F.T.; Enriquez, E.P. Controlled microwave-hydrolyzed starch as a stabilizer for green formulation of aqueous gold nanoparticle ink for flexible printed electronics. ACS Appl. Nano Mater. 2018, 1, 1247–1256. [Google Scholar] [CrossRef]
- Patil, S.A.; Ryu, C.-H.; Kim, H.-S. Synthesis and Characterization of Copper Nanoparticles (Cu-Nps) using Rongalite as Reducing Agent and Photonic Sintering of Cu-Nps Ink for Printed Electronics. Int. J. Precis. Eng. Manuf. Technol. 2018, 5, 239–245. [Google Scholar] [CrossRef]
- Ferri, J.; Llopis, R.L.; Moreno, J.; Lidón-Roger, J.V.; Garcia-Breijo, E. An investigation into the fabrication parameters of screen-printed capacitive sensors on e-textiles. Text. Res. J. 2020, 90, 1749–1769. [Google Scholar] [CrossRef]
- Gong, X.; Huang, K.; Wu, Y.-H.; Zhang, X.-S. Recent progress on screen-printed flexible sensors for human health monitoring. Sens. Actuators A Phys. 2022, 345, 113821. [Google Scholar] [CrossRef]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Nyberg, N.; Hourlier-Fargette, A.; Model, J.B.; Aranyosi, A.J.; et al. Soft, Skin-Integrated Multifunctional Microfluidic Systems for Accurate Colorimetric Analysis of Sweat Biomarkers and Temperature. ACS Sens. 2019, 4, 379–388. [Google Scholar] [CrossRef]
- Yin, L.; Cao, M.; Kim, K.N.; Lin, M.; Moon, J.-M.; Sempionatto, J.R.; Yu, J.; Liu, R.; Wicker, C.; Trifonov, A.; et al. A stretchable epidermal sweat sensing platform with an integrated printed battery and electrochromic display. Nat. Electron. 2022, 5, 694–705. [Google Scholar] [CrossRef]
- Kim, S.; Lee, B.; Reeder, J.T.; Seo, S.H.; Lee, S.-U.; Hourlier-Fargette, A.; Shin, J.; Sekine, Y.; Jeong, H.; Oh, Y.S.; et al. Soft, skin-interfaced microfluidic systems with integrated immunoassays, fluorometric sensors, and impedance measurement capabilities. Proc. Natl. Acad. Sci. USA 2020, 117, 27906–27915. [Google Scholar] [CrossRef]
- Pedro, B.G.; Marcôndes, D.W.C.; Bertemes-Filho, P. Analytical Model for Blood Glucose Detection Using Electrical Impedance Spectroscopy. Sensors 2020, 20, 6928. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Chinnadayyala, S.R.; Le, H.T.N.; Cho, S. Sensitive Electrochemical Non-Enzymatic Detection of Glucose Based on Wireless Data Transmission. Sensors 2022, 22, 2787. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yan, Z.; Liu, Q. Smartphone-Based Electrochemical Systems for Glucose Monitoring in Biofluids: A Review. Sensors 2022, 22, 5670. [Google Scholar] [CrossRef]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical Glucose Sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Sempionatto, J.R.; Barfidokht, A.; Mishra, R.K.; Campbell, A.S.; Hubble, L.J.; Wang, J. Wearable Bioelectronics: Enzyme-Based Body-Worn Electronic Devices. Accounts Chem. Res. 2018, 51, 2820–2828. [Google Scholar] [CrossRef]
- Khor, S.M.; Choi, J.; Won, P.; Ko, S.H. Challenges and Strategies in Developing an Enzymatic Wearable Sweat Glucose Biosensor as a Practical Point-Of-Care Monitoring Tool for Type II Diabetes. Nanomaterials 2022, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Katseli, V.; Economou, A.; Kokkinos, C. Smartphone-Addressable 3D-Printed Electrochemical Ring for Nonenzymatic Self-Monitoring of Glucose in Human Sweat. Anal. Chem. 2021, 93, 3331–3336. [Google Scholar] [CrossRef]
- Karyakin, A.A. Advances of Prussian blue and its analogues in (bio)sensors. Curr. Opin. Electrochem. 2017, 5, 92–98. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Chen, Y.; Fang, X.; Feng, X. Hydrogen peroxide sensor based on electrodeposited Prussian blue film. J. Appl. Electrochem. 2017, 47, 1261–1271. [Google Scholar] [CrossRef]
- Ahmadalinezhad, A.; Kafi, A.; Chen, A. Glucose biosensing based on the highly efficient immobilization of glucose oxidase on a Prussian blue modified nanostructured Au surface. Electrochem. Commun. 2009, 11, 2048–2051. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Moscone, D.; Palleschi, G.; Killard, A.J. Development of a Hydrogen Peroxide Sensor Based on Screen-Printed Electrodes Modified with Inkjet-Printed Prussian Blue Nanoparticles. Sensors 2014, 14, 14222–14234. [Google Scholar] [CrossRef] [PubMed]
- Aller-Pellitero, M.; Fremeau, J.; Villa, R.; Guirado, G.; Lakard, B.; Hihn, J.-Y.; del Campo, F.J. Electrochromic biosensors based on screen-printed Prussian Blue electrodes. Sens. Actuators B Chem. 2019, 290, 591–597. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef] [PubMed]
- De la Paz, E.; Barfidokht, A.; Rios, S.; Brown, C.; Chao, E.; Wang, J. Extended noninvasive glucose monitoring in the interstitial fluid using an epidermal biosensing patch. Anal. Chem. 2021, 93, 12767–12775. [Google Scholar] [CrossRef]
- Wiorek, A.; Parrilla, M.; Cuartero, M.; Crespo, G.A. Epidermal Patch with Glucose Biosensor: pH and Temperature Correction toward More Accurate Sweat Analysis during Sport Practice. Anal. Chem. 2020, 92, 10153–10161. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, J.; Reddy, V.S.; Ji, D.; Ramakrishna, S.; Xu, L. Flexible Textile-Based Sweat Sensors for Wearable Applications. Biosensors 2023, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Tai, L.-C.; Ngo, Q.P.; Chao, M.; Zhang, G.B.; Gao, W.; Bariya, M.; Bullock, J.; Kim, H.; Fahad, H.M.; et al. A Wearable Microfluidic Sensing Patch for Dynamic Sweat Secretion Analysis. ACS Sens. 2018, 3, 944–952. [Google Scholar] [CrossRef]
- Zhu, Y.; Nasiri, R.; Davoodi, E.; Zhang, S.; Saha, S.; Linn, M.; Jiang, L.; Haghniaz, R.; Hartel, M.C.; Jucaud, V.; et al. A Microfluidic Contact Lens to Address Contact Lens-Induced Dry Eye. Small 2022, 19, 2207017. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Y.; Haghniaz, R.; Kawakita, S.; Guan, S.; Chen, J.; Li, Z.; Mandal, K.; Bahari, J.; Shah, S.; et al. A Microchambers Containing Contact Lens for the Noninvasive Detection of Tear Exosomes. Adv. Funct. Mater. 2022, 32, 2206620. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed]
- Shajari, S.; Salahandish, R.; Zare, A.; Hassani, M.; Moossavi, S.; Munro, E.; Rashid, R.; Rosenegger, D.; Bains, J.S.; Nezhad, A.S. MicroSweat: A Wearable Microfluidic Patch for Noninvasive and Reliable Sweat Collection Enables Human Stress Monitoring. Adv. Sci. 2023, 10, 2204171. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Ma, H.J.H.; Baessler, P.; Balanay, R.K.; Ray, T.R. Skin-interfaced microfluidic systems with spatially engineered 3D fluidics for sweat capture and analysis. Sci. Adv. 2023, 9, eadg4272. [Google Scholar] [CrossRef]
- Boubin, M.; Shrestha, S. Microcontroller Implementation of Support Vector Machine for Detecting Blood Glucose Levels Using Breath Volatile Organic Compounds. Sensors 2019, 19, 2283. [Google Scholar] [CrossRef]
- Han, L.; Luo, S.; Yu, J.; Pan, L.; Chen, S. Rule Extraction from Support Vector Machines Using Ensemble Learning Approach: An Application for Diagnosis of Diabetes. IEEE J. Biomed. Health Inform. 2014, 19, 728–734. [Google Scholar] [CrossRef]
- Karan, O.; Bayraktar, C.; Gümüşkaya, H.; Karlık, B. Diagnosing diabetes using neural networks on small mobile devices. Expert Syst. Appl. 2012, 39, 54–60. [Google Scholar] [CrossRef]
- Temurtas, H.; Yumusak, N.; Temurtas, F. A comparative study on diabetes disease diagnosis using neural networks. Expert Syst. Appl. 2009, 36, 8610–8615. [Google Scholar] [CrossRef]
- Sarría-Santamera, A.; Orazumbekova, B.; Maulenkul, T.; Gaipov, A.; Atageldiyeva, K. The Identification of Diabetes Mellitus Subtypes Applying Cluster Analysis Techniques: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 9523. [Google Scholar] [CrossRef]
- Alamsyah, M.; Nafisah, Z.; Prayitno, E.; Afida, A.M.; Imah, E.M. The Classification of Diabetes Mellitus Using Kernel k-means. J. Phys. Conf. Ser. 2018, 947, 012003. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosravi, S.; Soltanian, S.; Servati, A.; Khademhosseini, A.; Zhu, Y.; Servati, P. Screen-Printed Textile-Based Electrochemical Biosensor for Noninvasive Monitoring of Glucose in Sweat. Biosensors 2023, 13, 684. https://doi.org/10.3390/bios13070684
Khosravi S, Soltanian S, Servati A, Khademhosseini A, Zhu Y, Servati P. Screen-Printed Textile-Based Electrochemical Biosensor for Noninvasive Monitoring of Glucose in Sweat. Biosensors. 2023; 13(7):684. https://doi.org/10.3390/bios13070684
Chicago/Turabian StyleKhosravi, Safoora, Saeid Soltanian, Amir Servati, Ali Khademhosseini, Yangzhi Zhu, and Peyman Servati. 2023. "Screen-Printed Textile-Based Electrochemical Biosensor for Noninvasive Monitoring of Glucose in Sweat" Biosensors 13, no. 7: 684. https://doi.org/10.3390/bios13070684
APA StyleKhosravi, S., Soltanian, S., Servati, A., Khademhosseini, A., Zhu, Y., & Servati, P. (2023). Screen-Printed Textile-Based Electrochemical Biosensor for Noninvasive Monitoring of Glucose in Sweat. Biosensors, 13(7), 684. https://doi.org/10.3390/bios13070684