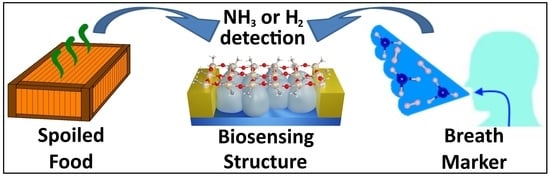
Two-in-One Sensor Based on PV4D4-Coated TiO2 Films for Food Spoilage Detection and as a Breath Marker for Several Diseases
Abstract
1. Introduction
2. Materials and Methods
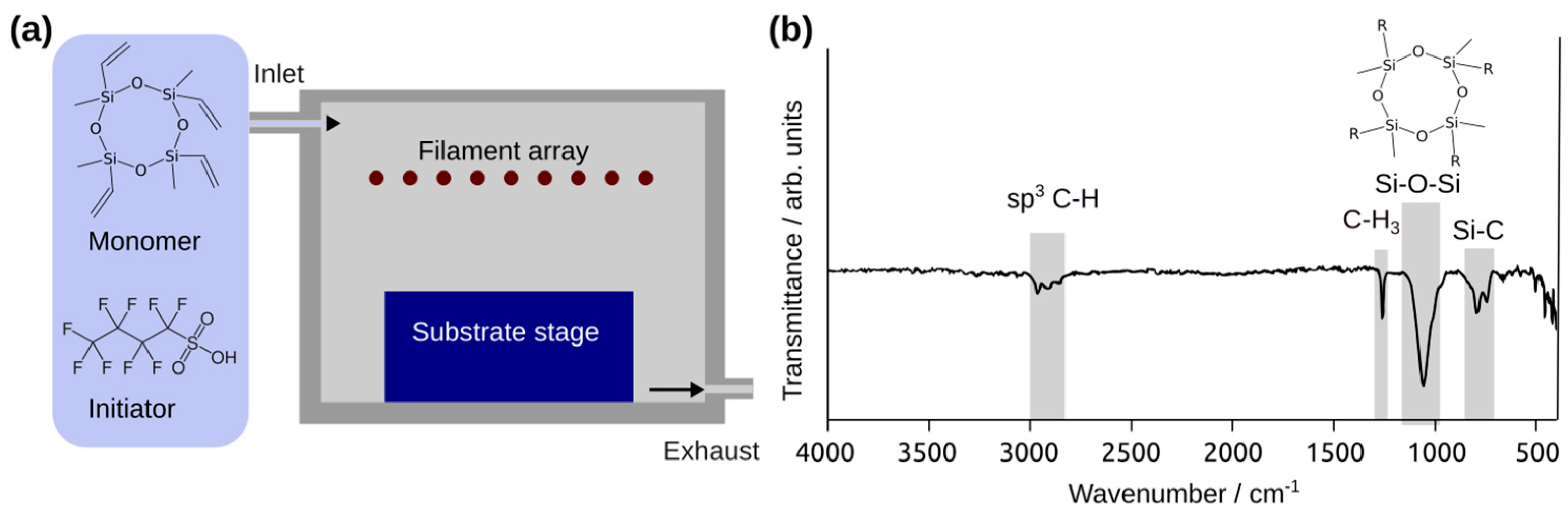
2.1. Sample Production
2.2. Computational
2.3. Sample Characterization
3. Results and Discussion
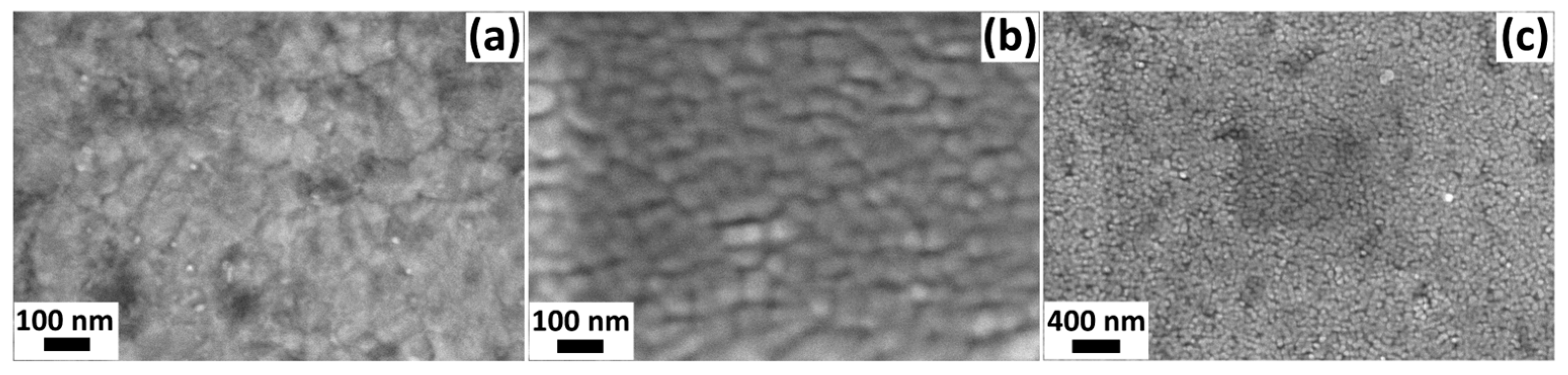
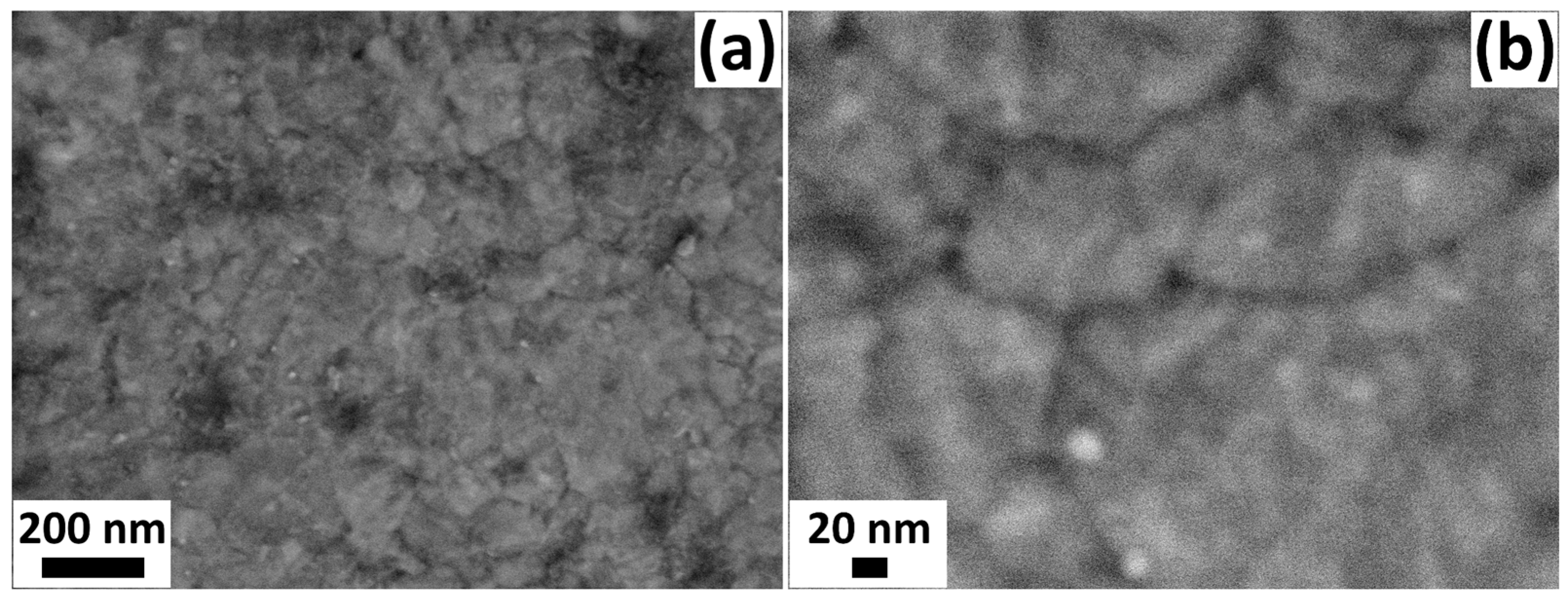
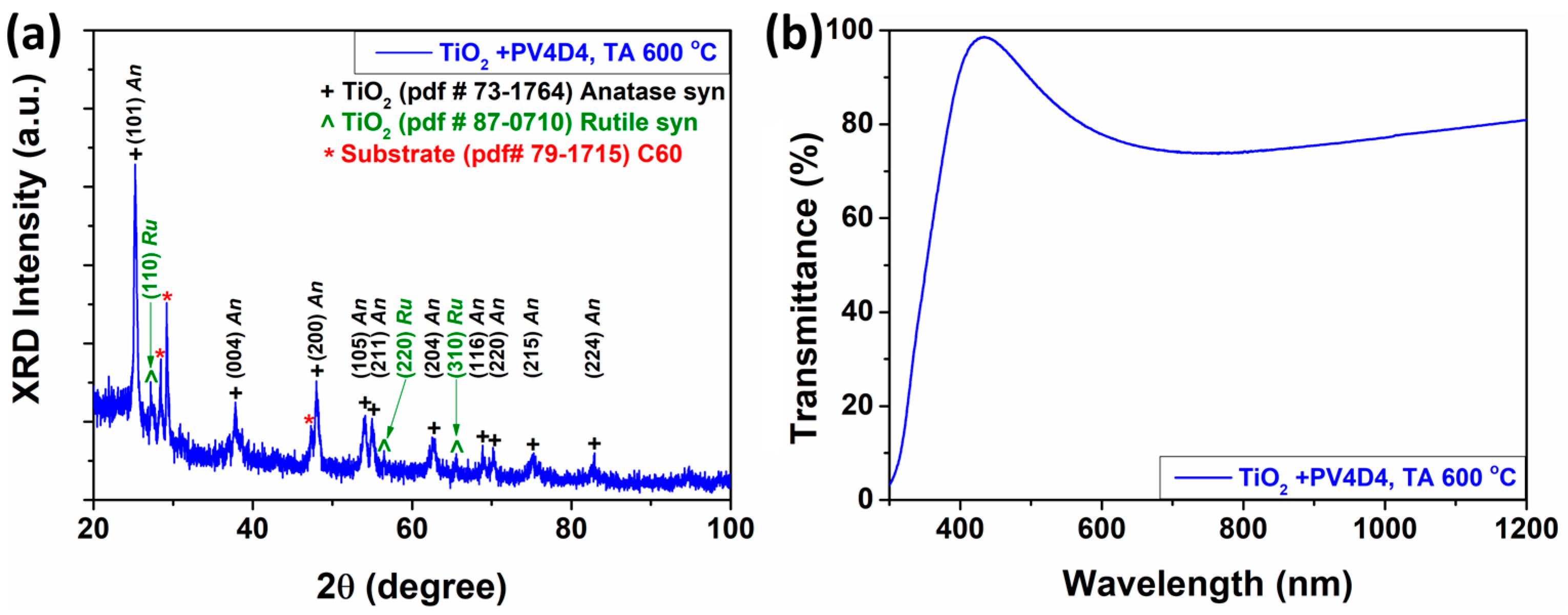
3.1. Characterization of the Fabricated Sensors
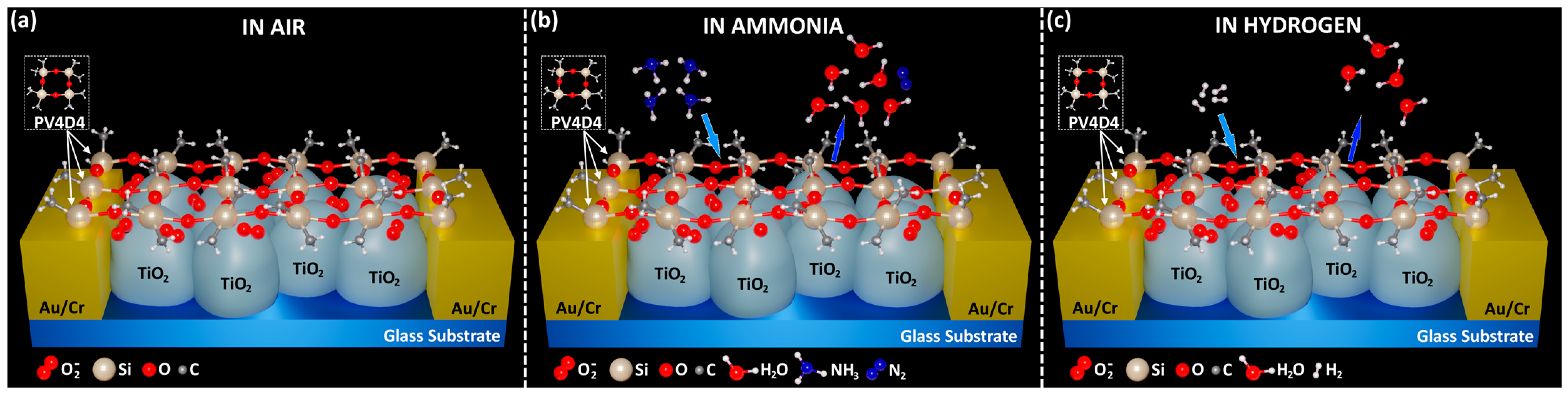
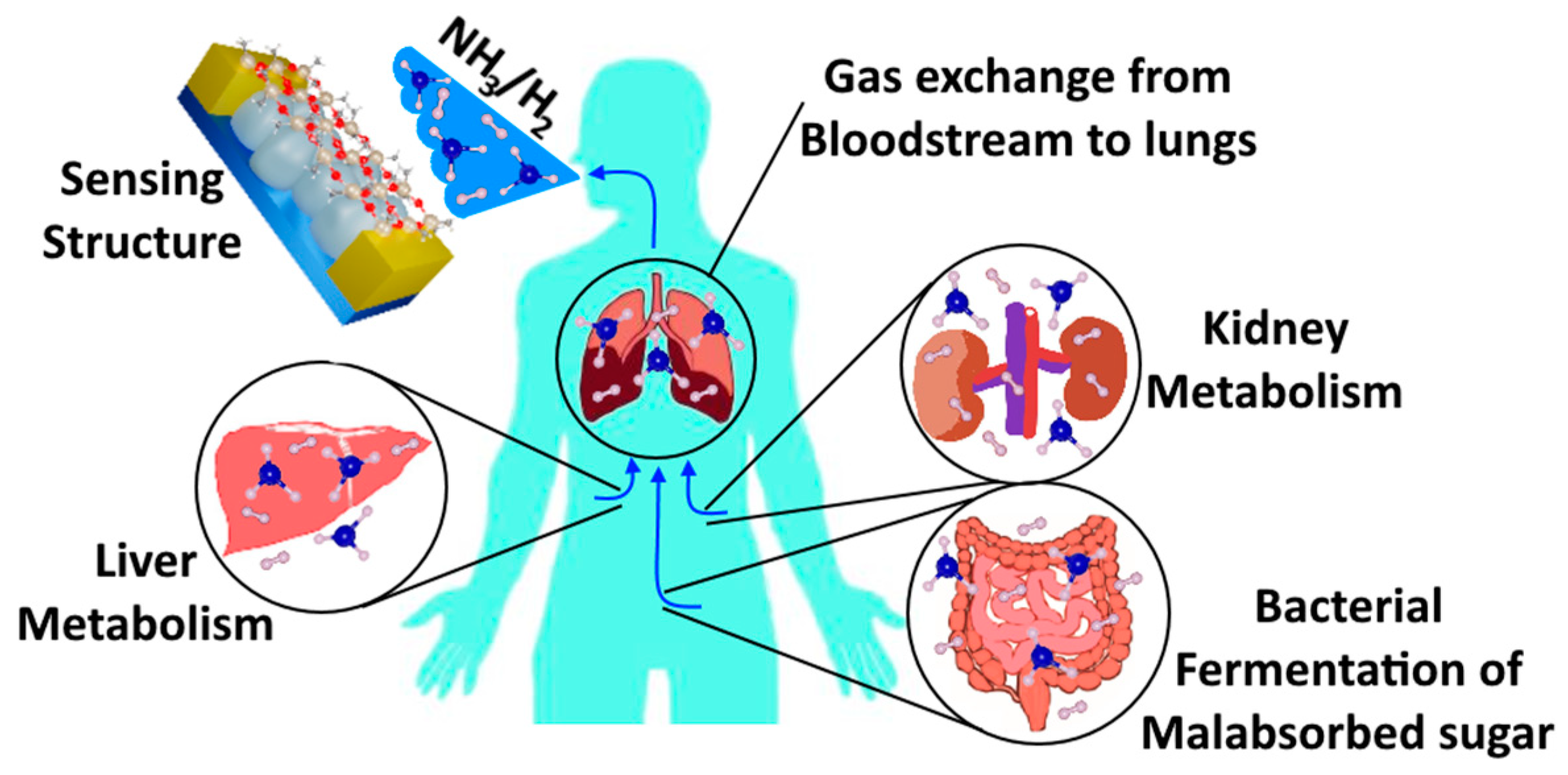
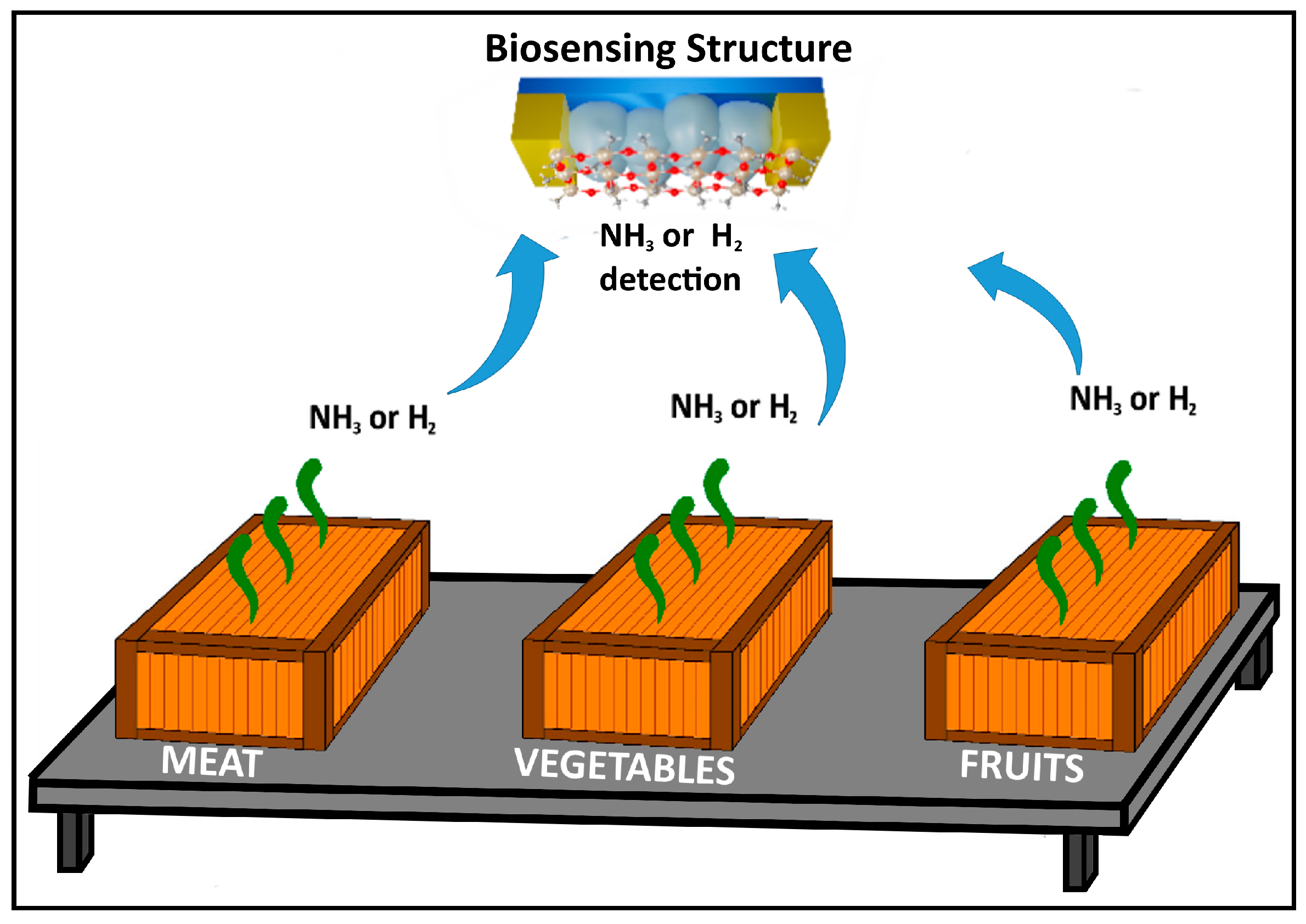
3.2. Gas-Sensing Measurements and Evaluation
| Sensor Material | Polymer | Response, (%) | Concentration, (ppm) | Working Temp, (°C) |
|---|---|---|---|---|
| Graphene heterostructures [52] | Polypyrrole | 45 | 10 | RT |
| Ti3C2Tx films [53] | - | 0.8 | 100 | 25 |
| Co3O4 nanorod [54] | - | 11.2 | 100 | 160 |
| TiO2 films [22] | - | 225 * | 100 | 200 |
| TiO2 films [23] | - | 270 * | 100 | 300 |
| SnO2/polypyrrole nanocomposite [55] | Polypyrrole | 57 | 0.1 | RT |
| PPy/MnO2 composites [56] | Polypyrrole | 3.79 | 100 | RT |
| PPy-coated WO3 nanofibers [57] | Polypyrrole | 6.3 | 1 | 100 |
| NiO/PPy hybrid films [58] | Polypyrrole | 246.6 | 350 | 25 |
| TiO2 (this work) | PV4D4 | 52 | 100 | RT |
| Sensor Material | Polymer | Response, (%) | Concentration, (ppm) | Working Temp, (°C) |
|---|---|---|---|---|
| TiO2 films [22] | - | 600 | 100 | 250 |
| TiO2 films [23] | - | 640 | 100 | 300 |
| SnO2 [59] | Teflon AF-2400 | 75 | 200 | 230 |
| CuO/Cu2O films [48] | - | 250 * | 1000 | 350 |
| TiO2/CuO/Cu2O films [48] | - | 140 | 1000 | 350 |
| TiO2 (this work) | PV4D4 | 100 | 100 | 300 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Sato, Y.; Takegami, Y.; Asamoto, T.; Ono, Y.; Hidetoshi, T.; Goto, R.; Kitamura, A.; Honda, S. Artificial Intelligence Improves the Accuracy of Residents in the Diagnosis of Hip Fractures: A Multicenter Study. BMC Musculoskelet. Disord. 2021, 22, 407. [Google Scholar] [CrossRef]
- Jin, K.; Yan, Y.; Wang, S.; Yang, C.; Chen, M.; Liu, X.; Terasaki, H.; Yeo, T.-H.; Singh, N.G.; Wang, Y.; et al. IERM: An Interpretable Deep Learning System to Classify Epiretinal Membrane for Different Optical Coherence Tomography Devices: A Multi-Center Analysis. J. Clin. Med. 2023, 12, 400. [Google Scholar] [CrossRef]
- Khakbaz, P.; Moshayedi, M.; Hajian, S.; Soleimani, M.; Narakathu, B.B.; Bazuin, B.J.; Pourfath, M.; Atashbar, M.Z. Titanium Carbide MXene as NH3 Sensor: Realistic First-Principles Study. J. Phys. Chem. C 2019, 123, 29794–29803. [Google Scholar] [CrossRef]
- Samotaev, N.; Etrekova, M.; Litvinov, A.; Mikhailov, A. Selective Ammonia Detection by Field Effect Gas Sensor as an Instrumentation Basis for HP-Infection Primary Diagnosis. In Proceedings of the 5th International Conference on Nanotechnologies and Biomedical Engineering, Online, 3–5 November 2021; Tiginyanu, I., Sontea, V., Railean, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 177–184, ISBN 978-3-030-92328-0. [Google Scholar]
- Shin, W. Medical Applications of Breath Hydrogen Measurements. Anal. Bioanal. Chem. 2014, 406, 3931–3939. [Google Scholar] [CrossRef]
- Schröder, S.; Ababii, N.; Brînză, M.; Magariu, N.; Zimoch, L.; Bodduluri, M.T.; Strunskus, T.; Adelung, R.; Faupel, F.; Lupan, O. Tuning the Selectivity of Metal Oxide Gas Sensors with Vapor Phase Deposited Ultrathin Polymer Thin Films. Polymers 2023, 15, 524. [Google Scholar] [CrossRef] [PubMed]
- Cloarec, D.; Bornet, F.; Gouilloud, S.; Barry, J.L.; Salim, B.; Galmiche, J.P. Breath Hydrogen Response to Lactulose in Healthy Subjects: Relationship to Methane Producing Status. Gut 1990, 31, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Urita, Y.; Watanabe, T.; Ishihara, S.; Maeda, T.; Sasaki, Y.; Hike, K.; Miura, Y.; Nanami, T.; Arai, K.-I.; Koshino, H.; et al. Breath Hydrogen and Methane Levels in a Patient with Volvulus of the Sigmoid Colon. J. Breath Res. 2008, 2, 037025. [Google Scholar] [CrossRef]
- Liu, F.; Kondo, T.; Toda, F. Measurement of Breath Hydrogen. Nagoya J. Health Phys. Fit. Sport. 1992, 15, 33–37. [Google Scholar]
- Kim, K.-H.; Jahan, S.A.; Kabir, E. A Review of Breath Analysis for Diagnosis of Human Health. TrAC Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Gahlot, A.P.S.; Paliwal, A.; Kapoor, A. Exploitation of SnO2/Polypyrrole Interface for Detection of Ammonia Vapors Using Conductometric and Optical Techniques: A Theoretical and Experimental Analysis. Sensors 2022, 22, 7252. [Google Scholar] [CrossRef]
- Amirjani, A.; Fatmehsari, D.H. Colorimetric Detection of Ammonia Using Smartphones Based on Localized Surface Plasmon Resonance of Silver Nanoparticles. Talanta 2018, 176, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Sotirov, S.; Demirci, S.; Marudova, M.; Sahiner, N. Trimesic Acid-Based Co(II) MOFs as Colorimetric Sensor for Detection of Ammonia Gas. IEEE Sens. J. 2022, 22, 3903–3910. [Google Scholar] [CrossRef]
- Simren, M. Use and Abuse of Hydrogen Breath Tests. Gut 2006, 55, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, S.-J.; Lee, I.; Youn, D.-Y.; Park, C.O.; Lee, J.-H.; Tuller, H.L.; Kim, I.-D. Thin-Wall Assembled SnO2 Fibers Functionalized by Catalytic Pt Nanoparticles and Their Superior Exhaled-Breath-Sensing Properties for the Diagnosis of Diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Di Stefano, M.; Corazza, G.R. Role of Hydrogen and Methane Breath Testing in Gastrointestinal Diseases. Dig. Liver Dis. Suppl. 2009, 3, 40–43. [Google Scholar] [CrossRef]
- Maity, A.; Raychaudhuri, A.K.; Ghosh, B. High Sensitivity NH3 Gas Sensor with Electrical Readout Made on Paper with Perovskite Halide as Sensor Material. Sci. Rep. 2019, 9, 7777. [Google Scholar] [CrossRef]
- Gleason, K.K. Nanoscale Control by Chemically Vapour-Deposited Polymers. Nat. Rev. Phys. 2020, 2, 347–364. [Google Scholar] [CrossRef]
- Coclite, A.M.; Howden, R.M.; Borrelli, D.C.; Petruczok, C.D.; Yang, R.; Yagüe, J.L.; Ugur, A.; Chen, N.; Lee, S.; Jo, W.J.; et al. 25th Anniversary Article: CVD Polymers: A New Paradigm for Surface Modifi Cation and Device Fabrication. Adv. Mater. 2013, 25, 5392–5423. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Ababii, N.; Reimer, T.; Shree, S.; Hoppe, M.; Polonskyi, O.; Sontea, V.; Chemnitz, S.; Faupel, F.; et al. Ultra-Thin TiO2 Films by Atomic Layer Deposition and Surface Functionalization with Au Nanodots for Sensing Applications. Mater. Sci. Semicond. Process 2018, 87, 44–53. [Google Scholar] [CrossRef]
- Ababii, N.; Hoppe, M.; Shree, S.; Vahl, A.; Ulfa, M.; Pauporté, T.; Viana, B.; Cretu, V.; Magariu, N.; Postica, V.; et al. Effect of Noble Metal Functionalization and Film Thickness on Sensing Properties of Sprayed TiO2 Ultra-Thin Films. Sens. Actuators A Phys. 2019, 293, 242–258. [Google Scholar] [CrossRef]
- Lee, W.-C.; Kim, K.-B.; Gurudatt, N.G.; Hussain, K.K.; Choi, C.S.; Park, D.-S.; Shim, Y.-B. Comparison of Enzymatic and Non-Enzymatic Glucose Sensors Based on Hierarchical Au-Ni Alloy with Conductive Polymer. Biosens. Bioelectron. 2019, 130, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shao, Z.; Ulfa, M.; Pauporté, T. Insights into the Hole Blocking Layer Effect on the Perovskite Solar Cell Performance and Impedance Response. J. Phys. Chem. C 2017, 121, 9131–9141. [Google Scholar] [CrossRef]
- Schröder, S.; Strunskus, T.; Rehders, S.; Gleason, K.K.; Faupel, F. Tunable Polytetrafluoroethylene Electret Films with Extraordinary Charge Stability Synthesized by Initiated Chemical Vapor Deposition for Organic Electronics Applications. Sci. Rep. 2019, 9, 2237. [Google Scholar] [CrossRef] [PubMed]
- Valiev, M.; Bylaska, E.J.; Govind, N.; Kowalski, K.; Straatsma, T.P.; Van Dam, H.J.J.; Wang, D.; Nieplocha, J.; Apra, E.; Windus, T.L.; et al. NWChem: A Comprehensive and Scalable Open-Source Solution for Large Scale Molecular Simulations. Comput. Phys. Commun. 2010, 181, 1477–1489. [Google Scholar] [CrossRef]
- Siebert, L.; Wolff, N.; Ababii, N.; Terasa, M.-I.; Lupan, O.; Vahl, A.; Duppel, V.; Qiu, H.; Tienken, M.; Mirabelli, M.; et al. Facile Fabrication of Semiconducting Oxide Nanostructures by Direct Ink Writing of Readily Available Metal Microparticles and Their Application as Low Power Acetone Gas Sensors. Nano Energy 2020, 70, 104420. [Google Scholar] [CrossRef]
- Lupan, O.; Cretu, V.; Postica, V.; Ababii, N.; Polonskyi, O.; Kaidas, V.; Schütt, F.; Mishra, Y.K.; Monaico, E.; Tiginyanu, I.; et al. Enhanced Ethanol Vapour Sensing Performances of Copper Oxide Nanocrystals with Mixed Phases. Sens. Actuators B Chem. 2016, 224, 434–448. [Google Scholar] [CrossRef]
- Lau, K.K.S.; Gleason, K.K. Initiated Chemical Vapor Deposition (ICVD) of Poly(Alkyl Acrylates): An Experimental Study. Macromolecules 2006, 39, 3688–3694. [Google Scholar] [CrossRef]
- Schröder, S.; Hinz, A.M.; Strunskus, T.; Faupel, F. Molecular Insight into Real-Time Reaction Kinetics of Free Radical Polymerization from the Vapor Phase by In-Situ Mass Spectrometry. J. Phys. Chem. A 2021, 125, 1661–1667. [Google Scholar] [CrossRef]
- Socrates, G. Alkane Group Residues: C-H Group. In Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons Ltd.: Chichester, UK, 2004; pp. 50–67. ISBN 978-0-470-09307-8. [Google Scholar]
- Socrates, G. Organic Silicon Compounds. In Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons Ltd.: Chichester, UK, 2004; pp. 241–246. ISBN 978-0-470-09307-8. [Google Scholar]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman Spectrum of Anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Enachi, M.; Lupan, O.; Braniste, T.; Sarua, A.; Chow, L.; Mishra, Y.K.; Gedamu, D.; Adelung, R.; Tiginyanu, I. Integration of Individual TiO2 Nanotube on the Chip: Nanodevice for Hydrogen Sensing. Phys. Status Solidi—Rapid Res. Lett. 2015, 9, 171–174. [Google Scholar] [CrossRef]
- Wetchakun, N.; Incessungvorn, B.; Wetchakun, K.; Phanichphant, S. Influence of Calcination Temperature on Anatase to Rutile Phase Transformation in TiO2 Nanoparticles Synthesized by the Modified Sol–Gel Method. Mater. Lett. 2012, 82, 195–198. [Google Scholar] [CrossRef]
- Kameya, Y.; Yabe, H. Optical and Superhydrophilic Characteristics of TiO2 Coating with Subwavelength Surface Structure Consisting of Spherical Nanoparticle Aggregates. Coatings 2019, 9, 547. [Google Scholar] [CrossRef]
- Morsella, M.; D’Alessandro, N.; Lanterna, A.E.; Scaiano, J.C. Improving the Sunscreen Properties of TiO2 through an Understanding of Its Catalytic Properties. ACS Omega 2016, 1, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Fogue, C.; Lemdani, M.; Huart, C. Nasal Chemosensory Tests: Biomarker between Dementia with Lewy Bodies and Parkinson Disease Dementia. Rhinol. J. 2020, 58, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Postica, V.; Wolff, N.; Polonskyi, O.; Duppel, V.; Kaidas, V.; Lazari, E.; Ababii, N.; Faupel, F.; Kienle, L.; et al. Localized Synthesis of Iron Oxide Nanowires and Fabrication of High Performance Nanosensors Based on a Single Fe2O3 Nanowire. Small 2017, 13, 1602868. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Cope, K.; Terence, R.H.; Diehl, A.M. Obesity and Female Gender Increase Breath Ethanol Concentration: Potential Implications for The Pathogenesis of Nonalcoholic Steatohepatitis. Am. J. Gastroenterol. 2001, 96, 1200–1204. [Google Scholar] [CrossRef]
- Koureas, M.; Kirgou, P.; Amoutzias, G.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons. Metabolites 2020, 10, 317. [Google Scholar] [CrossRef]
- Hwang, L.; Low, K.; Khoshini, R.; Melmed, G.; Sahakian, A.; Makhani, M.; Pokkunuri, V.; Pimentel, M. Evaluating Breath Methane as a Diagnostic Test for Constipation-Predominant IBS. Dig. Dis. Sci. 2010, 55, 398–403. [Google Scholar] [CrossRef]
- Afzal, A.; Cioffi, N.; Sabbatini, L.; Torsi, L. NOx Sensors Based on Semiconducting Metal Oxide Nanostructures: Progress and Perspectives. Sens. Actuators B Chem. 2012, 171–172, 25–42. [Google Scholar] [CrossRef]
- Lepselter, M.P.; Sze, S.M. Silicon Schottky Barrier Diode with Near-Ideal I-V Characteristics. Bell Syst. Tech. J. 1968, 47, 195–208. [Google Scholar] [CrossRef]
- NIST Standard Reference Database Number 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 19 February 2023).
- Lupan, O.; Santos-Carballal, D.; Ababii, N.; Magariu, N.; Hansen, S.; Vahl, A.; Zimoch, L.; Hoppe, M.; Pauporté, T.; Galstyan, V.; et al. TiO2/Cu2O/CuO Multi-Nanolayers as Sensors for H2 and Volatile Organic Compounds: An Experimental and Theoretical Investigation. ACS Appl. Mater. Interfaces 2021, 13, 32363–32380. [Google Scholar] [CrossRef]
- Chang, S. Oxygen Chemisorption on Tin Oxide: Correlation between Electrical Conductivity and EPR Measurements. J. Vac. Sci. Technol. 1980, 17, 366–369. [Google Scholar] [CrossRef]
- Lenaerts, S.; Roggen, J.; Maes, G. FT-IR Characterization of Tin Dioxide Gas Sensor Materials under Working Conditions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 883–894. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, H.; Li, F.; Yu, S.; Chen, Y. High Performance Ammonia Gas Detection Based on TiO2/WO3·H2O Heterojunction Sensor. Mater. Chem. Phys. 2021, 273, 125098. [Google Scholar] [CrossRef]
- Gao, J.; Qin, J.; Chang, J.; Liu, H.; Wu, Z.-S.; Feng, L. NH3 Sensor Based on 2D Wormlike Polypyrrole/Graphene Heterostructures for a Self-Powered Integrated System. ACS Appl. Mater. Interfaces 2020, 12, 38674–38681. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef]
- Srirattanapibul, S.; Nakarungsee, P.; Issro, C.; Tang, I.-M.; Thongmee, S. Enhanced Room Temperature NH3 Sensing of RGO/Co3O4 Nanocomposites. Mater. Chem. Phys. 2021, 272, 125033. [Google Scholar] [CrossRef]
- Beniwal, A. Sunny Electrospun SnO2/PPy Nanocomposite for Ultra-Low Ammonia Concentration Detection at Room Temperature. Sens. Actuators B Chem. 2019, 296, 126660. [Google Scholar] [CrossRef]
- Malook, K.; Khan, H.; Shah, M.; Haque, I.-U.-. Highly Selective and Sensitive Response of Polypyrrole–MnO2 Based Composites towards Ammonia Gas. Polym. Compos. 2019, 40, 1676–1683. [Google Scholar] [CrossRef]
- Ho, T.A.; Jun, T.-S.; Kim, Y.S. Material and NH3-Sensing Properties of Polypyrrole-Coated Tungsten Oxide Nanofibers. Sensors Actuators B Chem. 2013, 185, 523–529. [Google Scholar] [CrossRef]
- Thi Hien, H.; Thi Anh Thu, D.; Quang Ngan, P.; Hong Thai, G.; Thanh Trung, D.; Trung, T.; Minh Tan, M.; Truong Giang, H. High NH3 Sensing Performance of NiO/PPy Hybrid Nanostructures. Sens. Actuators B Chem. 2021, 340, 129986. [Google Scholar] [CrossRef]
- Tan, Y.; Du, B.; Liang, C.; Guo, X.; Zheng, H.; Liu, P.; Yang, X.; Li, S.; Jin, B.; Sun, J. Improving Anti-Humidity Property of a SnO2-Based Chemiresistive Hydrogen Sensor by a Breathable and Hydrophobic Fluoropolymer Coating. Langmuir 2022, 38, 13833–13840. [Google Scholar] [CrossRef] [PubMed]
- Piiper, J. Respiratory Gas Exchange at Lungs, Gills and Tissues: Mechanisms and Adjustments. J. Exp. Biol. 1982, 100, 5–22. [Google Scholar] [CrossRef]
- Yan, L.; Yin-He, S.; Qian, Y.; Zhi-Yu, S.; Chun-Zi, W.; Zi-Yun, L. Method of Reaching Consensus on Probability of Food Safety Based on the Integration of Finite Credible Data on Block Chain. IEEE Access 2021, 9, 123764–123776. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.-M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.M.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef]
- Yuan, Z.; Bariya, M.; Fahad, H.M.; Wu, J.; Han, R.; Gupta, N.; Javey, A. Trace-Level, Multi-Gas Detection for Food Quality Assessment Based on Decorated Silicon Transistor Arrays. Adv. Mater. 2020, 32, 1908385. [Google Scholar] [CrossRef]
- Evancho, G.M.; Tortorelli, S.; Scott, V.N. Microbiological Spoilage of Canned Foods. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Springer: New York, NY, USA, 2009; pp. 185–221. ISBN 978-1-4419-0826-1. [Google Scholar]
- Wang, Y.; Liu, S.; Yang, X.; Zhang, J.; Zhang, Y.; Liu, X.; Zhang, H.; Wang, H. Effect of Germination on Nutritional Properties and Quality Attributes of Glutinous Rice Flour and Dumplings. J. Food Compos. Anal. 2022, 108, 104440. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Yang, X.; Wang, W.; Liu, X.; Wang, H.; Zhang, H. Enhancing the Fermentation Performance of Frozen Dough by Ultrasonication: Effect of Starch Hierarchical Structures. J. Cereal Sci. 2022, 106, 103500. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinza, M.; Schröder, S.; Ababii, N.; Gronenberg, M.; Strunskus, T.; Pauporte, T.; Adelung, R.; Faupel, F.; Lupan, O. Two-in-One Sensor Based on PV4D4-Coated TiO2 Films for Food Spoilage Detection and as a Breath Marker for Several Diseases. Biosensors 2023, 13, 538. https://doi.org/10.3390/bios13050538
Brinza M, Schröder S, Ababii N, Gronenberg M, Strunskus T, Pauporte T, Adelung R, Faupel F, Lupan O. Two-in-One Sensor Based on PV4D4-Coated TiO2 Films for Food Spoilage Detection and as a Breath Marker for Several Diseases. Biosensors. 2023; 13(5):538. https://doi.org/10.3390/bios13050538
Chicago/Turabian StyleBrinza, Mihai, Stefan Schröder, Nicolai Ababii, Monja Gronenberg, Thomas Strunskus, Thierry Pauporte, Rainer Adelung, Franz Faupel, and Oleg Lupan. 2023. "Two-in-One Sensor Based on PV4D4-Coated TiO2 Films for Food Spoilage Detection and as a Breath Marker for Several Diseases" Biosensors 13, no. 5: 538. https://doi.org/10.3390/bios13050538
APA StyleBrinza, M., Schröder, S., Ababii, N., Gronenberg, M., Strunskus, T., Pauporte, T., Adelung, R., Faupel, F., & Lupan, O. (2023). Two-in-One Sensor Based on PV4D4-Coated TiO2 Films for Food Spoilage Detection and as a Breath Marker for Several Diseases. Biosensors, 13(5), 538. https://doi.org/10.3390/bios13050538