A UiO-66-NH2 MOF/PAMAM Dendrimer Nanocomposite for Electrochemical Detection of Tramadol in the Presence of Acetaminophen in Pharmaceutical Formulations
Abstract
1. Introduction
2. Experimental
2.1. Instruments and Reagents
2.2. Synthesis of the UiO-66-NH2 MOF/G3-PAMAM Nanocomposite
2.3. Preparation of GCE Modified with the UiO-66-NH2 MOF/G3-PAMAM Nanocomposite
2.4. Preparation of Pharmaceutical Formulations
3. Results and Discussion
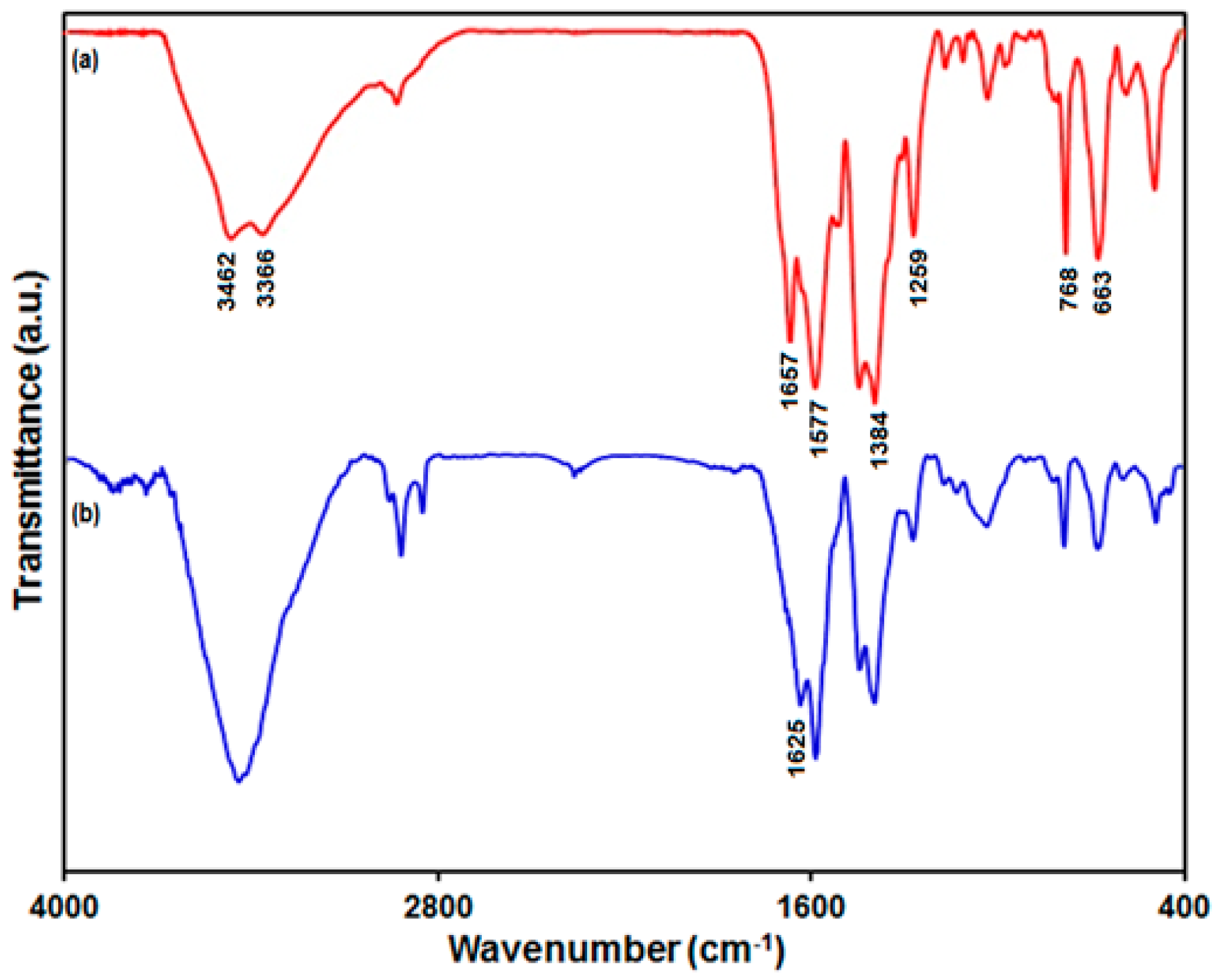
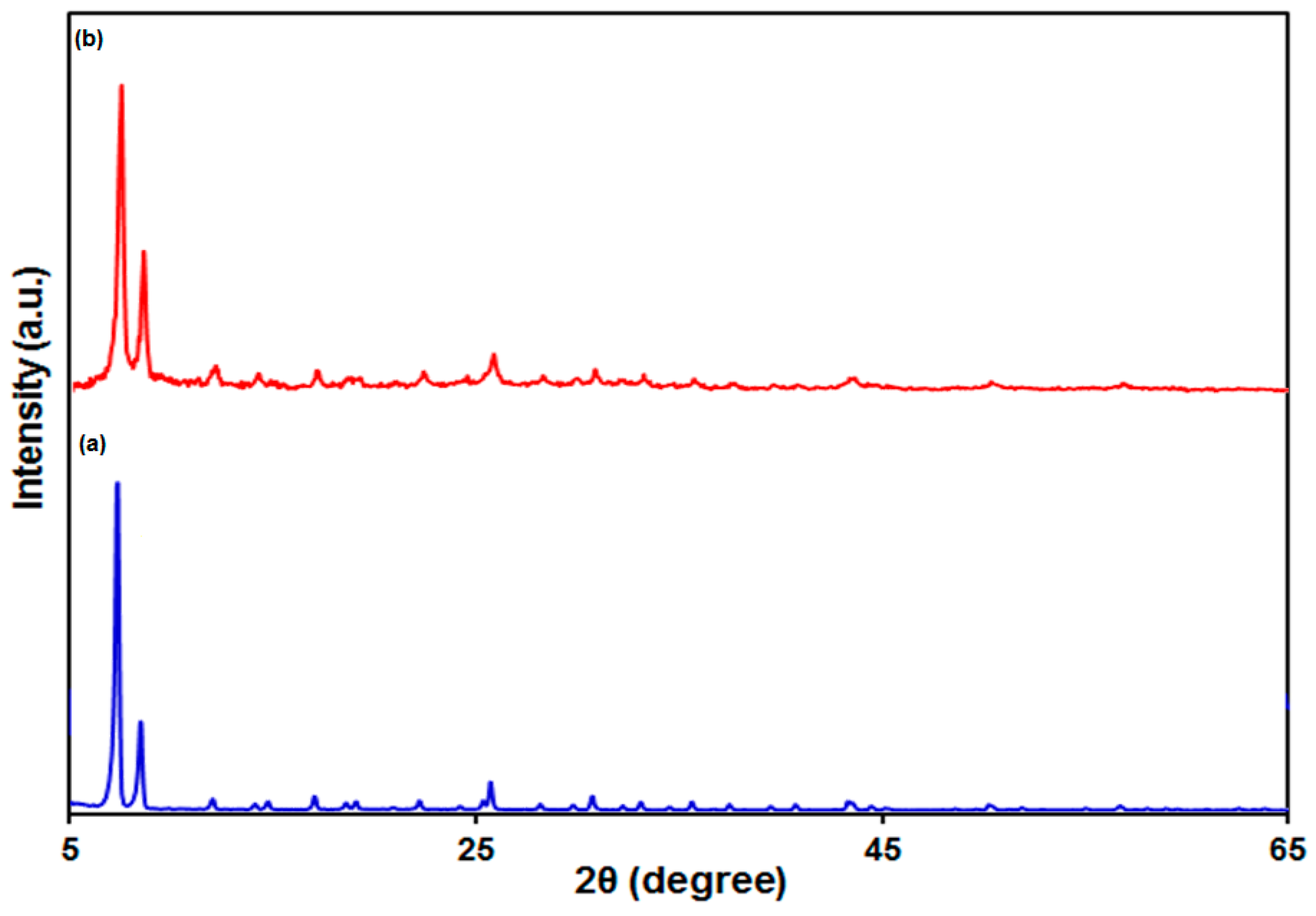
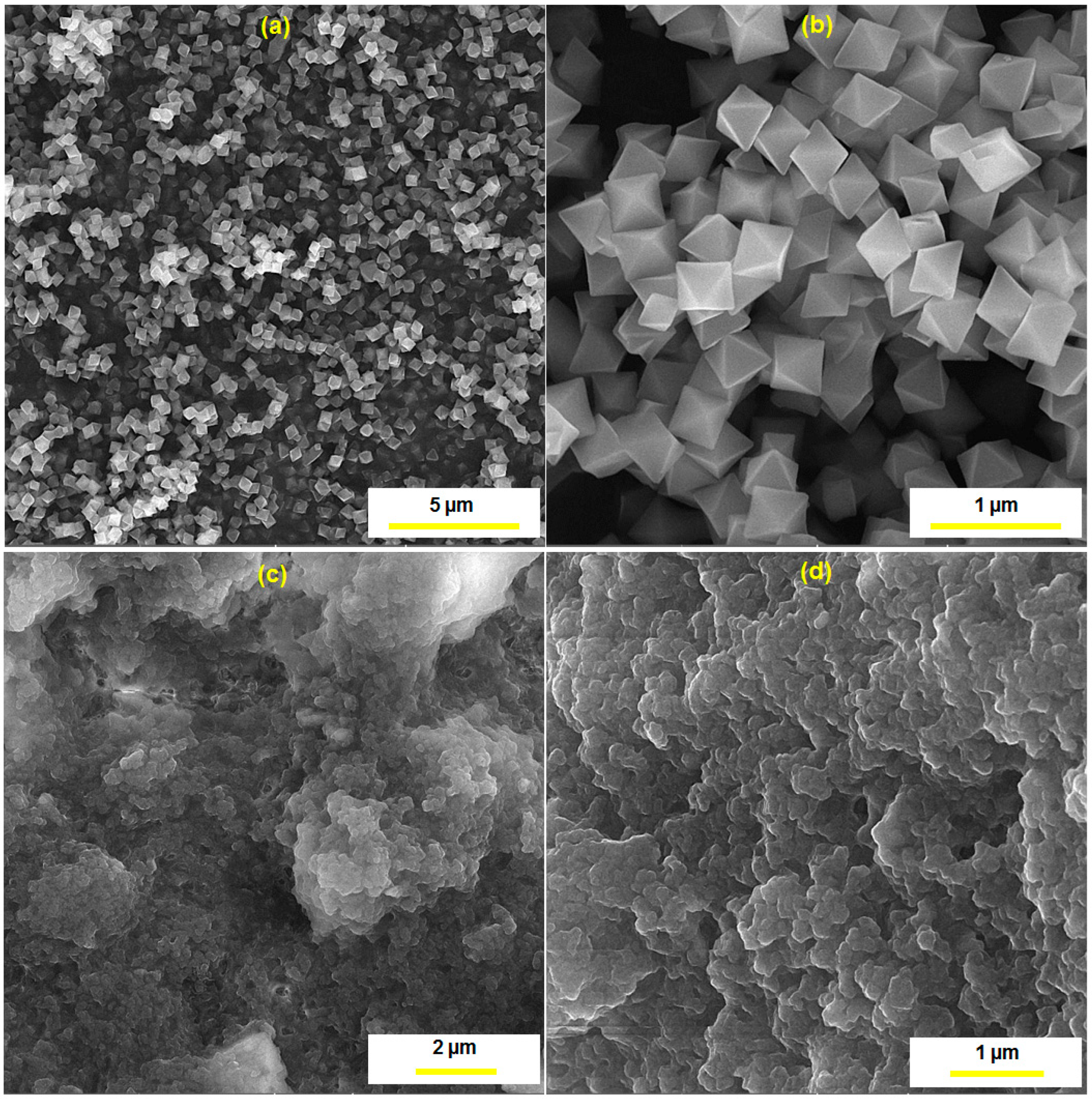
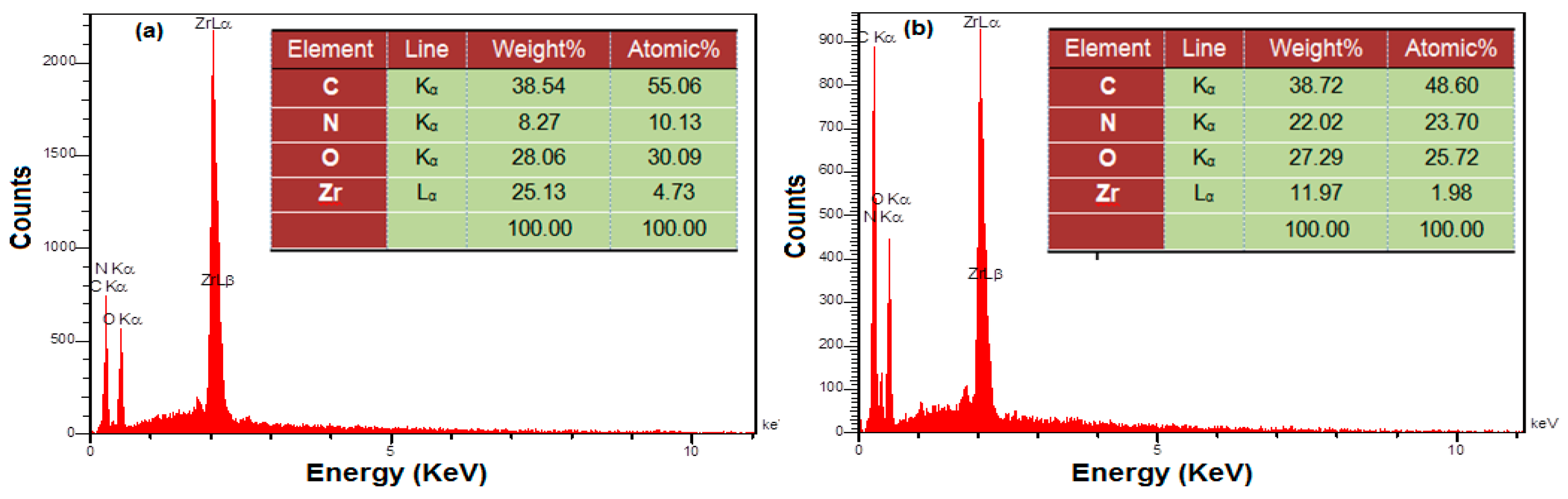
3.1. Characterization of the UiO-66-NH2 MOF/PAMAM Nanocomposite
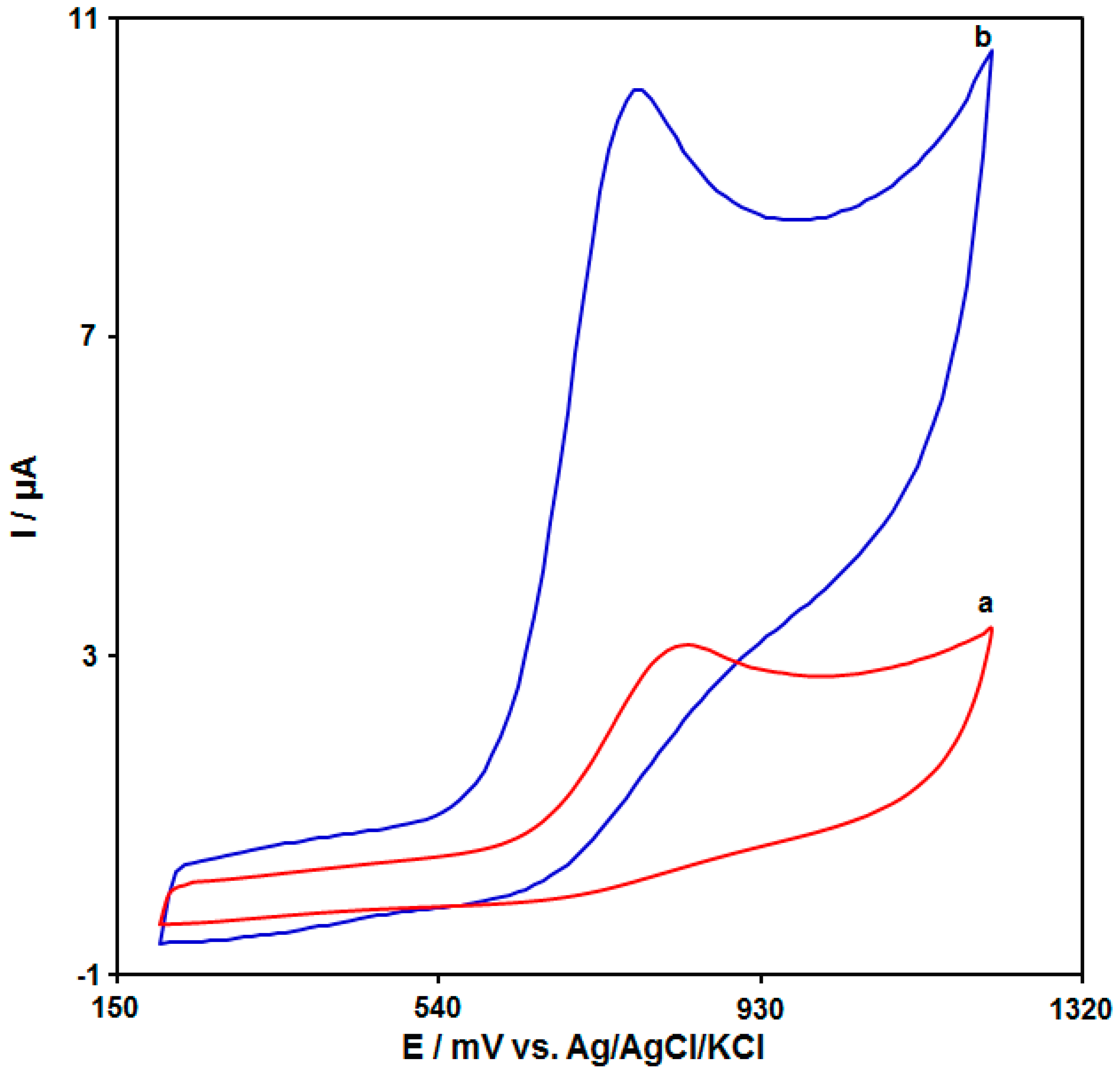
3.2. Investigating the Effect of the UiO-66-NH2 MOF/PAMAM Nanocomposite on the Electrochemical Behavior of Tramadol
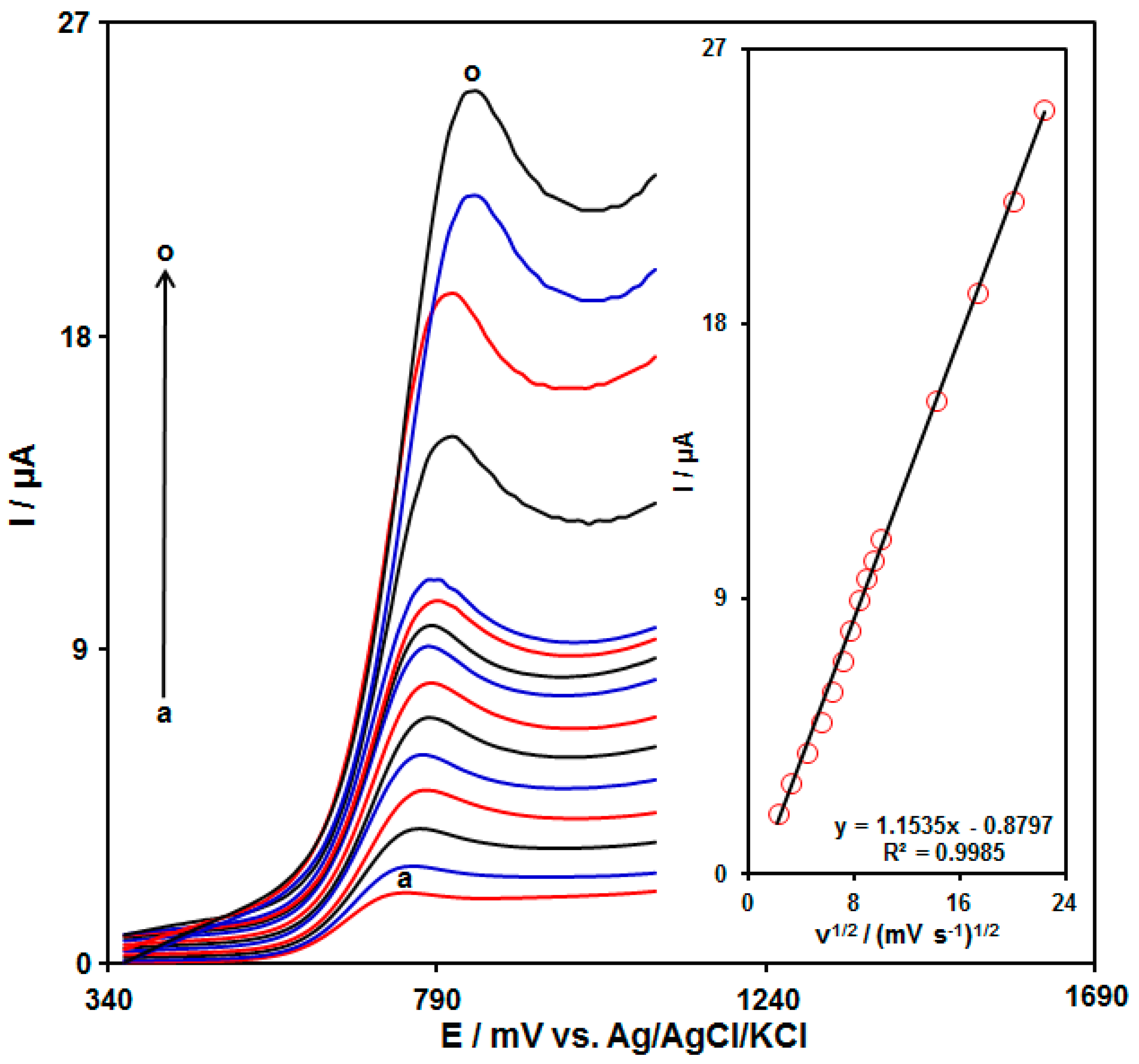
3.3. The Scan Rate Effect on the Oxidation of Tramadol on the UiO-66-NH2 MOF/PAMAM-Modified GCE
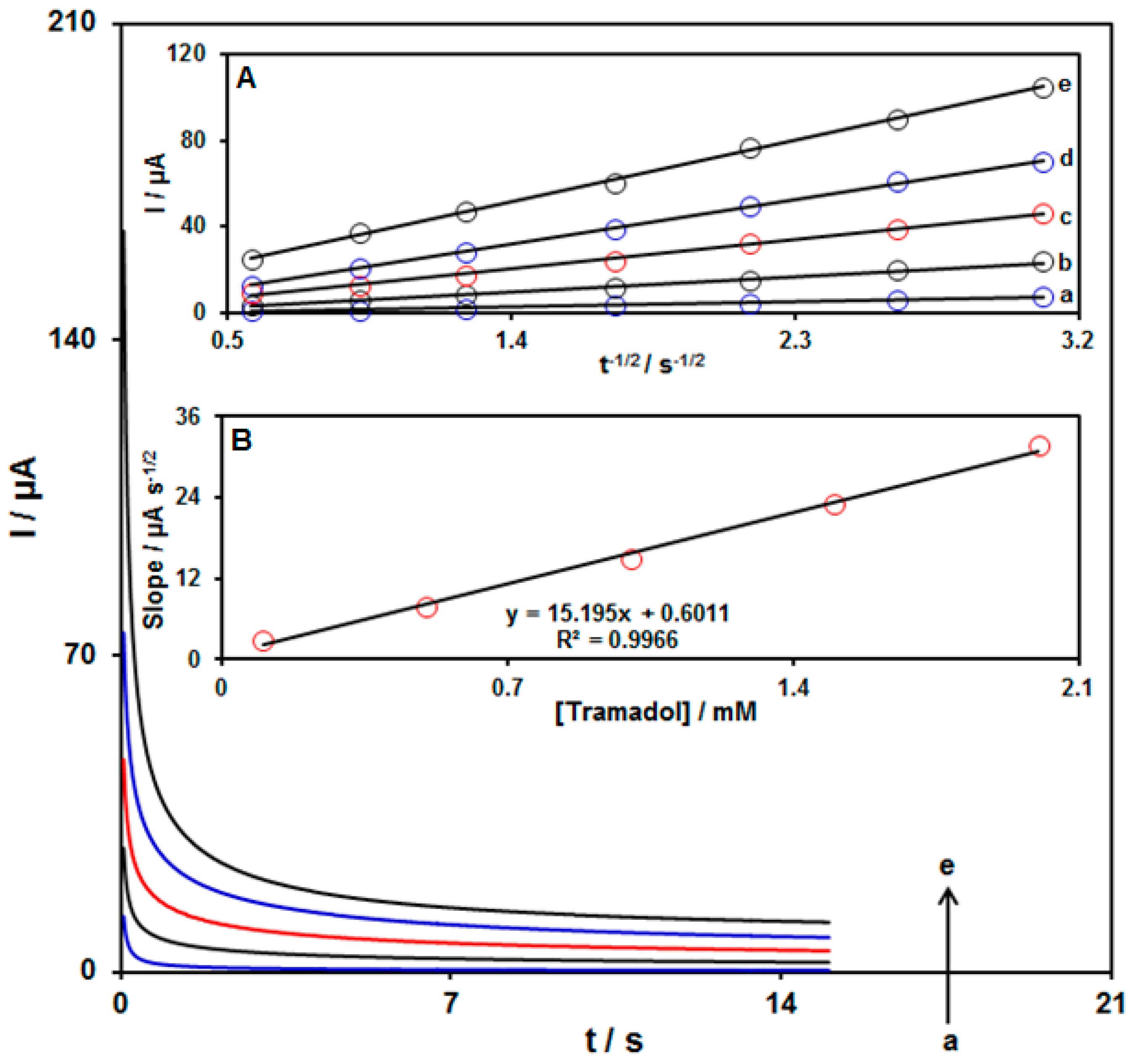
3.4. Chronoamperometric Determinations
3.5. Quantitative Determination of Tramadol by the DPV Method
3.6. Quantitative Determination of Tramadol in the Presence of Acetaminophen
3.7. The Stability, Repeatability, and Reproducibility Studies of the UiO-66-NH2 MOF/PAMAM-Modified GCE for Tramadol Analysis
3.8. The Selectivity of the UiO-66-NH2 MOF-PAMAM/GCE for the Detection of Tramadol
3.9. Application of the UiO-66-NH2 MOF/PAMAM-Modified GCE Sensor for the Analysis of Acetaminophen and Tramadol in Pharmaceutical Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palakollu, V.N.; Chiwunze, T.E.; Liu, C.; Karpoormath, R. Electrochemical sensitive determination of acetaminophen in pharmaceutical formulations at iron oxide/graphene composite modified electrode. Arab. J. Chem. 2020, 13, 4350–4357. [Google Scholar] [CrossRef]
- Houshmand, M.; Jabbari, A.; Heli, H.; Hajjizadeh, M.; Moosavi-Movahedi, A.A. Electrocatalytic oxidation of aspirin and acetaminophen on a cobalt hydroxide nanoparticles modified glassy carbon electrode. J. Solid State Electrochem. 2008, 12, 1117–1128. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Asadian, E. Simultaneous voltammetric determination of ascorbic acid, acetaminophen and isoniazid using thionine immobilized multi-walled carbon nanotube modified carbon paste electrode. Electrochim. Acta 2010, 55, 666–672. [Google Scholar] [CrossRef]
- Habibi, B.; Jahanbakhshi, M.; Pournaghi-Azar, M.H. Simultaneous determination of acetaminophen and dopamine using SWCNT modified carbon–ceramic electrode by differential pulse voltammetry. Electrochim. Acta 2011, 56, 2888–2894. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, J.; Xi, Q.; Yang, W. A high performance electrochemical sensor for acetaminophen based on single-walled carbon nanotube–graphene nanosheet hybrid films. Sens. Actuators B Chem. 2012, 161, 648–654. [Google Scholar] [CrossRef]
- Xu, C.X.; Huang, K.J.; Fan, Y.; Wu, Z.W.; Li, J. Electrochemical determination of acetaminophen based on TiO2–graphene/poly (methyl red) composite film modified electrode. J. Mol. Liq. 2012, 165, 32–37. [Google Scholar] [CrossRef]
- Subedi, M.; Bajaj, S.; Kumar, M.S.; Mayur, Y.C. An overview of tramadol and its usage in pain management and future perspective. Biomed. Pharmacother. 2019, 111, 443–451. [Google Scholar] [CrossRef]
- Memon, S.A.; Hassan, D.; Buledi, J.A.; Solangi, A.R.; Memon, S.Q.; Palabiyik, I.M. Plant material protected cobalt oxide nanoparticles: Sensitive electro-catalyst for tramadol detection. Microchem. J. 2020, 159, 105480. [Google Scholar] [CrossRef]
- Abu-Shawish, H.M.; Ghalwa, N.A.; Zaggout, F.R.; Saadeh, S.M.; Al-Dalou, A.R.; Abou Assi, A.A. Improved determination of tramadol hydrochloride in biological fluids and pharmaceutical preparations utilizing a modified carbon paste electrode. Biochem. Eng. J. 2010, 48, 237–245. [Google Scholar] [CrossRef]
- Deiminiat, B.; Rounaghi, G.H.; Arbab-Zavar, M.H. Development of a new electrochemical imprinted sensor based on poly-pyrrole, sol–gel and multiwall carbon nanotubes for determination of tramadol. Sens. Actuators B Chem. 2017, 238, 651–659. [Google Scholar] [CrossRef]
- Atta, N.F.; Ahmed, R.A.; Amin, H.M.; Galal, A. Monodispersed gold nanoparticles decorated carbon nanotubes as an enhanced sensing platform for nanomolar detection of tramadol. Electroanalysis 2012, 24, 2135–2146. [Google Scholar] [CrossRef]
- Çidem, E.; Teker, T.; Aslanoglu, M. A sensitive determination of tramadol using a voltammetric platform based on antimony oxide nanoparticles. Microchem. J. 2019, 147, 879–885. [Google Scholar] [CrossRef]
- Ghorbani-Bidkorbeh, F.; Shahrokhian, S.; Mohammadi, A.; Dinarvand, R. Simultaneous voltammetric determination of tramadol and acetaminophen using carbon nanoparticles modified glassy carbon electrode. Electrochim. Acta 2010, 55, 2752–2759. [Google Scholar] [CrossRef]
- Korduba, C.A.; Petruzzi, R.F. High-performance liquid chromatographic method for the determination of trace amounts of acetaminophen in plasma. J. Pharm. Sci. 1984, 73, 117–119. [Google Scholar] [CrossRef]
- Belal, T.; Awad, T.; Clark, R. Determination of paracetamol and tramadol hydrochloride in pharmaceutical mixture using HPLC and GC-MS. J. Chromatogr. Sci. 2009, 47, 849–854. [Google Scholar] [CrossRef]
- Song, S.M.; Marriott, P.; Kotsos, A.; Drummer, O.H.; Wynne, P. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry (GC×GC-TOFMS) for drug screening and confirmation. Forensic Sci. Int. 2004, 143, 87–101. [Google Scholar] [CrossRef]
- Cunha, R.R.; Ribeiro, M.M.; Muñoz, R.A.; Richter, E.M. Fast determination of codeine, orphenadrine, promethazine, scopolamine, tramadol, and paracetamol in pharmaceutical formulations by capillary electrophoresis. J. Sep. Sci. 2017, 40, 1815–1823. [Google Scholar] [CrossRef]
- Khairy, M.; Banks, C.E. A screen-printed electrochemical sensing platform surface modified with nanostructured ytterbium oxide nanoplates facilitating the electroanalytical sensing of the analgesic drugs acetaminophen and tramadol. Microchim. Acta 2020, 187, 126. [Google Scholar] [CrossRef]
- Miraki, M.; Karimi-Maleh, H.; Taher, M.A.; Cheraghi, S.; Karimi, F.; Agarwal, S.; Gupta, V.K. Voltammetric amplified platform based on ionic liquid/NiO nanocomposite for determination of benserazide and levodopa. J. Mol. Liq. 2019, 278, 672–676. [Google Scholar] [CrossRef]
- Cerdà, V.; Araujo, R.G.O.; Ferreira, S.L.C. revising flow-through cells for amperometric and voltammetric detections using stationary mercury and bismuth screen printed electrodes. Prog. Chem. Biochem. Res. 2022, 5, 351–366. [Google Scholar]
- Scandurra, A.; Ruffino, F.; Urso, M.; Grimaldi, M.G.; Mirabella, S. Disposable and low-cost electrode based on graphene paper-nafion-bi nanostructures for ultra-trace determination of pb (II) and cd (II). Nanomaterials 2020, 10, 1620. [Google Scholar] [CrossRef] [PubMed]
- Ismaeel, S.A.; Al-Bayati, Y.K. Determination of trace metformin in pharmaceutical preparation using molecularly imprinted polymer based pvc-membrane. Eurasian Chem. Commun. 2021, 3, 812–830. [Google Scholar]
- Shiva Ariavand, S.; Ebrahimi, M.; Foladi, E. Design and construction of a novel and an efficient potentiometric sensor for determination of sodium ion in urban water samples. Chem. Methodol. 2022, 6, 886–904. [Google Scholar]
- Alavi-Tabari, S.A.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Chitravathi, S.; Munichandraiah, N. Voltammetric determination of paracetamol, tramadol and caffeine using poly (Nile blue) modified glassy carbon electrode. J. Electroanal. Chem. 2016, 764, 93–103. [Google Scholar] [CrossRef]
- Tajik, S.; Taher, M.A.; Beitollahi, H. Simultaneous determination of droxidopa and carbidopa using a carbon nanotubes paste electrode. Sens. Actuators B Chem. 2013, 188, 923–930. [Google Scholar] [CrossRef]
- Vilian, A.E.; Hwang, S.K.; Bhaskaran, G.; Alhammadi, M.; Kim, S.; Tiwari, J.N.; Han, Y.K. Polypyrrole-MXene supported gold nanoparticles for the trace-level detection of nitrofurantoin. Chem. Eng. J. 2023, 454, 139980. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Shojaei, A.F.; Tabatabaeian, K.; Karimi, F.; Shakeri, S.; Moradi, R. Simultaneous determination of 6-mercaptopruine, 6-thioguanine and dasatinib as three important anticancer drugs using nanostructure voltammetric sensor employing Pt/MWCNTs and 1-butyl-3-methylimidazolium hexafluoro phosphate. Biosens. Bioelectron. 2016, 86, 879–884. [Google Scholar] [CrossRef]
- Mehdizadeh, Z.; Shahidi, S.A.; Ghorbani-HasanSaraei, A.; Limooei, M.B.; Bijad, M. Monitoring of amaranth in drinking samples using voltammetric amplified electroanalytical sensor. Chem. Methodol. 2022, 6, 246–252. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Shayegan, H.; Safarifard, V.; Taherkhani, H.; Rezvani, M.A. Efficient removal of cobalt(II) ion from aqueous solution using amide-functionalized metal-organic framework. J. Appl. Organomet. Chem. 2022, 2, 109–118. [Google Scholar]
- Tallapaneni, V.; Mude, L.; Pamu, D.; Karri, V.V.S.R. Formulation, characterization and in vitro evaluation of dual-drug loaded biomimetic chitosan-collagen hybrid nanocomposite scaffolds. J. Med. Chem. Sci. 2022, 5, 1059–1074. [Google Scholar]
- Sharma, M.; Yadav, S.; Srivastava, M.; Ganesh, N.; Srivastava, S. Promising anti-inflammatory bio-efficacy of saponin loaded silver nanoparticles prepared from the plant Madhuca longifolia. Asian J. Nanosci. Mater. 2022, 5, 313–326. [Google Scholar]
- Janitabar Darzi, S.; Bastami, H. Au decorated mesoporous TiO2 as a high performance photocatalyst towards crystal violet dye. Adv. J. Chem. A 2022, 5, 22–30. [Google Scholar]
- Kavade, R.; Khanapure, R.; Gawali, U.; Patil, A.; Patil, S. Degradation of methyl orange under visible light by ZnO-polyaniline nanocomposites. J. Appl. Organomet. Chem. 2022, 2, 101–112. [Google Scholar]
- Dormanesh, R.; Mohseni, S. Green synthesis of zinc oxide nanoparticles and their application in ring opening of epoxides. Prog. Chem. Biochem. Res. 2021, 4, 403–413. [Google Scholar]
- Muhi-Alden, Y.Y.; Saleh, K.A. Removing methylene blue dye from industrial wastewater using polyacrylonitrile/iron oxide nanocomposite. Eurasian Chem. Commun. 2021, 3, 755–762. [Google Scholar]
- Shete, R.C.; Fernandes, P.R.; Borhade, B.R.; Pawar, A.A.; Sonawane, M.C.; Warude, N.S. Review of cobalt oxide nanoparticles: Green synthesis, biomedical applications, and toxicity studies. J. Chem. Rev. 2022, 4, 331–345. [Google Scholar]
- Abadi, M.A.A.; Masrournia, M.; Abedi, M.R. Simultaneous extraction and preconcentration of benzene, toluene, ethylbenzene and xylenes from aqueous solutions using magnetite-graphene oxide composites. Chem. Methodol. 2021, 5, 11–20. [Google Scholar]
- Martins, E.C.; Santana, E.R.; Spinelli, A. Nitrogen and sulfur co-doped graphene quantum dot-modified electrode for monitoring of multivitamins in energy drinks. Talanta 2023, 252, 123836. [Google Scholar] [CrossRef]
- Hosseini Fakhrabad, A.; Sanavi Khoshnood, R.; Abedi, M.R.; Ebrahimi, M. Fabrication a composite carbon paste electrodes (CPEs) modified with multi-wall carbon nano-tubes (MWCNTs/N,N-Bis (salicyliden)-1,3-propandiamine) for determination of lanthanum (III). Eurasian Chem. Commun. 2021, 3, 627–634. [Google Scholar]
- Hasanpour, F.; Taei, M.; Fouladgar, M.; Salehi, M. Au nano dendrites/composition optimized Nd-dopped cobalt oxide as an efficient electrocatalyst for ethanol oxidation. J. Appl. Organomet. Chem. 2022, 2, 203–211. [Google Scholar]
- Zhou, Y.; Lü, H.; Zhang, D.; Xu, K.; Hui, N.; Wang, J. Electrochemical biosensors based on conducting polymer composite and PAMAM dendrimer for the ultrasensitive detection of acetamiprid in vegetables. Microchem. J. 2022, 185, 108284. [Google Scholar] [CrossRef]
- Mohammadi, S.; Beitollahi, H.; Mohadesi, A. Electrochemical behaviour of a modified carbon nanotube paste electrode and its application for simultaneous determination of epinephrine, uric acid and folic acid. Sens. Lett. 2013, 11, 388–394. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Shabani-Nooshabadi, M.; Baghayeri, M.; Karimi, F.; Rouhi, J.; Karaman, C. Determination of D&C Red 33 and Patent Blue V Azo dyes using an impressive electrochemical sensor based on carbon paste electrode modified with ZIF-8/g-C3N4/Co and ionic liquid in mouthwash and toothpaste as real samples. Food Chem. Toxicol. 2022, 162, 112907. [Google Scholar] [PubMed]
- Saritha, D.; Koirala, A.R.; Venu, M.; Reddy, G.D.; Reddy, A.V.B.; Sitaram, B.; Aruna, K. A simple, highly sensitive and stable electrochemical sensor for the detection of quercetin in solution, onion and honey buckwheat using zinc oxide supported on carbon nanosheet (ZnO/CNS/MCPE) modified carbon paste electrode. Electrochim. Acta 2019, 313, 523–531. [Google Scholar] [CrossRef]
- Ardakani, M.M.; Taleat, Z.; Beitollahi, H.; Salavati-Niasari, M.; Mirjalili, B.B.F.; Taghavinia, N. Electrocatalytic oxidation and nanomolar determination of guanine at the surface of a molybdenum (VI) complex–TiO2 nanoparticle modified carbon paste electrode. J. Electroanal. Chem. 2008, 624, 73–78. [Google Scholar] [CrossRef]
- Sánchez, A.; Villalonga, A.; Martínez-García, G.; Parrado, C.; Villalonga, R. Dendrimers as soft nanomaterials for electrochemical immunosensors. Nanomaterials 2019, 9, 1745. [Google Scholar] [CrossRef]
- Elancheziyan, M.; Senthilkumar, S. Redox-active gold nanoparticle-encapsulated poly (amidoamine) dendrimer for electrochemical sensing of 4-aminophenol. J. Mol. Liq. 2021, 325, 115131. [Google Scholar] [CrossRef]
- Elancheziyan, M.; Senthilkumar, S. Covalent immobilization and enhanced electrical wiring of hemoglobin using gold nanoparticles encapsulated PAMAM dendrimer for electrochemical sensing of hydrogen peroxide. Appl. Surf. Sci. 2019, 495, 143540. [Google Scholar] [CrossRef]
- Cui, H.; Cui, S.; Tian, Q.; Zhang, S.; Wang, M.; Zhang, P.; Li, X. Electrochemical sensor for the detection of 1-hydroxypyrene based on composites of PAMAM-regulated chromium-centered metal–organic framework nanoparticles and graphene oxide. ACS Omega 2021, 6, 31184–31195. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Mathew, G.; Anpo, M.; Neppolian, B. MOF based electrochemical sensors for the detection of physiologically relevant biomolecules: An overview. Coord. Chem. Rev. 2022, 468, 214627. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Zhang, H.W.; Yuan, R.; He, H. Ingenious fabrication of metal–organic framework/graphene oxide composites as aptasensors with superior electrochemical recognition capability. J. Mater. Chem. C 2020, 8, 15823–15829. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Q.; Zhang, Q.; Wang, H.; Wang, F.; Liu, J.; Song, K. Research progress of UiO-66-based electrochemical biosensors. Front. Chem. 2022, 10, 19. [Google Scholar] [CrossRef]
- Zhang, H.W.; Li, H.K.; Han, Z.Y.; Yuan, R.; He, H. Incorporating fullerenes in nanoscale metal–organic matrixes: An ultrasensitive platform for impedimetric aptasensing of tobramycin. ACS Appl. Mater. Interfaces 2022, 14, 7350–7357. [Google Scholar] [CrossRef]
- Rong, S.; Zou, L.; Meng, L.; Yang, X.; Dai, J.; Wu, M.; Pan, H. Dual function metal-organic frameworks based ratiometric electrochemical sensor for detection of doxorubicin. Anal. Chim. Acta 2022, 1196, 339545. [Google Scholar] [CrossRef]
- Duan, S.; Wu, X.; Shu, Z.; Xiao, A.; Chai, B.; Pi, F.; Liu, X. Curcumin-enhanced MOF electrochemical sensor for sensitive detection of methyl parathion in vegetables and fruits. Microchem. J. 2023, 184, 108182. [Google Scholar] [CrossRef]
- Li, Z.Y.; Huang, L.; Hu, R.; Yang, T.; Yang, Y.H. An electrochemical aptasensor based on intelligent walking DNA nanomachine with cascade signal amplification powered by nuclease for Mucin 1 assay. Anal. Chim. Acta 2022, 1214, 339964. [Google Scholar] [CrossRef]
- Zhang, H.W.; Zhu, Q.Q.; Yuan, R.; He, H. Crystal engineering of MOF@COF core-shell composites for ultra-sensitively electrochemical detection. Sens. Actuators B Chem. 2021, 329, 129144. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Y.; Zhang, Y.; Zeng, T.; Wan, Q.; Lai, G.; Yang, N. A UiO-66-NH2/carbon nanotube nanocomposite for simultaneous sensing of dopamine and acetaminophen. Anal. Chim. Acta 2021, 1158, 338419. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Li, Y.; Li, Y.; Li, Z.; Zhang, W.; Li, W. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef] [PubMed]
- Garkani-Nejad, F.; Sheikhshoaie, I.; Beitollahi, H. Simultaneous detection of carmoisine and tartrazine in food samples using GO-Fe3O4-PAMAM and ionic liquid based electrochemical sensor. Food Chem. Toxicol. 2022, 162, 112864. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A metal–organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wang, T.; Fan, Z.; Zhang, G. A thin film nanocomposite membrane with pre-immobilized UiO-66-NH2 toward enhanced nanofiltration performance. RSC Adv. 2019, 9, 24802–24810. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, L.; Zhao, Q.; Wang, X.; Jia, X. Highly selective visible-light photocatalytic benzene hydroxylation to phenol using a new heterogeneous photocatalyst UiO-66-NH2-SA-V. Catal. Lett. 2019, 149, 2408–2414. [Google Scholar] [CrossRef]
- Arabali, V.; Malekmohammadi, S.; Karimi, F. Surface amplification of pencil graphite electrode using CuO nanoparticle/polypyrrole nanocomposite; a powerful electrochemical strategy for determination of tramadol. Microchem. J. 2020, 158, 105179. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Jafari, M.; Mohammadzadeh-Jahani, P.; Ahmadi, S.A.; Mashayekh, R. Electrochemical determination of tramadol using modified screen-printed electrode. J. Electrochem. Sci. Eng. 2022, 12, 127–135. [Google Scholar]
- Mohamed, M.A.; Atty, S.A.; Salama, N.N.; Banks, C.E. Highly selective sensing platform utilizing graphene oxide and multiwalled carbon nanotubes for the sensitive determination of tramadol in the presence of co-formulated drugs. Electroanalysis 2017, 29, 1038–1048. [Google Scholar] [CrossRef]
- Babaei, A.; Taheri, A.R.; Afrasiabi, M. A multi-walled carbon nanotube-modified glassy carbon electrode as a new sensor for the sensitive simultaneous determination of paracetamol and tramadol in pharmaceutical preparations and biological fluids. J. Braz. Chem. Soc. 2011, 22, 1549–1558. [Google Scholar] [CrossRef]
- Manokaran, J.; Narendranath, J.; Muruganantham, R.; Balasubramanian, N. Nitrogen doped graphene supported Pt-Pd nanoparticle modified GC electrode for electrochemical determination of tramadol and paracetamol. Indian J. Chem. A 2017, 56, 63–68. [Google Scholar]
- Madrakian, T.; Alizadeh, S.; Bahram, M.; Afkhami, A. A novel electrochemical sensor based on magneto LDH/Fe3O4 nanoparticles@glassy carbon electrode for voltammetric determination of tramadol in real samples. Ionics 2017, 23, 1005–1015. [Google Scholar] [CrossRef]
- Hassannezhad, M.; Hosseini, M.; Ganjali, M.R.; Arvand, M. A graphitic carbon nitride (gC3N4/Fe3O4) nanocomposite: An efficient electrode material for the electrochemical determination of tramadol in human biological fluids. Anal. Methods 2019, 11, 2064–2071. [Google Scholar] [CrossRef]
- Hassanvand, Z.; Jalali, F. Gold nanoparticles/cysteic acid modified electrode for simultaneous electrochemical determination of tramadol and paracetamol. Anal. Bioanal. Chem. Res. 2019, 6, 393–404. [Google Scholar]
- Jahromi, Z.; Mirzaei, E.; Savardashtaki, A.; Afzali, M.; Afzali, Z. A rapid and selective electrochemical sensor based on electrospun carbon nanofibers for tramadol detection. Microchem. J. 2020, 157, 104942. [Google Scholar] [CrossRef]
- Mynttinen, E.; Wester, N.; Lilius, T.; Kalso, E.; Koskinen, J.; Laurila, T. Simultaneous electrochemical detection of tramadol and O-desmethyltramadol with Nafion-coated tetrahedral amorphous carbon electrode. Electrochim. Acta 2019, 295, 347–353. [Google Scholar] [CrossRef]
- Aflatoonian, M.R.; Tajik, S.; Aflatoonian, B.; Beitollahi, H.; Zhang, K.; Le, Q.V.; Cha, J.H.; Jang, H.W.; Shokouhimehr, M.; Peng, W. A screen-printed electrode modified with graphene/Co3O4 nanocomposite for electrochemical detection of tramadol. Front. Chem. 2020, 8, 562308. [Google Scholar] [CrossRef]
- Hajmalek, S.; Jahani, S.; Foroughi, M.M. Simultaneous voltammetric determination of tramadol and paracetamol exploiting glassy carbon electrode modified with FeNi3 nanoalloy in biological and pharmaceutical media. ChemistrySelect 2021, 6, 8797–8808. [Google Scholar] [CrossRef]


| Electrochemical Sensor | Electrochemical Technique | Linear Range | Limit of Detection | Sample Type | Ref. |
|---|---|---|---|---|---|
| Poly(Nile blue)/glassy carbon electrode | DPV | 1–310 μM | 0.5 µM | Ultracet® tablets | [25] |
| La3+/ZnO nano-flowers and multi-walled carbon nanotubes/screen-printed electrode | DPV | 0.5–800.0 μM | 0.08 μM | Tramadol tablets and urine | [68] |
| Multi-walled carbon nanotubes/glassy carbon electrode | DPV | 2–300 μM | 0.361 µM | Human serum, urine, and ZAFIN® tablets | [70] |
| Pt-Pd bimetallic nanoparticles/poly(diallyldimethylammonium chloride)/nitrogen-doped graphene/glassy carbon electrode | 1 SWV | 12.0–240.0 µM | 5.7 µM | Infected urine | [71] |
| Magneto layer double hydroxide (LDH)/Fe3O4/glassy carbon electrode | DPV | 1.0–200.0 µM | 0.3 µM | Human serum and human urine | [72] |
| Graphitic carbon nitride/Fe3O4 nanocomposite/carbon paste electrode | DPV | 0.2–14.0 µM and 14.0–120.0 µM | 0.1 µM | Human blood serum, human blood plasma, and urine | [73] |
| Au nanoparticles/cysteic acid/glassy carbon electrode | SWV | 0.5–63.5 µM | 0.17 µM | Human blood plasma | [74] |
| Electrospun carbon nanofibers/screen-printed electrode | SWV | 0.05–1.0 nM and 1.0–100 nM | 0.016 nM | Urine | [75] |
| Nafion-coated tetrahedral amorphous carbon electrode | DPV | 1–12.5 µM | 131 nM | Human plasma | [76] |
| Graphene/Co3O4 nanocomposite/screen-printed electrode | DPV | 0.1–500.0 μM | 0.03 μM | Tramadol tablets, Acetaminophen tablets, and urine | [77] |
| FeNi3 nanoalloy/glassy carbon electrode | DPV | 0.1–900.0 μM | 8.2 nM | Ultracet® tablets, tramadol tablets, acetaminophen tablets, serum, and urine | [78] |
| UiO-66-NH2 MOF-PAMAM/GCE | DPV | 0.5–500.0 µM | 0.2 µM | Tramadol tablets | This work |
| Sample | Spiked | Found | Recovery (%) | |||
|---|---|---|---|---|---|---|
| Tramadol | Acetaminophen | Tramadol | Acetaminophen | Tramadol | Acetaminophen | |
| Tramadol tablets | 0 | 0 | 4.0 ± 0.05 | - | - | - |
| 1.0 | 4.0 | 4.9 ± 0.04 | 4.1 ± 0.07 | 98.0 | 102.5 | |
| 2.0 | 6.0 | 6.2 ± 0.05 | 5.8 ± 0.05 | 103.3 | 96.7 | |
| 3.0 | 8.0 | 7.1 ± 0.03 | 7.9 ± 0.04 | 101.4 | 98.7 | |
| 4.0 | 10.0 | 7.9 ± 0.06 | 10.1 ± 0.04 | 98.7 | 101.0 | |
| Acetaminophen tablets | 0 | 0 | - | 3.5 ± 0.05 | - | - |
| 5.0 | 1.0 | 5.1 ± 0.05 | 4.4 ± 0.06 | 102.0 | 97.8 | |
| 7.0 | 2.0 | 6.8 ± 0.03 | 5.6 ± 0.03 | 97.1 | 101.8 | |
| 9.0 | 3.0 | 9.1 ± 0.04 | 6.4 ± 0.06 | 101.1 | 98.5 | |
| 11.0 | 4.0 | 10.9 ± 0.05 | 7.6 ± 0.04 | 99.1 | 101.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garkani Nejad, F.; Beitollahi, H.; Sheikhshoaie, I. A UiO-66-NH2 MOF/PAMAM Dendrimer Nanocomposite for Electrochemical Detection of Tramadol in the Presence of Acetaminophen in Pharmaceutical Formulations. Biosensors 2023, 13, 514. https://doi.org/10.3390/bios13050514
Garkani Nejad F, Beitollahi H, Sheikhshoaie I. A UiO-66-NH2 MOF/PAMAM Dendrimer Nanocomposite for Electrochemical Detection of Tramadol in the Presence of Acetaminophen in Pharmaceutical Formulations. Biosensors. 2023; 13(5):514. https://doi.org/10.3390/bios13050514
Chicago/Turabian StyleGarkani Nejad, Fariba, Hadi Beitollahi, and Iran Sheikhshoaie. 2023. "A UiO-66-NH2 MOF/PAMAM Dendrimer Nanocomposite for Electrochemical Detection of Tramadol in the Presence of Acetaminophen in Pharmaceutical Formulations" Biosensors 13, no. 5: 514. https://doi.org/10.3390/bios13050514
APA StyleGarkani Nejad, F., Beitollahi, H., & Sheikhshoaie, I. (2023). A UiO-66-NH2 MOF/PAMAM Dendrimer Nanocomposite for Electrochemical Detection of Tramadol in the Presence of Acetaminophen in Pharmaceutical Formulations. Biosensors, 13(5), 514. https://doi.org/10.3390/bios13050514






