A 512-Ch Dual-Mode Microchip for Simultaneous Measurements of Electrophysiological and Neurochemical Activities
Abstract
1. Introduction
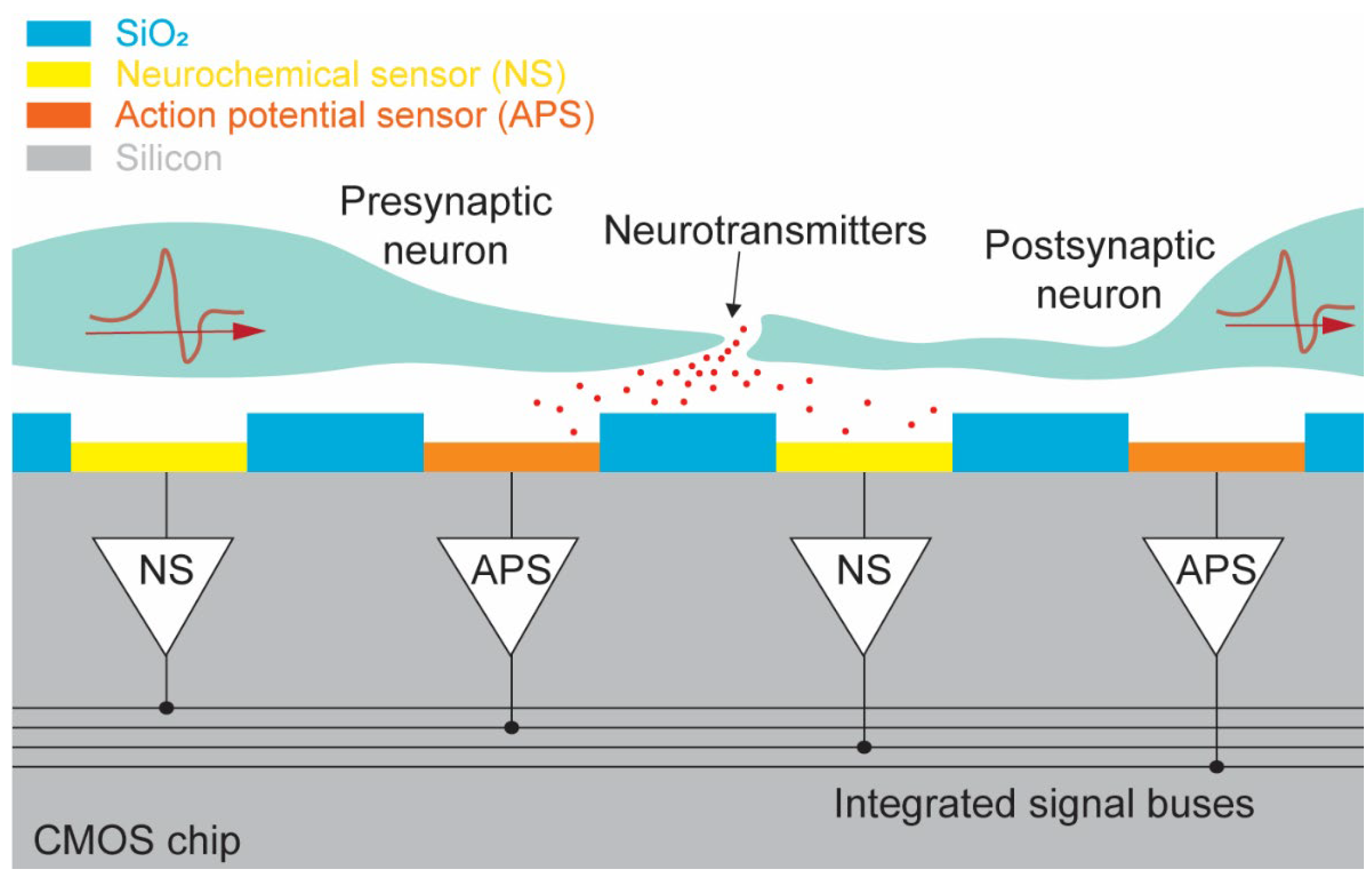
2. Dual-Mode Chip Concept
2.1. System Design
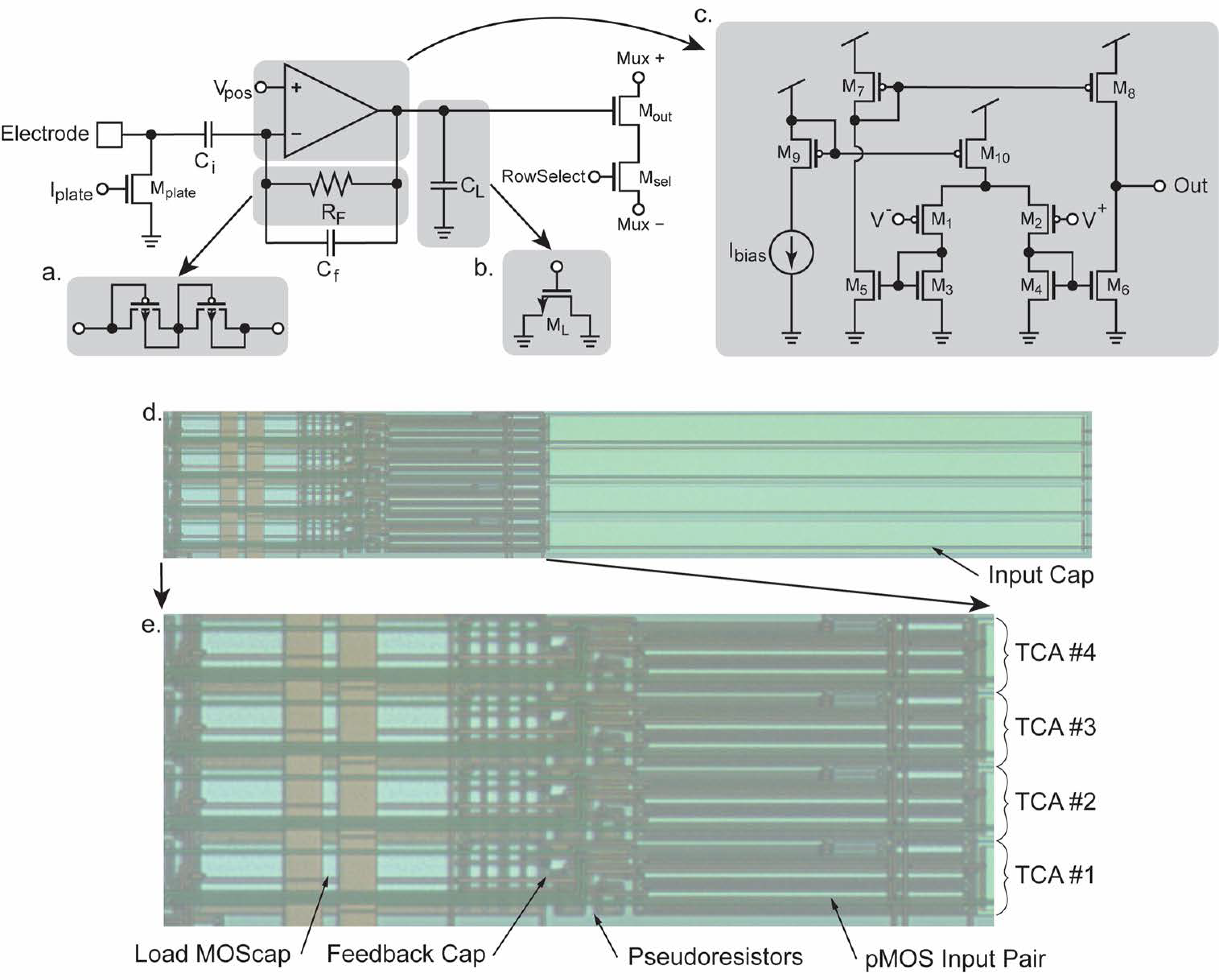
2.2. Fundamental Transimpedance Amplifier Concept
2.3. Fundamental Transconductance Amplifier Concept
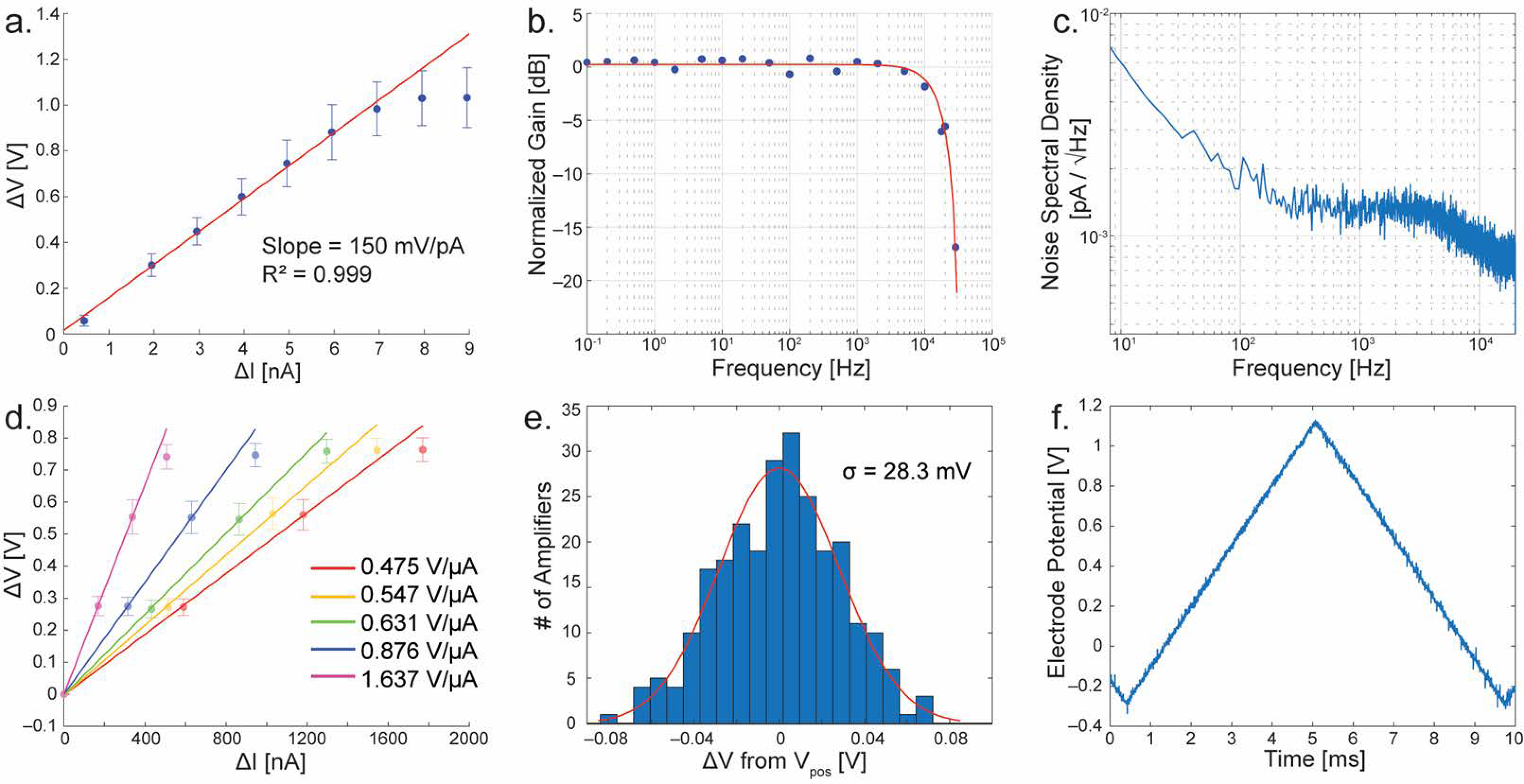
3. Electrochemical Amplifier
3.1. Amperometry Mode
3.2. Fast Scan Cyclic Voltammetry Mode
3.3. Electrochemical Amplifier Performance
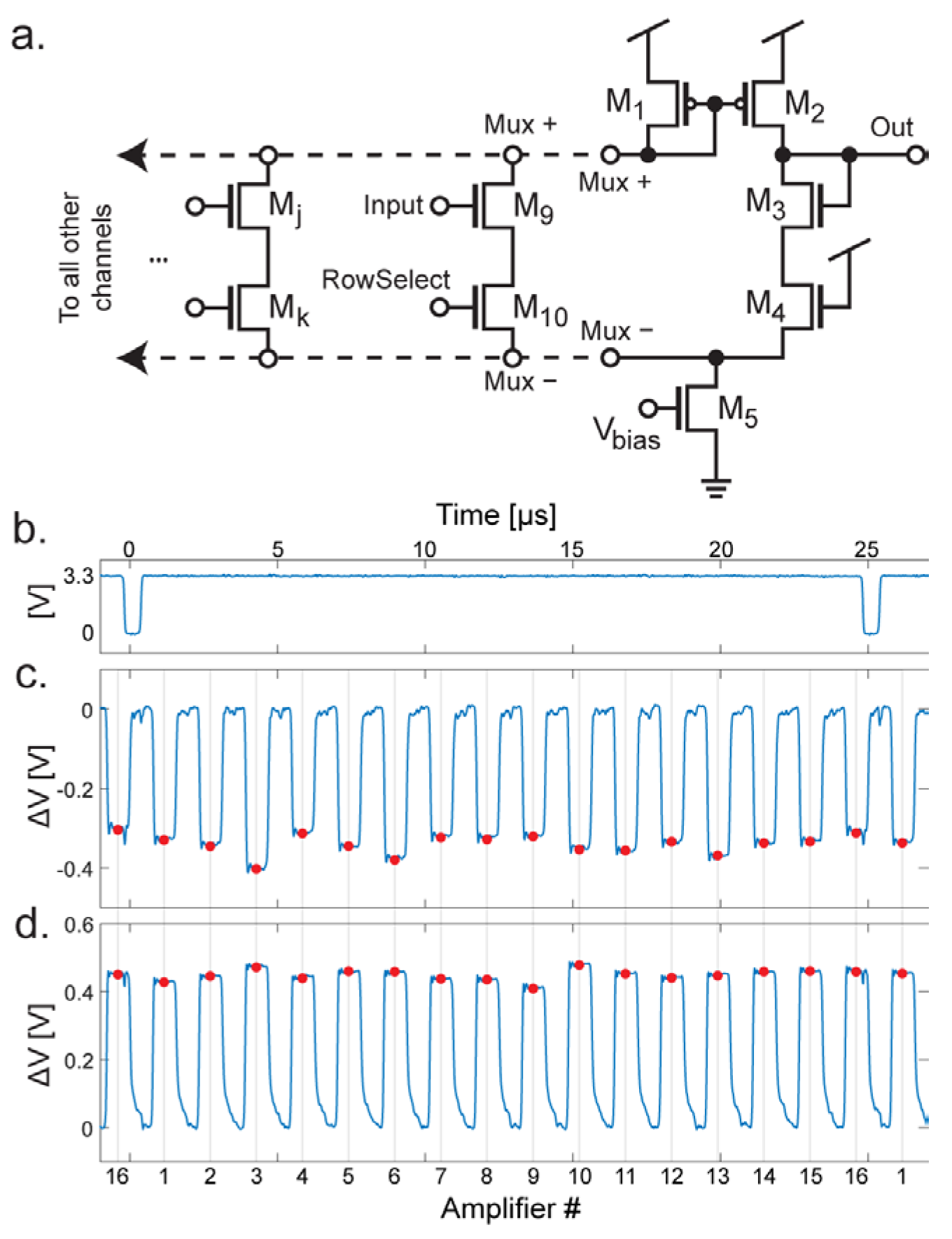
3.4. Multiplexing
| Chip | Current Amplifiers | Voltage Amplifiers | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Year | Dual-Mode | Process Node | Die Size | Voltage | Total Power | Electrodes | Electrode Pitch | Parallel Channels | Size/Ch | Sample Rate | Bandwidth | Noise (RMS) | Current | Power/Ch | Parallel Channels | Size/Ch | Sample Rate | Bandwidth | Noise (RMS) | Current | Power/Ch |
| [33] | 2003 | No | 1.5 μm | 2.2 × 2.2 mm | 5 V | - | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 6 | 160,000 μm2 | - | 7.5 kHz | 2.2 μV | 8 μA | 40 μW |
| [39] | 2016 | No | 0.13 μm | 9 × 5 mm | 1.8 V | - | 966 | ~70 μm | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 384 | - | 30 kHz | 10 kHz | 6.4 μV | - | 49.1 μW |
| [40] | 2017 | No | 0.35 μm | - | - | - | 36 | 25 μm | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 36 | 70,000 μm2 | 25 kHz | 300 Hz–10 kHz | 2.2 μV | - | 30 μW |
| [41] | 2020 | No | 90 nm | 32.5 × 25.1 mm | - | 4420 mW | 236,880 | 11.72 μm | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 33,840 | - | 10 kHz | 10 kHz | 5.5 μV | - | - |
| [42] | 2020 | No | 0.18 µm | 5.6 × 5.6 mm | - | - | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 32 | 245,000 μm2 | - | 200 Hz | 1.49 μV | - | - |
| [43] | 2021 | No | 0.18 µm | 6 × 9 mm | - | - | 19,584 | 18.0 μm | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 19,584 | - | 11.6 kHz | 5 kHz | 10.4 μV | - | 5.9 μW |
| [44] | 2022 | No | - | 5.9 × 5.24 mm | - | 30.7 mW | 24,320 | 17.7 μm | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 380 | - | 20 kHz | 300 Hz–10 kHz | 5.4 μV | - | - |
| [23] | 2015 | Yes | 0.18 μm | 5 × 2.65 mm | 1.8 V | 3.21 mW | 200 * | 200 μm * | 100 | 30,000 μm2 | 20 kHz | 110 Hz–10 kHz | 480 fA ** | - | 12.1 μW | 100 | 30,000 μm2 | 20 kHz | 10 kHz | 4.07 μV | N/A | 9.1 μW |
| [29] | 2017 | Yes | 0.18 μm | 12 × 8.9 mm | - | 86 mW | 59,760 | 13.5 μm | 28 | 40,000 μm2 | 20 kHz | 16 kHz | 120 pA | - | 178 μW | 2048 | - | 20 kHz | 300 Hz–10 kHz | 5.4 μV | - | 16 μW |
| [24] | 2017 | Yes | 0.13 μm | 3 × 1.85 mm | - | - | 1024 | 58 μm | 4 | 8000 μm2 | - | 700 Hz | 56 pA | - | - | 1024 | - | - | 300 Hz–6 kHz | 7.1 μV | ~3–30 μA | - |
| [45] | 2020 | Yes | 0.18 µm | ~10 × 20 mm | - | - | 4096 | 20 µm | 4096 *** | 25,000 μm2 | 9.4 kHz | 4.7 kHz | ~1 pA | - | - | 4096 *** | 25,000 μm2 | 9.415 kHz | 4.7 kHz | 20μV | - | - |
| This Work | 2022 | Yes | 0.35 μm | 5 × 5 mm | 3.3 V | 11.5 mW | 512 | 16 μm | 256 | 3070 μm2 | 40 kHz | 10.3 kHz | 4.51 pA | 4.8 μA | 16 μW | 256 | 11,000 μm2 | 40 kHz | 0.2 Hz–10 kHz | 24.9 μV | 8 μA | 26 μW |
| [46] | 2006 | No | 0.5 μm | - | 5 V | - | 25 | 15 µm | 25 | 525 μm2 | - | 2 kHz | ~110 fA | - | 1 μW | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| [13] | 2012 | No | 0.5 μm | 3 × 3 mm | 5 V | - | 100 | ~50 μm | 100 | 900 μm2 | 2 kHz | ~1 kHz | ~100 fA | - | - | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| [47] | 2015 | No | 0.6 μm | - | - | - | 1,048,576 | 3.6 × 4.45 µm | - | - | - | 10 kHz | 21.8 pA | - | - | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| [48] | 2018 | No | 0.5 µm | 2.5 × 2.5 mm | - | 2.1 mW | 100 | - | 25 | 60,000 μm2 | - | 11.5 kHz | 7.2 pA | - | - | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| [35] | 2018 | No | 0.5 µm | - | 5 V | 4.85 mW | 64 | - | 64 | 1350 μm2 | 10 kHz | 10 kHz | 443 fA | - | 13.5 μW | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| [32] | 2019 | No | 0.35 μm | 5 × 5 mm | - | 12.5 mW | 1024 | - | 1024 | 90 μm2 | 10 kHz | 4.4 kHz | 415 fA | - | - | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| [49] | 2022 | No | 0.18 µm | 25 mm2 | - | 58.8 mW | 131,072 | 10 μm | 131,072 | - | 0.062 Hz | - | - | - | - | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
4. Electrophysiology Amplifier
4.1. Transconductance Amplifier Design
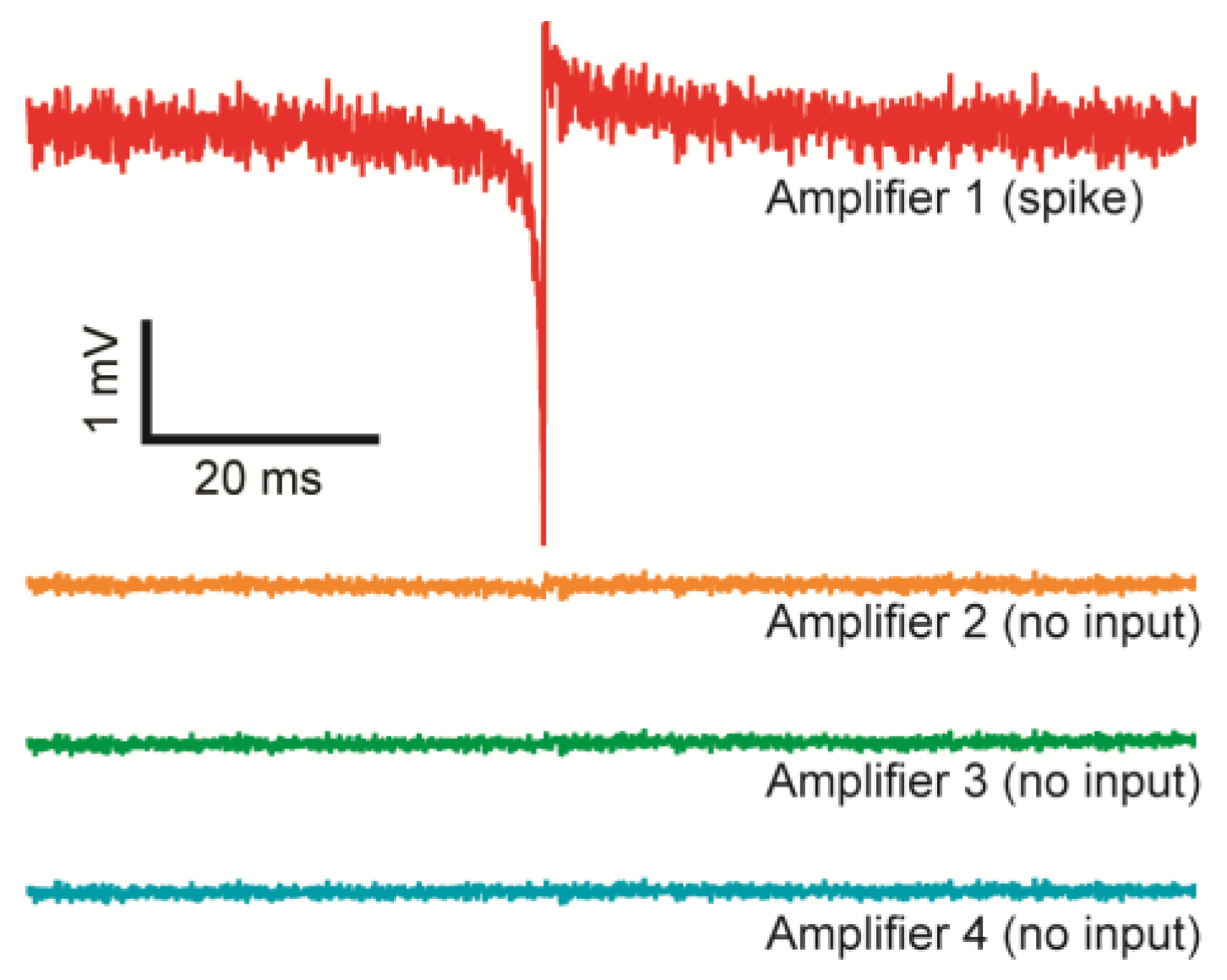
4.2. Transconductance Amplifier Performance
5. CMOS Implementation
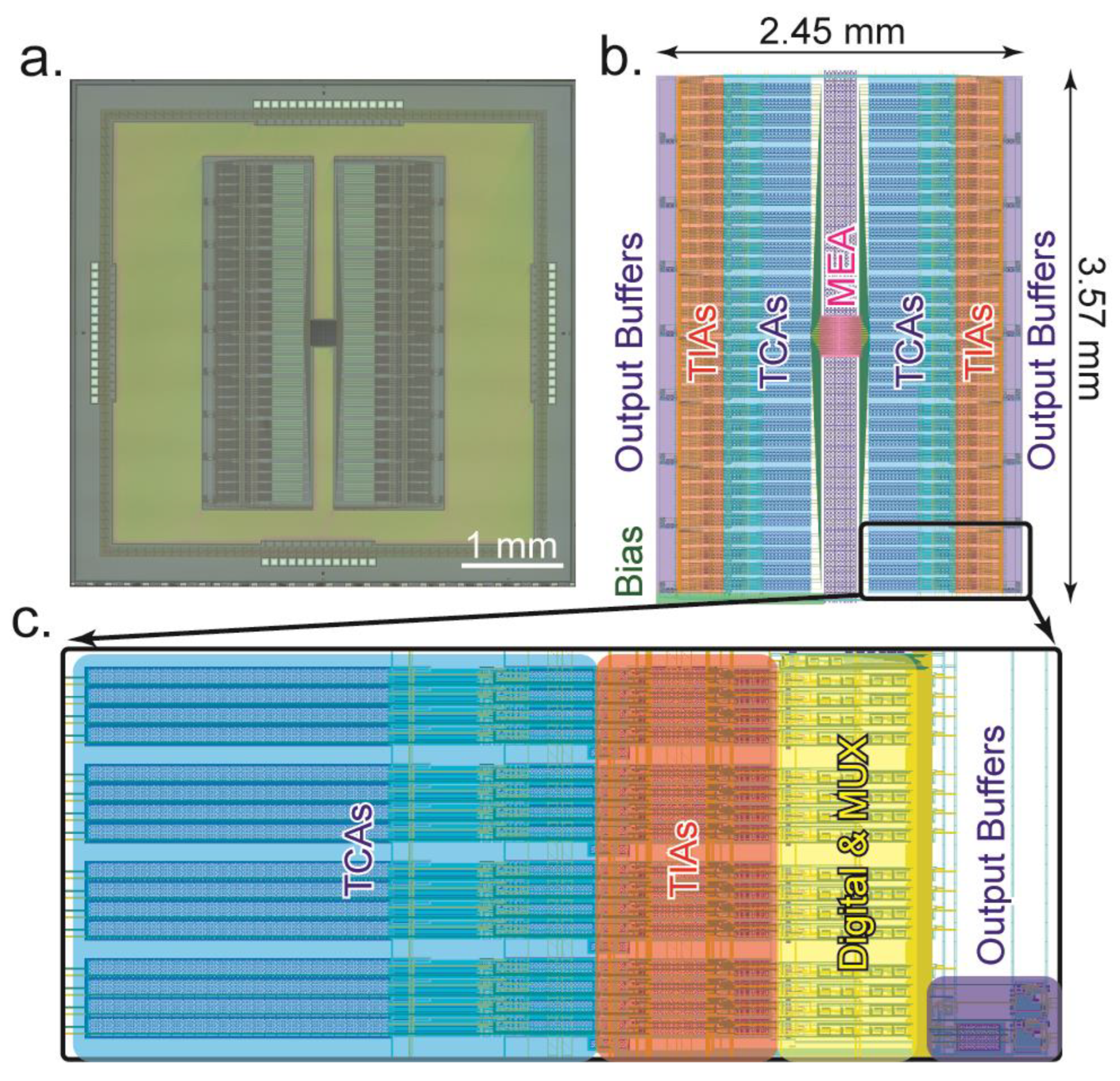
5.1. Chip Layout
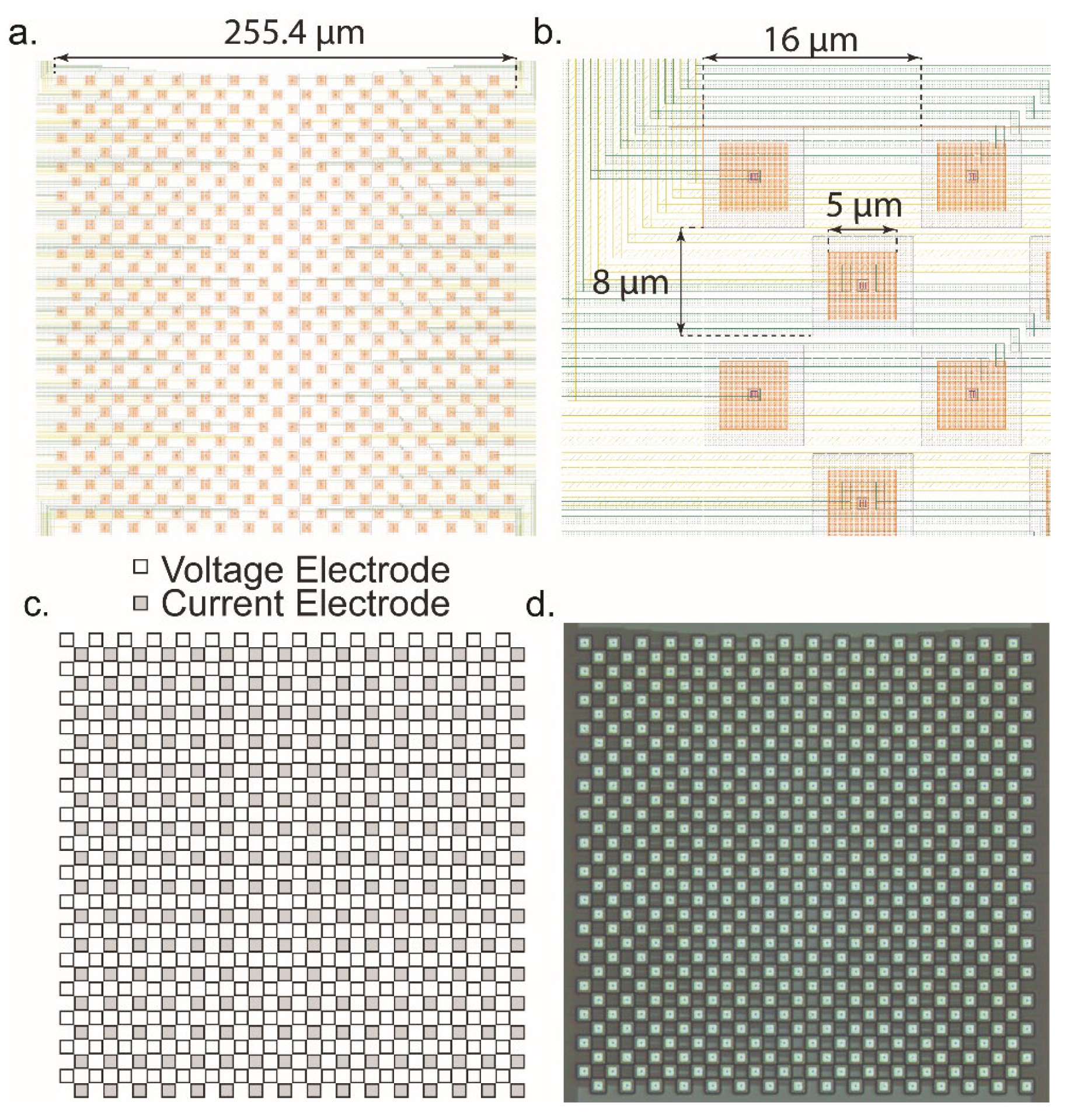
5.2. Microelectrode Array
6. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cleary, J.P.; Walsh, D.M.; Hofmeister, J.J.; Shankar, G.M.; Kuskowski, M.A.; Selkoe, D.J.; Ashe, K.H. Natural Oligomers of the Amyloid-β Protein Specifically Disrupt Cognitive Function. Nat. Neurosci. 2005, 8, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s Disease: Genes, Proteins, and Therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical Basis of Cognitive Alterations in Alzheimer’s Disease: Synapse Loss Is the Major Correlate of Cognitive Impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Taoufik, E.; Kouroupi, G.; Zygogianni, O.; Matsas, R. Synaptic Dysfunction in Neurodegenerative and Neurodevelopmental Diseases: An Overview of Induced Pluripotent Stem-Cell-Based Disease Models. Open Biol. 2018, 8, 180138. [Google Scholar] [CrossRef] [PubMed]
- Staal, R.G.W.; Rayport, S.; Sulzer, D. Amperometric Detection of Dopamine Exocytosis from Synaptic Terminals; CRC Press: Boca Raton, FL, USA, 2007; ISBN 0849340756. [Google Scholar]
- Braak, H.; Braak, E.; Bohl, J. Staging of Alzheimer-Related Cortical Destruction. Eur. Neurol. 1993, 33, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. The Molecular Pathology of Alzheimer’s Disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Stefanis, L. α-Synuclein in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, F.; Wang, D.; Li, C.; Fu, Y.; He, W.; Zhang, J. Tau Pathology in Parkinson’s Disease. Front. Neurol. 2018, 9, 809. [Google Scholar] [CrossRef]
- Duka, T.; Duka, V.; Joyce, J.N.; Sidhu, A. A-Synuclein Contributes to GSK-3β-catalyzed Tau Phosphorylation in Parkinson’s Disease Models. FASEB J. 2009, 23, 2820–2830. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; DeKosky, S.T.; Mufson, E.J. Synaptic Alterations in CA1 in Mild Alzheimer Disease and Mild Cognitive Impairment. Neurology 2007, 68, 1501–1508. [Google Scholar] [CrossRef]
- Maestú, F.; Peña, J.-M.; Garcés, P.; González, S.; Bajo, R.; Bagic, A.; Cuesta, P.; Funke, M.; Mäkelä, J.P.; Menasalvas, E.; et al. A Multicenter Study of the Early Detection of Synaptic Dysfunction in Mild Cognitive Impairment Using Magnetoencephalography-Derived Functional Connectivity. Neuroimage Clin. 2015, 9, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.N.; Herbst, A.D.; Kim, S.J.; Minch, B.A.; Lindau, M. Parallel Recording of Neurotransmitters Release from Chromaffin Cells Using a 10×10 CMOS IC Potentiostat Array with On-Chip Working Electrodes. Biosens. Bioelectron. 2013, 41, 736–744. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Mulberry, G.; Smith, J.; Lindau, M.; Minch, B.A.; Sugaya, K.; Kim, B.N. Single-Cell Recording of Vesicle Release From Human Neuroblastoma Cells Using 1024-Ch Monolithic CMOS Bioelectronics. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.E.; Schmitz, Y.; Troyer, M.D.; Mosharov, E.; Dietrich, P.; Quazi, A.Z.; Savalle, M.; Nemani, V.; Chaudhry, F.A.; Edwards, R.H.; et al. Alpha-Synuclein Overexpression in PC12 and Chromaffin Cells Impairs Catecholamine Release by Interfering with a Late Step in Exocytosis. J. Neurosci. 2006, 26, 11915–11922. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Reim, K.; Matti, U.; Brose, N.; Binz, T.; Rettig, J.; Neher, E.; Sørensen, J.B. Regulation of Releasable Vesicle Pool Sizes by Protein Kinase A-Dependent Phosphorylation of SNAP-25. Neuron 2004, 41, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Klenchin, V.A.; Martin, T.F.J. Priming in Exocytosis: Attaining Fusion-Competence after Vesicle Docking. Biochimie 2000, 82, 399–407. [Google Scholar] [CrossRef]
- Stefanis, L.; Larsen, K.E.; Rideout, H.J.; Sulzer, D.; Greene, L.A. Expression of A53T Mutant but Not Wild-Type Alpha-Synuclein in PC12 Cells Induces Alterations of the Ubiquitin-Dependent Degradation System, Loss of Dopamine Release, and Autophagic Cell Death. J. Neurosci. 2001, 21, 9549–9560. [Google Scholar] [CrossRef]
- Snider, R.S. Neurophysiology: A Primer. Neurology 1967, 17, 111. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K. Animal Physiology: Adaptation and Environment, 5th ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Bullock, T.H. Introduction to Nervous Systems; W. H. Freeman: San Francisco, CA, USA, 1977. [Google Scholar]
- Amin, H.; Nieus, T.; Lonardoni, D.; Maccione, A.; Berdondini, L. High-Resolution Bioelectrical Imaging of Aβ-Induced Network Dysfunction on CMOS-MEAs for Neurotoxicity and Rescue Studies. Sci. Rep. 2017, 7, 2460. [Google Scholar] [CrossRef]
- Guo, J.; Ng, W.; Yuan, J.; Li, S.; Chan, M. A 200-Channel Area-Power-Efficient Chemical and Electrical Dual-Mode Acquisition IC for the Study of Neurodegenerative Diseases. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 567–578. [Google Scholar] [CrossRef]
- Park, J.S.; Aziz, M.K.; Li, S.; Chi, T.; Grijalva, S.I.; Sung, J.H.; Cho, H.C.; Wang, H. 1024-Pixel CMOS Multimodality Joint Cellular Sensor/Stimulator Array for Real-Time Holistic Cellular Characterization and Cell-Based Drug Screening. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 80–94. [Google Scholar] [CrossRef]
- Kouhalvandi, L.; Matekovits, L.; Peter, I. Amplifiers in Biomedical Engineering: A Review from Application Perspectives. Sensors 2023, 23, 2277. [Google Scholar] [CrossRef] [PubMed]
- Nordi, T.M.; Gounella, R.H.; Luppe, M.; Junior, J.N.S.; Fonoff, E.T.; Colombari, E.; Romero, M.A.; Do Carmo, J.P.P. Low-Noise Amplifier for Deep-Brain Stimulation (DBS). Electronics 2022, 11, 939. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Q.; Chen, K.; Zhang, H.; Yang, Y.; Zheng, N.; Hong, H. An Ultra-Low-Noise, Low Power and Miniaturized Dual-Channel Wireless Neural Recording Microsystem. Biosensors 2022, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Vijay, V.; Raziyeh, B.; Amir, S.; Jelena, D.; Alicia, B.J.; Axel, B.; Jan, M.; Yihui, C.; Andreas, H. High-Density CMOS Microelectrode Array System for Impedance Spectroscopy and Imaging of Biological Cells. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Dragas, J.; Viswam, V.; Shadmani, A.; Chen, Y.; Bounik, R.; Stettler, A.; Radivojevic, M.; Geissler, S.; Obien, M.E.J.; Müller, J.; et al. In Vitro Multi-Functional Microelectrode Array Featuring 59,760 Electrodes, 2048 Electrophysiology Channels, Stimulation, Impedance Measurement, and Neurotransmitter Detection Channels. IEEE J. Solid-State Circuits 2017, 52, 1576–1590. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Kim, B.N. Quantifying Neurotransmitter Secretion at Single-Vesicle Resolution Using High-Density Complementary Metal–Oxide–Semiconductor Electrode Array. Nat. Commun. 2021, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Mulberry, G.; Kim, B.N. Rapid 1024-Pixel Electrochemical Imaging at 10,000 Frames Per Second Using Monolithic CMOS Sensor and Multifunctional Data Acquisition System. IEEE Sens. J. 2018, 18, 5507–5514. [Google Scholar] [CrossRef]
- Mulberry, G.; White, K.A.; Kim, B.N. Analysis of Simple Half-Shared Transimpedance Amplifier for Picoampere Biosensor Measurements. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 387–395. [Google Scholar] [CrossRef]
- Harrison, R.R.; Charles, C. A Low-Power Low-Noise CMOS Amplifier for Neural Recording Applications. IEEE J. Solid-State Circuits 2003, 38, 958–965. [Google Scholar] [CrossRef]
- White, K.A.; Mulberry, G.; Kim, B.N. Parallel 1024-Ch Cyclic Voltammetry on Monolithic CMOS Electrochemical Detector Array. IEEE Sens. J. 2020, 20, 4395–4402. [Google Scholar] [CrossRef]
- Dorta-Quinones, C.I.; Huang, M.; Ruelas, J.C.; Delacruz, J.; Apsel, A.B.; Minch, B.A.; Lindau, M. A Bidirectional-Current CMOS Potentiostat for Fast-Scan Cyclic Voltammetry Detector Arrays. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Kudur Jayaprakash, G.; Swamy, B.E.K.; Flores-Moreno, R.; Pineda-Urbina, K. Theoretical and Cyclic Voltammetric Analysis of Asparagine and Glutamine Electrocatalytic Activities for Dopamine Sensing Applications. Catalysts 2023, 13, 100. [Google Scholar] [CrossRef]
- Heien, M.L.; Johnson, M.A.; Wightman, R.M. Resolving Neurotransmitters Detected by Fast-Scan Cyclic Voltammetry. Anal. Chem. 2004, 76, 5697–5704. [Google Scholar] [CrossRef] [PubMed]
- Mora Lopez, C.; Putzeys, J.; Raducanu, B.C.; Ballini, M.; Wang, S.; Andrei, A.; Rochus, V.; Vandebriel, R.; Severi, S.; van Hoof, C.; et al. A Neural Probe With Up to 966 Electrodes and Up to 384 Configurable Channels in 0.13 μm SOI CMOS. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 510–522. [Google Scholar] [CrossRef]
- Angotzi, G.N.; Malerba, M.; Maccione, A.; Boi, F.; Crepaldi, M.; Bonanno, A.; Berdondini, L. A High Temporal Resolution Multiscale Recording System for in Vivo Neural Studies. In Proceedings of the 2017 IEEE International Symposium on Circuits and Systems (ISCAS), Baltimore, MD, USA, 28–31 May 2017; pp. 1–4. [Google Scholar]
- Kato, Y.; Matoba, Y.; Honda, K.; Ogawa, K.; Shimizu, K.; Maehara, M.; Fujiwara, A.; Odawara, A.; Yamane, C.; Kimizuka, N.; et al. High-Density and Large-Scale MEA System Featuring 236,880 Electrodes at 11.72 μm Pitch for Neuronal Network Analysis. In Proceedings of the 2020 IEEE Symposium on VLSI Circuits, Honolulu, HI, USA, 16–19 June 2020; pp. 1–2. [Google Scholar]
- Malekzadeh-Arasteh, O.; Pu, H.; Lim, J.; Liu, C.Y.; Do, A.H.; Nenadic, Z.; Heydari, P. An Energy-Efficient CMOS Dual-Mode Array Architecture for High-Density ECoG-Based Brain-Machine Interfaces. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 332–342. [Google Scholar] [CrossRef]
- Yuan, X.; Hierlemann, A.; Frey, U. Extracellular Recording of Entire Neural Networks Using a Dual-Mode Microelectrode Array With 19 584 Electrodes and High SNR. IEEE J. Solid-State Circuits 2021, 56, 2466–2475. [Google Scholar] [CrossRef]
- Cha, J.-H.; Park, J.-H.; Park, Y.; Shin, H.; Hwang, K.S.; Cho, I.-J.; Kim, S.-J. A Reconfigurable Sub-Array Multiplexing Microelectrode Array System With 24,320 Electrodes and 380 Readout Channels for Investigating Neural Communication. In Proceedings of the 2022 IEEE International Solid- State Circuits Conference (ISSCC), San Francisco, CA, USA, 20–26 February 2022; pp. 342–344. [Google Scholar]
- Abbott, J.; Ye, T.; Krenek, K.; Gertner, R.S.; Ban, S.; Kim, Y.; Qin, L.; Wu, W.; Park, H.; Ham, D. A Nanoelectrode Array for Obtaining Intracellular Recordings from Thousands of Connected Neurons. Nat. Biomed. Eng. 2020, 4, 232–241. [Google Scholar] [CrossRef]
- Ayers, S.; Gillis, K.D.; Lindau, M.; Minch, B.A. Design of a CMOS Potentiostat Circuit for Electrochemical Detector Arrays. IEEE Trans. Circuits Syst. I Regul. Pap. 2007, 54, 736–744. [Google Scholar] [CrossRef]
- Niitsu, K.; Ota, S.; Gamo, K.; Kondo, H.; Hori, M.; Nakazato, K. Development of Microelectrode Arrays Using Electroless Plating for CMOS-Based Direct Counting of Bacterial and HeLa Cells. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 607–619. [Google Scholar] [CrossRef]
- Li, H.; Parsnejad, S.; Ashoori, E.; Thompson, C.; Purcell, E.K.; Mason, A.J. Ultracompact Microwatt CMOS Current Readout with Picoampere Noise and Kilohertz Bandwidth for Biosensor Arrays. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Incandela, J.; Lian, X.; Larkin, J.W.; Rosenstein, J.K. A 13.1 mm 2512 × 256 Multimodal CMOS Array for Spatiochemical Imaging of Bacterial Biofilms. In Proceedings of the 2022 IEEE Custom Integrated Circuits Conference (CICC), Newport Beach, CA, USA, 24–27 April 2022; pp. 1–2. [Google Scholar]
- Enz, C.C.; Krummenacher, F.; Vittoz, E.A. An Analytical MOS Transistor Model Valid in All Regions of Operation and Dedicated to Low-Voltage and Low-Current Applications. Analog. Integr. Circuits Signal. Process. 1995, 8, 83–114. [Google Scholar] [CrossRef]
- Najafi, K.; Wise, K.D. An Implantable Multielectrode Array with On-Chip Signal Processing. IEEE J. Solid-State Circuits 1986, 21, 1035–1044. [Google Scholar] [CrossRef]
- Metting van Rijn, A.C.; Peper, A.; Grimbergen, C.A. High-Quality Recording of Bioelectric Events. Med. Biol. Eng. Comput. 1990, 28, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Izhikevich, E.M. Simple Model of Spiking Neurons. IEEE Trans. Neural Netw. 2003, 14, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Emmenegger, V.; Obien, M.E.J.; Hierlemann, A.; Frey, U. Dual-Mode Microelectrode Array Featuring 20k Electrodes and High SNR for Extracellular Recording of Neural Networks. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 1–4. [Google Scholar]
- Steinmetz, N.A.; Aydin, C.; Lebedeva, A.; Okun, M.; Pachitariu, M.; Bauza, M.; Beau, M.; Bhagat, J.; Böhm, C.; Broux, M.; et al. Neuropixels 2.0: A Miniaturized High-Density Probe for Stable, Long-Term Brain Recordings. Science 2021, 372, eabf4588. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulberry, G.; White, K.A.; Crocker, M.A.; Kim, B.N. A 512-Ch Dual-Mode Microchip for Simultaneous Measurements of Electrophysiological and Neurochemical Activities. Biosensors 2023, 13, 502. https://doi.org/10.3390/bios13050502
Mulberry G, White KA, Crocker MA, Kim BN. A 512-Ch Dual-Mode Microchip for Simultaneous Measurements of Electrophysiological and Neurochemical Activities. Biosensors. 2023; 13(5):502. https://doi.org/10.3390/bios13050502
Chicago/Turabian StyleMulberry, Geoffrey, Kevin A. White, Matthew A. Crocker, and Brian N. Kim. 2023. "A 512-Ch Dual-Mode Microchip for Simultaneous Measurements of Electrophysiological and Neurochemical Activities" Biosensors 13, no. 5: 502. https://doi.org/10.3390/bios13050502
APA StyleMulberry, G., White, K. A., Crocker, M. A., & Kim, B. N. (2023). A 512-Ch Dual-Mode Microchip for Simultaneous Measurements of Electrophysiological and Neurochemical Activities. Biosensors, 13(5), 502. https://doi.org/10.3390/bios13050502





