A Comprehensive Review on Electrochemical Nano Biosensors for Precise Detection of Blood-Based Oncomarkers in Breast Cancer
Abstract
1. Introduction
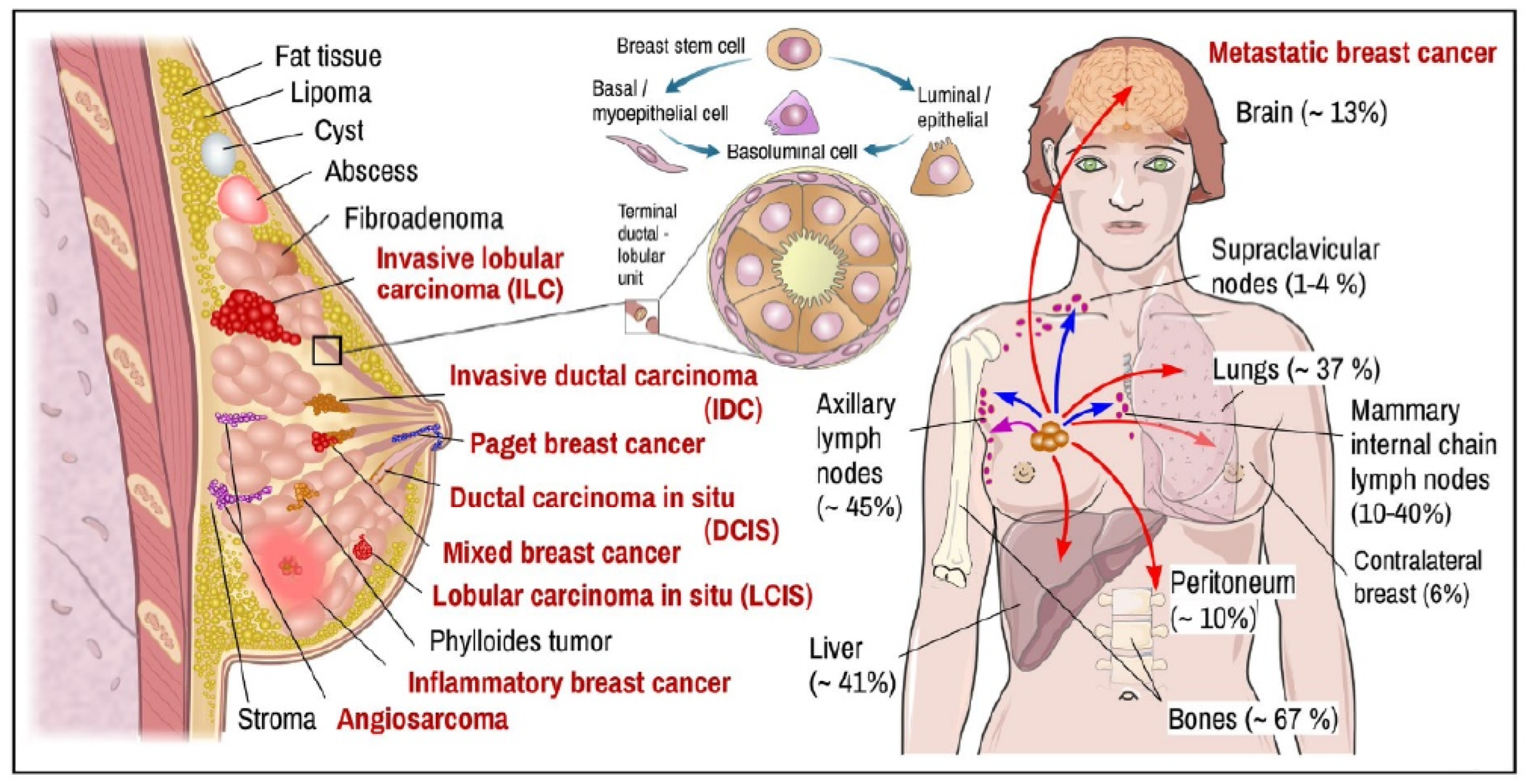
2. Breast Tissue Pathobiology
3. Blood-Based Oncomarkers of BC
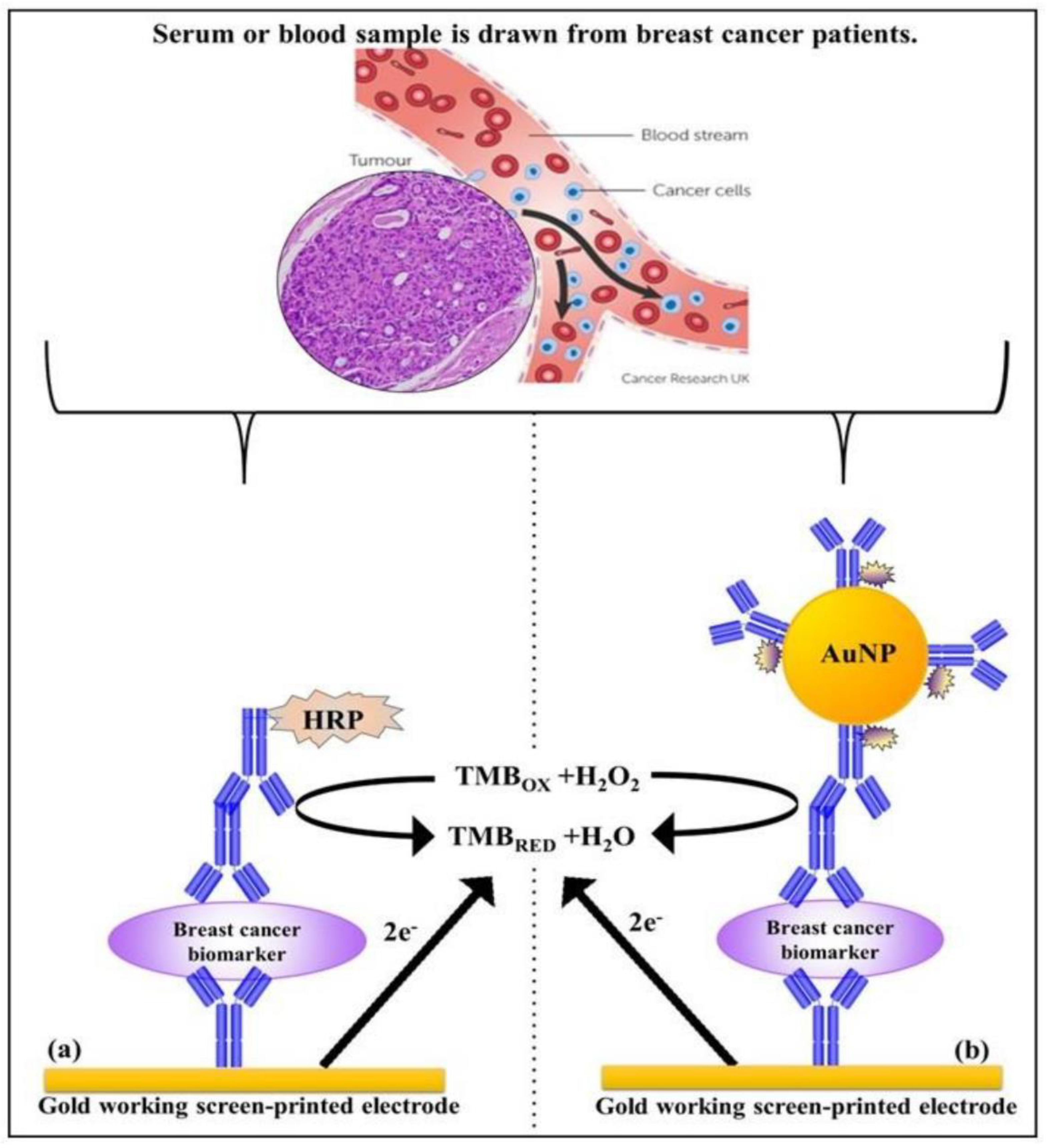
3.1. Proteins of Carcinoma in the Circulation
3.2. Circulating Tumor Cells (CTC)
3.3. Circulating Tumor-Specific DNA (ctDNA)
3.4. Circulating miRNAs
3.5. Extracellular Vesicles (Evs)
4. Electrochemical Biosensors Based on Nanostructured Materials as Ultrasensitive Platforms for Detecting BC Oncomarkers
4.1. Carcinoma Proteins in the Circulation
4.1.1. CA15-3
4.1.2. Carbohydrate Antigen 125 (CA 125)
4.1.3. CA 27-29
4.1.4. CD44
4.1.5. CEA
4.1.6. HER2
4.1.7. EGFR
4.1.8. EpCAM
4.2. Circulating Tumor Cells (CTC)
4.3. Circulating Tumor-Specific DNA (ctDNA)
4.4. Circulating miRNAs
4.5. Extracellular Vesicles (EVs)
5. Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreno-Sánchez, R.; Saavedra, E.; Gallardo-Pérez, J.C.; Rumjanek, F.D.; Rodríguez-Enríquez, S. Understanding the Cancer Cell Phenotype beyond the Limitations of Current Omics Analyses. FEBS J. 2016, 283, 54–73. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhou, M.; Chen, S.; Li, D.; Cao, X.; Liu, B. Effects of PH Alterations on Stress-and Aging-Induced Protein Phase Separation. Cell Mol. Life Sci. 2022, 79, 380. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Cox, T.R. The Matrix in Cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- Cominetti, M.R.; Altei, W.F.; Selistre-de-Araujo, H.S. Metastasis Inhibition in Breast Cancer by Targeting Cancer Cell Extravasation. Breast Cancer Targets Ther. 2019, 11, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Gaganpreet, K.; Hardik, G.; Heena, K.; Singh, L.; Kaur, N.; Kumar, S.; Alam, S. Role of Medical Image Analysis in Oncology. Biomed. Data Min. Inf. Retr. Methodol. Tech. Appl. 2021, 351–381. [Google Scholar] [CrossRef]
- Lakshmanakumar, M.; JBB, A.J.; Nesakumar, N. An Introduction to Cancer Biomarkers. In Biomarkers and Biosensors for Cervical Cancer Diagnosis; Springer: Singapore, 2021; pp. 1–12. [Google Scholar]
- Mauriz, E. Low-Fouling Substrates for Plasmonic Sensing of Circulating Biomarkers in Biological Fluids. Biosensors 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-H.; Tsai, K.; Kim, S.; Wu, Y.-J.; Demissie, K. Exposure to Tomographic Scans and Cancer Risks. JNCI cancer Spectr. 2020, 4, pkz072. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W. Radiation Exposure from CT Scans in Childhood and Subsequent Risk of Leukaemia and Brain Tumours: A Retrospective Cohort Study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- De González, A.B.; Mahesh, M.; Kim, K.-P.; Bhargavan, M.; Lewis, R.; Mettler, F.; Land, C. Projected Cancer Risks from Computed Tomographic Scans Performed in the United States in 2007. Arch. Intern. Med. 2009, 169, 2071–2077. [Google Scholar] [CrossRef]
- Henry, N.L.; Braun, T.M.; Breslin, T.M.; Gorski, D.H.; Silver, S.M.; Griggs, J.J. Variation in the Use of Advanced Imaging at the Time of Breast Cancer Diagnosis in a Statewide Registry. Cancer 2017, 123, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Mathews, J.D.; Forsythe, A.V.; Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A. Cancer Risk in 680 000 People Exposed to Computed Tomography Scans in Childhood or Adolescence: Data Linkage Study of 11 Million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef]
- Vajhadin, F.; Ahadian, S.; Travas-Sejdic, J.; Lee, J.; Mazloum-Ardakani, M.; Salvador, J.; Aninwene II, G.E.; Bandaru, P.; Sun, W.; Khademhossieni, A. Electrochemical Cytosensors for Detection of Breast Cancer Cells. Biosens. Bioelectron. 2020, 151, 111984. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, S.; Mishra, P. Molecularly Imprinted Polymer-Based Sensors for Cancer Biomarker Detection. Sens. Actuators Rep. 2021, 3, 100061. [Google Scholar] [CrossRef]
- Ebrahimi, G.; Samadi Pakchin, P.; Shamloo, A.; Mota, A.; de la Guardia, M.; Omidian, H.; Omidi, Y. Label-Free Electrochemical Microfluidic Biosensors: Futuristic Point-of-Care Analytical Devices for Monitoring Diseases. Microchim. Acta 2022, 189, 252. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Xie, C.; Li, Q.; Yang, M.; Li, S.; Li, H.; Xia, F. Electrochemical Biosensors for the Analysis of Breast Cancer Biomarkers: From Design to Application. Anal. Chem. 2021, 94, 269–296. [Google Scholar] [CrossRef]
- Li, J.; Guan, X.; Fan, Z.; Ching, L.-M.; Li, Y.; Wang, X.; Cao, W.-M.; Liu, D.-X. Non-Invasive Biomarkers for Early Detection of Breast Cancer. Cancers 2020, 12, 2767. [Google Scholar] [CrossRef]
- Wu, J.; Hu, S.; Zhang, L.; Xin, J.; Sun, C.; Wang, L.; Ding, K.; Wang, B. Tumor Circulome in the Liquid Biopsies for Cancer Diagnosis and Prognosis. Theranostics 2020, 10, 4544. [Google Scholar] [CrossRef]
- Hussain, S.H.; Huertas, C.S.; Mitchell, A.; Deman, A.-L.; Laurenceau, E. Biosensors for Circulating Tumor Cells (CTCs)-Biomarker Detection in Lung and Prostate Cancer: Trends and Prospects. Biosens. Bioelectron. 2022, 197, 113770. [Google Scholar] [CrossRef]
- Restrepo, R.; Cervantes, L.F.; Swirsky, A.M.; Diaz, A. Breast Development in Pediatric Patients from Birth to Puberty: Physiology, Pathology and Imaging Correlation. Pediatr. Radiol. 2021, 51, 1959–1969. [Google Scholar] [CrossRef]
- Bazira, P.J.; Ellis, H.; Mahadevan, V. Anatomy and Physiology of the Breast. Surgery 2022, 40, 79–83. [Google Scholar] [CrossRef]
- Lawrence, R.A. Anatomy of the Breast. In Breastfeeding; Elsevier: Amsterdam, The Netherlands, 2022; pp. 38–57. [Google Scholar]
- Ng, W.; Pinder, S.E. The Breasts. In Muir’s Textbook of Pathology; CRC Press: Boca Raton, FL, USA, 2020; pp. 427–446. ISBN 0429053010. [Google Scholar]
- Gupta, M.; Goyal, N. Applied Anatomy of Breast Cancer. In Breast Cancer: Comprehensive Management; Springer: Berlin/Heidelberg, Germany, 2022; pp. 23–35. [Google Scholar]
- O’Sullivan, C.C.; Loprinzi, C.L.; Haddad, T.C. Updates in the Evaluation and Management of Breast Cancer. Mayo Clin. Proc. 2018, 93, 794–807. [Google Scholar] [CrossRef]
- Jenkins, S.; Kachur, M.E.; Rechache, K.; Wells, J.M.; Lipkowitz, S. Rare Breast Cancer Subtypes. Curr. Oncol. Rep. 2021, 23, 54. [Google Scholar] [CrossRef]
- Harbeck, N. Breast Cancer Is a Systemic Disease Optimally Treated by a Multidisciplinary Team. Nat. Rev. Dis. Prim. 2020, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Beňačka, R.; Szabóová, D.; Guľašová, Z.; Hertelyová, Z.; Radoňák, J. Classic and New Markers in Diagnostics and Classification of Breast Cancer. Cancers 2022, 14, 5444. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Tsugawa, K.; Akiyama, F.; Horii, R.; Kurosumi, M.; Moriya, T.; Takano, T.; Takei, H.; Nakayama, T.; Miyagi, Y.; et al. Histological Classification of Breast Tumors in the General Rules for Clinical and Pathological Recording of Breast Cancer (18th Edition). Breast Cancer 2020, 27, 309–321. [Google Scholar] [CrossRef]
- marie Sopik, V.A. The Natural History of Ductal Carcinoma in Situ and Early-Stage Breast Cancer. Ph.D. Thesis, University of Toronto, Toronto, Canada, 2022. [Google Scholar]
- Chan, P.F.; Abd Hamid, R. An Overview of Breast Cancer: Classification and Related Signaling Pathways. Prog. Microbes Mol. Biol. 2021, 4. [Google Scholar] [CrossRef]
- Lam, D.L.; Smith, J.; Partridge, S.C.; Kim, A.; Javid, S.H.; Hippe, D.S.; Lehman, C.D.; Lee, J.M.; Rahbar, H. The Impact of Preoperative Breast MRI on Surgical Management of Women with Newly Diagnosed Ductal Carcinoma In Situ. Acad. Radiol. 2020, 27, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, D.; Yin, Y.; Yang, T.; You, Z.; Li, D.; Chen, Y.; Jiang, Y.; Xu, S.; Geng, J.; et al. Circulating Cell-Free DNA-Based Methylation Patterns for Breast Cancer Diagnosis. NPJ Breast Cancer 2021, 7, 106. [Google Scholar] [CrossRef]
- Shaikh, K.; Krishnan, S.; Thanki, R. Types, Diagnosis, and Treatment of Breast Cancer BT. In Artificial Intelligence in Breast Cancer Early Detection and Diagnosis; Shaikh, K., Krishnan, S., Thanki, R., Eds.; Springer: Cham, Switzerland, 2021; pp. 21–35. ISBN 978-3-030-59208-0. [Google Scholar]
- Caparica, R.; Bruzzone, M.; Agostinetto, E.; Franzoi, M.A.; Ceppi, M.; Radosevic-Robin, N.; Penault-Llorca, F.; Willard-Gallo, K.; Loi, S.; Salgado, R.; et al. Tumour-Infiltrating Lymphocytes in Non-Invasive Breast Cancer: A Systematic Review and Meta-Analysis. Breast 2021, 59, 183–192. [Google Scholar] [CrossRef]
- Das, A.K.; Biswas, S.K.; Bhattacharya, A.; Alam, E. Introduction to Breast Cancer and Awareness. In Proceedings of the 7th International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India, 9–20 March 2021; pp. 227–232. [Google Scholar]
- Agre, A.M.; Upade, A.C.; Yadav, M.A.; Kumbhar, S.B. A Review on Breasr Cancer and Its Management. World J. Pharm. Res. 2021, 10, 408–437. [Google Scholar]
- Kao, Y.; Wu, Y.-J.; Hsu, C.-C.; Lin, H.-J.; Wang, J.-J.; Tian, Y.-F.; Weng, S.-F.; Huang, C.-C. Short-and Long-Term Recurrence of Early-Stage Invasive Ductal Carcinoma in Middle-Aged and Old Women with Different Treatments. Sci. Rep. 2022, 12, 4422. [Google Scholar] [CrossRef] [PubMed]
- Tarighati, E.; Keivan, H.; Mahani, H. A Review of Prognostic and Predictive Biomarkers in Breast Cancer. Clin. Exp. Med. 2022, 23, 1–16. [Google Scholar] [CrossRef]
- Tadayyon, H.; Sannachi, L.; Gangeh, M.J.; Kim, C.; Ghandi, S.; Trudeau, M.; Pritchard, K.; Tran, W.T.; Slodkowska, E.; Sadeghi-Naini, A.; et al. A Priori Prediction of Neoadjuvant Chemotherapy Response and Survival in Breast Cancer Patients Using Quantitative Ultrasound. Sci. Rep. 2017, 7, 45733. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Y.; Soto-Rubio, A.; Ramos-Campos, M.; Escriche-Saura, A.; Martínez, M.; Gavilá, J. Impact of Hormone Therapy Side Effects on Health-Related Quality of Life, Distress, and Well-Being of Breast Cancer Survivors. Sci. Rep. 2022, 12, 18673. [Google Scholar] [CrossRef] [PubMed]
- Aysal, A.; Gundogdu, B.; Pehlivanoglu, B.; Ekmekci, S.; Toper, M.H.; Talu, C.K.; Erdogdu, I.H.; Gurel, D.; Durak, M.G.; Ulukus, E.C.; et al. Diagnostic Approach According to More Frequent Metastatic Sites: Liver, Lung, Bone, and Lymph Nodes. In Biomarkers in Carcinoma of Unknown Primary; Sarioglu, S., Sagol, O., Aysal, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 335–379. ISBN 978-3-030-84432-5. [Google Scholar]
- Jabbarzadeh, M.; Hamblin, M.R.; Pournaghi-Azar, F.; Vakili Saatloo, M.; Kouhsoltani, M.; Vahed, N. Ki-67 Expression as a Diagnostic Biomarker in Odontogenic Cysts and Tumors: A Systematic Review and Meta-Analysis. J. Dent. Res. Dent. Clin. Dent. Prospects 2021, 15, 66–75. [Google Scholar] [CrossRef] [PubMed]
- van Uden, D.J.P.; van Maaren, M.C.; Bult, P.; Strobbe, L.J.A.; van der Hoeven, J.J.M.; Blanken-Peeters, C.F.J.M.; Siesling, S.; de Wilt, J.H.W. Pathologic Complete Response and Overall Survival in Breast Cancer Subtypes in Stage III Inflammatory Breast Cancer. Breast Cancer Res. Treat. 2019, 176, 217–226. [Google Scholar] [CrossRef]
- Naorem, L.D.; Muthaiyan, M.; Bhattacharyya, I.; Ampasala, D.R.; Venkatesan, A. Chapter 2—Identification of Potential Drug Candidates for the Treatment of Triple-Negative Breast Cancer. In A Theranostic and Precision Medicine Approach for Female Specific Cancers; Malla, R.R., Nagaraju, Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 31–47. ISBN 978-0-12-822009-2. [Google Scholar]
- Basu, A.; Ramamoorthi, G.; Jia, Y.; Faughn, J.; Wiener, D.; Awshah, S.; Kodumudi, K.; Czerniecki, B.J. Chapter Six—Immunotherapy in Breast Cancer: Current Status and Future Directions. In Immunotherapy of Cancer; Wang, X.-Y., Fisher, Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 143, pp. 295–349. ISBN 0065-230X. [Google Scholar]
- Badve, S.; Dabbs, D.J.; Schnitt, S.J.; Baehner, F.L.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Basal-like and Triple-Negative Breast Cancers: A Critical Review with an Emphasis on the Implications for Pathologists and Oncologists. Mod. Pathol. 2011, 24, 157–167. [Google Scholar] [CrossRef]
- Ahn, S.K.; Jung, S.-Y. Current Biomarkers for Precision Medicine in Breast Cancer BT—Translational Research in Breast Cancer. In Translational Research in Breast Cancer; Noh, D.-Y., Han, W., Toi, M., Eds.; Springer: Singapore, 2021; pp. 363–379. ISBN 978-981-32-9620-6. [Google Scholar]
- Liang, Q.; Ma, D.; Gao, R.-F.; Yu, K.-D. Effect of Ki-67 Expression Levels and Histological Grade on Breast Cancer Early Relapse in Patients with Different Immunohistochemical-Based Subtypes. Sci. Rep. 2020, 10, 7648. [Google Scholar] [CrossRef]
- Coiro, S.; Gasparini, E.; Falco, G.; Santandrea, G.; Foroni, M.; Besutti, G.; Iotti, V.; Di Cicilia, R.; Foroni, M.; Mele, S.; et al. Biomarkers Changes after Neoadjuvant Chemotherapy in Breast Cancer: A Seven-Year Single Institution Experience. Diagnostics 2021, 11, 2249. [Google Scholar] [CrossRef]
- Andrahennadi, S.; Sami, A.; Manna, M.; Pauls, M.; Ahmed, S. Current Landscape of Targeted Therapy in Hormone Receptor-Positive and HER2-Negative Breast Cancer. Curr. Oncol. 2021, 28, 168. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.L.B.; Czerniecki, B.J. Clinical Development of Immunotherapies for HER2+ Breast Cancer: A Review of HER2-Directed Monoclonal Antibodies and Beyond. NPJ Breast Cancer 2020, 6, 10. [Google Scholar] [CrossRef]
- Fancellu, A.; Houssami, N.; Sanna, V.; Porcu, A.; Ninniri, C.; Marinovich, M.L. Outcomes after Breast-Conserving Surgery or Mastectomy in Patients with Triple-Negative Breast Cancer: Meta-Analysis. Br. J. Surg. 2021, 108, 760–768. [Google Scholar] [CrossRef]
- Shen, M.; Pan, H.; Chen, Y.; Xu, Y.H.; Yang, W.; Wu, Z. A Review of Current Progress in Triple-Negative Breast Cancer Therapy. Open Med. 2020, 15, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, L.; Zhang, R.; Han, J.; Qin, W.; Gu, Y.; Sha, J.; Xu, X.; Feng, Y.; Ren, Z.; et al. Screening and Diagnosis of Colorectal Cancer and Advanced Adenoma by Bionic Glycome Method and Machine Learning. Am. J. Cancer Res. 2021, 11, 3002–3020. [Google Scholar]
- Fu, Y.; Zhang, Y.; Khoo, B.L. Liquid Biopsy Technologies for Hematological Diseases. Med. Res. Rev. 2021, 41, 246–274. [Google Scholar] [CrossRef]
- Wei, J.; Hu, M.; Huang, K.; Lin, S.; Du, H. Roles of Proteoglycans and Glycosaminoglycans in Cancer Development and Progression. Int. J. Mol. Sci. 2020, 21, 5983. [Google Scholar] [CrossRef] [PubMed]
- Núñez, C. Blood-Based Protein Biomarkers in Breast Cancer. Clin. Chim. Acta 2019, 490, 113–127. [Google Scholar] [CrossRef]
- Paul, D. The Systemic Hallmarks of Cancer. J. Cancer Metastasis Treat. 2020, 6, 29. [Google Scholar] [CrossRef]
- Eroglu, Z.; Fielder, O.; Somlo, G. Analysis of Circulating Tumor Cells in Breast Cancer. J. Natl. Compr. Cancer Netw. J. Natl. Compr. Canc. Netw. 2013, 11, 977–985. [Google Scholar] [CrossRef]
- Tellez-Gabriel, M.; Knutsen, E.; Perander, M. Current Status of Circulating Tumor Cells, Circulating Tumor DNA, and Exosomes in Breast Cancer Liquid Biopsies. Int. J. Mol. Sci. 2020, 21, 9457. [Google Scholar] [CrossRef] [PubMed]
- Campos-Carrillo, A.; Weitzel, J.N.; Sahoo, P.; Rockne, R.; Mokhnatkin, J.V.; Murtaza, M.; Gray, S.W.; Goetz, L.; Goel, A.; Schork, N.; et al. Circulating Tumor DNA as an Early Cancer Detection Tool. Pharmacol. Ther. 2020, 207, 107458. [Google Scholar] [CrossRef]
- Mesquita, A.; Costa, J.L.; Schmitt, F. Utility of Circulating Tumor DNA in Different Clinical Scenarios of Breast Cancer. Cancers 2020, 12, 3797. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, H.; An, K.; Liu, Z.; Hai, L.; Li, R.; Zhou, Y.; Zhao, W.; Jia, Y.; Wu, N.; et al. Whole-Genome Bisulfite Sequencing Analysis of Circulating Tumour DNA for the Detection and Molecular Classification of Cancer. Clin. Transl. Med. 2022, 12, e1014. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-Free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, I.; Ačkar, L.; Mossahebi Mohammadi, P.; Pantel, K. Liquid Biopsies: Potential and Challenges. Int. J. Cancer 2021, 148, 528–545. [Google Scholar] [CrossRef]
- van Schooneveld, E.; Wildiers, H.; Vergote, I.; Vermeulen, P.B.; Dirix, L.Y.; Van Laere, S.J. Dysregulation of MicroRNAs in Breast Cancer and Their Potential Role as Prognostic and Predictive Biomarkers in Patient Management. Breast Cancer Res. 2015, 17, 21. [Google Scholar] [CrossRef]
- Ahmadzada, T.; Kao, S.; Reid, G.; Clarke, S.; Grau, G.E.; Hosseini-Beheshti, E. Extracellular Vesicles as Biomarkers in Malignant Pleural Mesothelioma: A Review. Crit. Rev. Oncol. Hematol. 2020, 150, 102949. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, S.; Liu, C.; Han, Z.; Liu, Y.; Deng, J.; Li, Y.; Wu, X.; Cai, L.; Qin, L.; et al. Protein Analysis of Extracellular Vesicles to Monitor and Predict Therapeutic Response in Metastatic Breast Cancer. Nat. Commun. 2021, 12, 2536. [Google Scholar] [CrossRef]
- Sadeghi, M.; Kashanian, S.; Naghib, S.M.; Haghiralsadat, F.; Tofighi, D. An Efficient Electrochemical Biosensor Based on Pencil Graphite Electrode Mediated by 2D Functionalized Graphene Oxide to Detect HER2 Breast Cancer Biomarker. Int. J. Electrochem. Sci 2022, 17, 2. [Google Scholar] [CrossRef]
- Li, J.; Meng, Q.H.; Ramanathan, L.V. Chapter 1—Overview of Traditional and Nontraditional Tumor Markers. In Clinical Aspects and Laboratory Determination; Ramanathan, L.V., Fleisher, M., Duffy, Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–29. ISBN 978-0-12-824302-2. [Google Scholar]
- Fakhari, A.; Gharepapagh, E.; Dabiri, S.; Gilani, N. Correlation of Cancer Antigen 15-3 (CA15-3) Serum Level and Bony Metastases in Breast Cancer Patients. Med. J. Islam. Repub. Iran 2019, 33, 142. [Google Scholar] [CrossRef] [PubMed]
- Kuntamung, K.; Jakmunee, J.; Ounnunkad, K. A Label-Free Multiplex Electrochemical Biosensor for the Detection of Three Breast Cancer Biomarker Proteins Employing Dye/Metal Ion-Loaded and Antibody-Conjugated Polyethyleneimine-Gold Nanoparticles. J. Mater. Chem. B 2021, 9, 6576–6585. [Google Scholar] [CrossRef]
- Rebelo, T.S.C.R.; Ribeiro, J.A.; Sales, M.G.F.; Pereira, C.M. Electrochemical Immunosensor for Detection of CA 15-3 Biomarker in Point-of-Care. Sens. Bio-Sens. Res. 2021, 33, 100445. [Google Scholar] [CrossRef]
- Martins, T.S.; Bott-Neto, J.L.; Oliveira, O.N.; Machado, S.A.S. A Sandwich-Type Electrochemical Immunosensor Based on Au-RGO Composite for CA15-3 Tumor Marker Detection. Microchim. Acta 2021, 189, 38. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Pereira, A.C.; Ferreira, L.F. Disposable Electropolymerized Molecularly Imprinted Electrochemical Sensor for Determination of Breast Cancer Biomarker CA 15-3 in Human Serum Samples. Talanta 2023, 252, 123819. [Google Scholar] [CrossRef] [PubMed]
- Shawky, A.M.; El-Tohamy, M. Signal Amplification Strategy of Label-Free Ultrasenstive Electrochemical Immunosensor Based Ternary Ag/TiO2/RGO Nanocomposites for Detecting Breast Cancer Biomarker CA 15-3. Mater. Chem. Phys. 2021, 272, 124983. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Feng, Y.-G.; Cen, S.-Y.; Wang, A.-J.; Mei, L.-P.; Luo, X.; Feng, J.-J. A Label-Free Electrochemical Immnunosensor Based on Signal Magnification of Oxygen Reduction Reaction Catalyzed by Uniform PtCo Nanodendrites for Highly Sensitive Detection of Carbohydrate Antigen 15-3. Anal. Chim. Acta 2021, 1176, 338750. [Google Scholar] [CrossRef]
- Gajdosova, V.P.; Lorencova, L.; Kasak, P.; Jerigova, M.; Velic, D.; Orovcik, L.; Barath, M.; Farkas, P.; Tkac, J. Redox Features of Hexaammineruthenium(III) on MXene Modified Interface: Three Options for Affinity Biosensing. Anal. Chim. Acta 2022, 1227, 340310. [Google Scholar] [CrossRef]
- Funston, G.; Mounce, L.T.A.; Price, S.; Rous, B.; Crosbie, E.J.; Hamilton, W.; Walter, F.M. CA-125 Test Result, Test-to-Diagnosis Interval, and Stage in Ovarian Cancer at Diagnosis: A Retrospective Cohort Study Using Electronic Health Records. Br. J. Gen. Pract. 2021, 71, e465–e472. [Google Scholar] [CrossRef]
- Er, O.F.; Kivrak, H.; Ozok, O.; Çelik, S.; Kivrak, A. A Novel Electrochemical Sensor for Monitoring Ovarian Cancer Tumor Protein CA 125 on Benzothiophene Derivative Based Electrodes. J. Electroanal. Chem. 2022, 904, 115854. [Google Scholar] [CrossRef]
- Öndeş, B.; Evli, S.; Uygun, M.; Aktaş Uygun, D. Boron Nitride Nanosheet Modified Label-Free Electrochemical Immunosensor for Cancer Antigen 125 Detection. Biosens. Bioelectron. 2021, 191, 113454. [Google Scholar] [CrossRef] [PubMed]
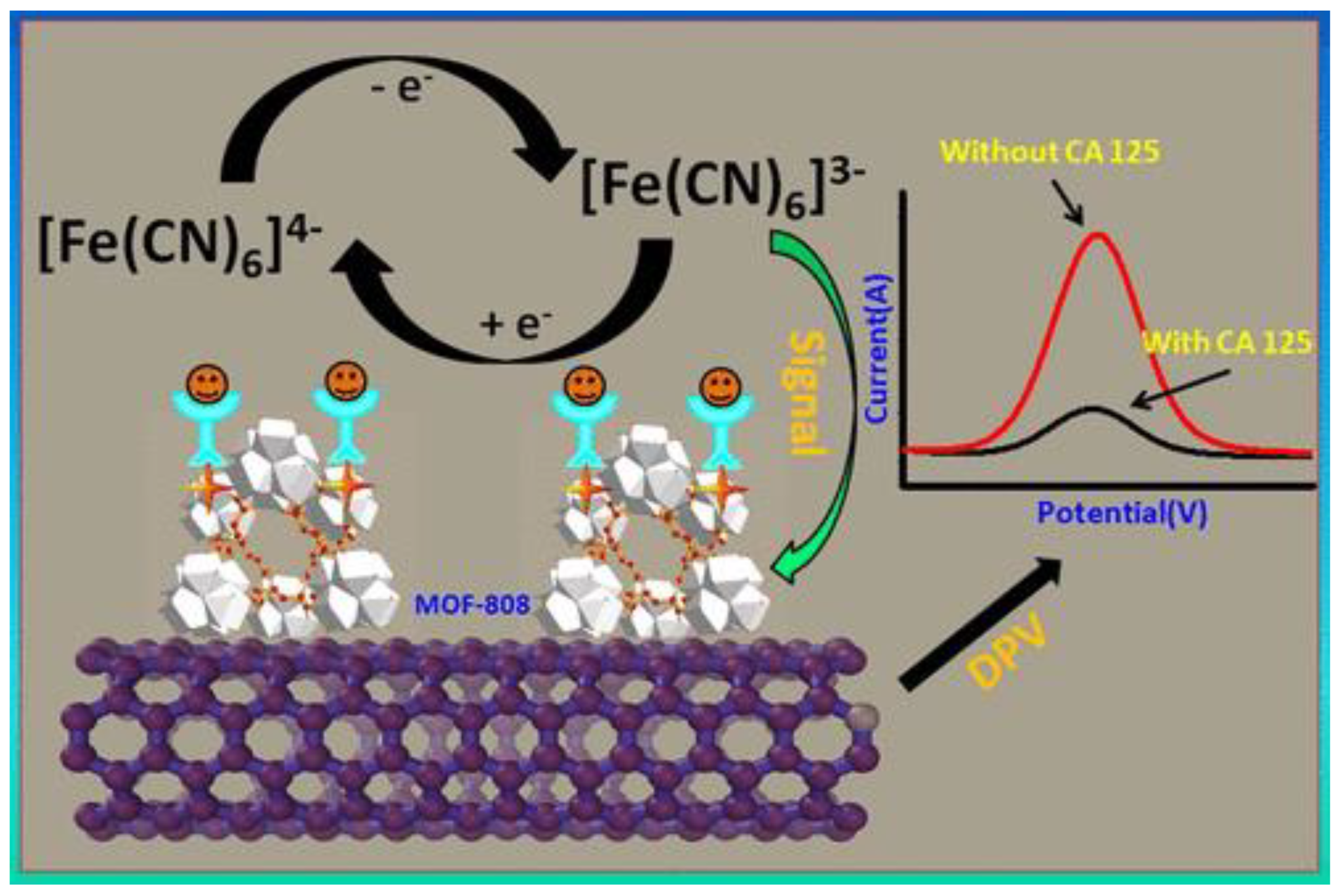
- Biswas, S.; Lan, Q.; Xie, Y.; Sun, X.; Wang, Y. Label-Free Electrochemical Immunosensor for Ultrasensitive Detection of Carbohydrate Antigen 125 Based on Antibody-Immobilized Biocompatible MOF-808/CNT. ACS Appl. Mater. Interfaces 2021, 13, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Saadati, A.; Hassanpour, S.; Bahavarnia, F.; Hasanzadeh, M. A Novel Biosensor for the Monitoring of Ovarian Cancer Tumor Protein CA 125 in Untreated Human Plasma Samples Using a Novel Nano-Ink: A New Platform for Efficient Diagnosis of Cancer Using Paper Based Microfluidic Technology. Anal. Methods 2020, 12, 1639–1649. [Google Scholar] [CrossRef]
- Chen, M.; Han, R.; Wang, W.; Li, Y.; Luo, X. Antifouling Aptasensor Based on Self-Assembled Loop-Closed Peptides with Enhanced Stability for CA-125 Assay in Complex Biofluids. Anal. Chem. 2021, 93, 13555–13563. [Google Scholar] [CrossRef]
- Li, S.; Hu, C.; Chen, C.; Zhang, J.; Bai, Y.; Tan, C.S.; Ni, G.; He, F.; Li, W.; Ming, D. Molybdenum Disulfide Supported on Metal–Organic Frameworks as an Ultrasensitive Layer for the Electrochemical Detection of the Ovarian Cancer Biomarker CA-125. ACS Appl. Bio Mater. 2021, 4, 5494–5502. [Google Scholar] [CrossRef]
- Chen, M.; Song, Z.; Yang, X.; Song, Z.; Luo, X. Antifouling Peptides Combined with Recognizing DNA Probes for Ultralow Fouling Electrochemical Detection of Cancer Biomarkers in Human Bodily Fluids. Biosens. Bioelectron. 2022, 206, 114162. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. Smartphone-Based Electrochemical System with Multi-Walled Carbon Nanotubes/Thionine/Gold Nanoparticles Modified Screen-Printed Immunosensor for Cancer Antigen 125 Detection. Microchem. J. 2022, 174, 107044. [Google Scholar] [CrossRef]
- Ni, Y.; Ouyang, H.; Yu, L.; Ling, C.; Zhu, Z.; He, A.; Liu, R. Label-Free Electrochemical Aptasensor Based on Magnetic α-Fe2O3/Fe3O4 Heterogeneous Hollow Nanorods for the Detection of Cancer Antigen 125. Bioelectrochemistry 2022, 148, 108255. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.S.; Fu-Ming, W.; Rajangam, I. Electrochemical Detection of CA-125 Using Thionine and Gold Nanoparticles Supported on Heteroatom-Doped Graphene Nanocomposites. Appl. Nanosci. 2021, 11, 2167–2180. [Google Scholar] [CrossRef]
- Kabel, A.M. Tumor Markers of Breast Cancer: New Prospectives. J. Oncol. Sci. 2017, 3, 5–11. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H. New Label-Free Ultrasensitive Electrochemical Immunosensor-Based Au/MoS2/RGO Nanocomposites for CA 27-29 Breast Cancer Antigen Detection. New J. Chem. 2018, 42, 11046–11053. [Google Scholar] [CrossRef]
- Jang Hye, M.; Kang Jong, H.; Jang Seok, K.; Paik Sam, S.; Kim Seop, W. Clinicopathological Analysis of CD44 and CD24 Expression in Invasive Breast Cancer. Oncol. Lett. 2016, 12, 2728–2733. [Google Scholar] [CrossRef] [PubMed]
- El-benhawy, S.; Ebeid, S.; Abd El Moneim, N.; Ahmed, A.; Ahmed, S.S.; Wezza, H. Soluble CD44 Is a Promising Biomarker with a Prognostic Value in Breast Cancer Patients. Int. J. Cancer Biomed. Res. 2021, 5, 77–86. [Google Scholar] [CrossRef]
- Kong, Y.; Lyu, N.; Wu, J.; Tang, H.; Xie, X.; Yang, L.; Li, X.; Wei, W.; Xie, X. Breast Cancer Stem Cell Markers CD44 and ALDH1A1 in Serum: Distribution and Prognostic Value in Patients with Primary Breast Cancer. J. Cancer 2018, 9, 3728–3735. [Google Scholar] [CrossRef]
- Xu, H.; Tian, Y.; Yuan, X.; Liu, Y.; Wu, H.; Liu, Q.; Wu, G.S.; Wu, K. Enrichment of CD44 in Basal-Type Breast Cancer Correlates with EMT, Cancer Stem Cell Gene Profile, and Prognosis. OncoTargets Ther. 2016, 9, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Yadav, S.; Sadique, M.A.; Khan, R. Electrochemically Exfoliated Graphene Quantum Dots Based Biosensor for CD44 Breast Cancer Biomarker. Biosensors 2022, 12, 966. [Google Scholar] [CrossRef]
- Cui, M.; Sun, X.; Liu, R.; Du, M.; Song, X.; Wang, S.; Hu, W.; Luo, X. A Dual-Responsive Electrochemical Biosensor Based on Artificial Protein Imprinted Polymers and Natural Hyaluronic Acid for Sensitive Recognition towards Biomarker CD44. Sens. Actuators B Chem. 2022, 371, 132554. [Google Scholar] [CrossRef]
- Lian, M.; Shi, Y.; Chen, L.; Qin, Y.; Zhang, W.; Zhao, J.; Chen, D. Cell Membrane and V2C MXene-Based Electrochemical Immunosensor with Enhanced Antifouling Capability for Detection of CD44. ACS Sensors 2022, 7, 2701–2709. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, Y.; Yu, M.; Yuan, X.; Zhang, C. Hyaluronic Acid-Based Label-Free Electrochemical Impedance Analysis for Cancer Cell Quantification and CD44 Expression. Microchem. J. 2021, 160, 105622. [Google Scholar] [CrossRef]
- Niu, C.; Lin, X.; Jiang, X.; Guo, F.; Liu, J.; Liu, X.; Huang, H.; Huang, Y. An Electrochemical Aptasensor for Highly Sensitive Detection of CEA Based on Exonuclease III and Hybrid Chain Reaction Dual Signal Amplification. Bioelectrochemistry 2022, 143, 107986. [Google Scholar] [CrossRef]
- Aslan, S. An Electrochemical Immunosensor Modified with Titanium IV Oxide/Polyacrylonitrile Nanofibers for the Determination of a Carcinoembryonic Antigen. New J. Chem. 2021, 45, 5391–5398. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Wang, H.; Sun, S. Dual-Signal Amplified Electrochemical Biosensor Based on EATRP and PEI for Early Detection of Lung Cancer. Bioelectrochemistry 2022, 148, 108224. [Google Scholar] [CrossRef] [PubMed]
- Taheri, N.; Khoshsafar, H.; Ghanei, M.; Ghazvini, A.; Bagheri, H. Dual-Template Rectangular Nanotube Molecularly Imprinted Polypyrrole for Label-Free Impedimetric Sensing of AFP and CEA as Lung Cancer Biomarkers. Talanta 2022, 239, 123146. [Google Scholar] [CrossRef]
- Bal Altuntaş, D.; Nalkıran, H.S.; Aslan, S.; Yolcu, Z. Development of MoS2 and Au Nanoparticle Including Disposable CEA-Based Immuno-Cytosensor Platforms. Chem. Pap. 2022, 76, 5217–5229. [Google Scholar] [CrossRef]
- Ranjan, P.; Yadav, S.; Sadique, M.A.; Khan, R.; Srivastava, A.K. Ionic Liquid-Functionalized ZrO2/Reduced Graphene Oxide Nanocomposites for Carcinoembryonic Antigen Electrochemical Detection. ACS Appl. Nano Mater. 2022, 5, 14999–15010. [Google Scholar] [CrossRef]
- Shekari, Z.; Zare, H.R.; Falahati, A. Dual Assaying of Breast Cancer Biomarkers by Using a Sandwich–Type Electrochemical Aptasensor Based on a Gold Nanoparticles–3D Graphene Hydrogel Nanocomposite and Redox Probes Labeled Aptamers. Sensors Actuators B Chem. 2021, 332, 129515. [Google Scholar] [CrossRef]
- Song, J.; Teng, H.; Xu, Z.; Liu, N.; Xu, L.; Liu, L.; Gao, F.; Luo, X. Free-Standing Electrochemical Biosensor for Carcinoembryonic Antigen Detection Based on Highly Stable and Flexible Conducting Polypyrrole Nanocomposite. Microchim. Acta 2021, 188, 217. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Han, R.; Chen, Q.; Jiang, L.; Luo, X. Click Reaction-Assisted Construction of Antifouling Immunosensors for Electrochemical Detection of Cancer Biomarkers in Human Serum. Sens. Actuators B Chem. 2022, 363, 131810. [Google Scholar] [CrossRef]
- Biswas, S.; Lan, Q.; Li, C.; Xia, X.-H. Morphologically Flex Sm-MOF Based Electrochemical Immunosensor for Ultrasensitive Detection of a Colon Cancer Biomarker. Anal. Chem. 2022, 94, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bao, J.; Huo, D.; Zeng, Y.; Wang, X.; Samalo, M.; Zhao, J.; Zhang, S.; Shen, C.; Hou, C. Au Doped Poly-Thionine and Poly-m-Cresol Purple: Synthesis and Their Application in Simultaneously Electrochemical Detection of Two Lung Cancer Markers CEA and CYFRA21-1. Talanta 2021, 224, 121816. [Google Scholar] [CrossRef]
- Shamsuddin, S.H.; Gibson, T.D.; Tomlinson, D.C.; McPherson, M.J.; Jayne, D.G.; Millner, P.A. Reagentless Affimer- and Antibody-Based Impedimetric Biosensors for CEA-Detection Using a Novel Non-Conducting Polymer. Biosens. Bioelectron. 2021, 178, 113013. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.P.T.; Ferreira, N.S.; Tavares, A.P.M.; Pinto, A.M.F.R.; Mendes, A.; Sales, M.G.F. A Passive Direct Methanol Fuel Cell as Transducer of an Electrochemical Sensor, Applied to the Detection of Carcinoembryonic Antigen. Biosens. Bioelectron. 2021, 175, 112877. [Google Scholar] [CrossRef]
- Shamshirian, A.; Aref, A.R.; Yip, G.W.; Ebrahimi Warkiani, M.; Heydari, K.; Razavi Bazaz, S.; Hamzehgardeshi, Z.; Shamshirian, D.; Moosazadeh, M.; Alizadeh-Navaei, R. Diagnostic Value of Serum HER2 Levels in Breast Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2020, 20, 1049. [Google Scholar] [CrossRef]
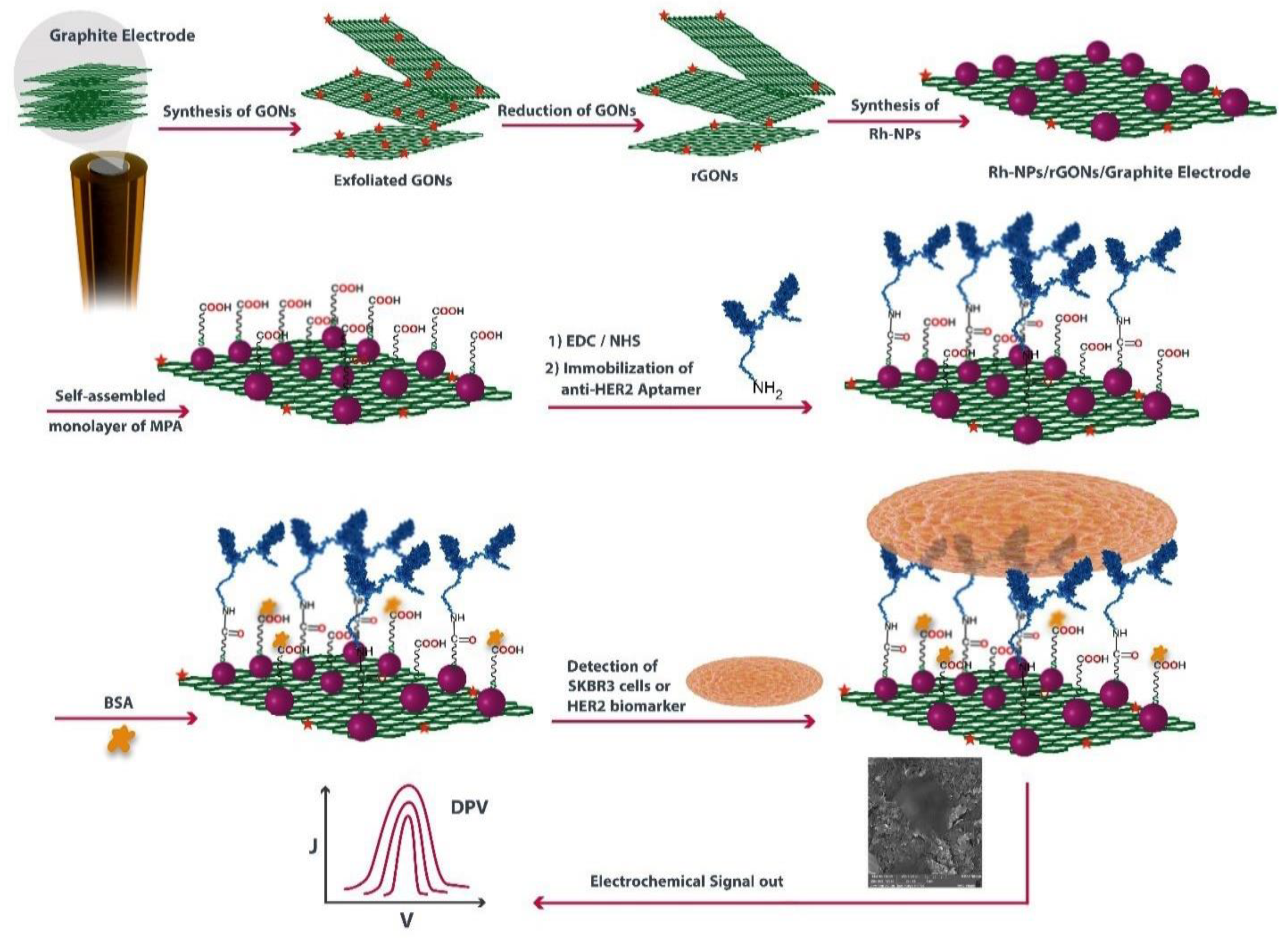
- Sadeghi, M.; Kashanian, S.; Naghib, S.M.; Arkan, E. A High-Performance Electrochemical Aptasensor Based on Graphene-Decorated Rhodium Nanoparticles to Detect HER2-ECD Oncomarker in Liquid Biopsy. Sci. Rep. 2022, 12, 3299. [Google Scholar] [CrossRef]
- Wang, W.; Han, R.; Chen, M.; Luo, X. Antifouling Peptide Hydrogel Based Electrochemical Biosensors for Highly Sensitive Detection of Cancer Biomarker HER2 in Human Serum. Anal. Chem. 2021, 93, 7355–7361. [Google Scholar] [CrossRef]
- Nasrollahpour, H.; Naseri, A.; Rashidi, M.-R.; Khalilzadeh, B. Application of Green Synthesized WO3-Poly Glutamic Acid Nanobiocomposite for Early Stage Biosensing of Breast Cancer Using Electrochemical Approach. Sci. Rep. 2021, 11, 23994. [Google Scholar] [CrossRef]
- Rauf, S.; Lahcen, A.A.; Aljedaibi, A.; Beduk, T.; Ilton de Oliveira Filho, J.; Salama, K.N. Gold Nanostructured Laser-Scribed Graphene: A New Electrochemical Biosensing Platform for Potential Point-of-Care Testing of Disease Biomarkers. Biosens. Bioelectron. 2021, 180, 113116. [Google Scholar] [CrossRef]
- Centane, S.; Nyokong, T. Impedimetric Aptasensor for HER2 Biomarker Using Graphene Quantum Dots, Polypyrrole and Cobalt Phthalocyanine Modified Electrodes. Sens. Bio-Sens. Res. 2021, 34, 100467. [Google Scholar] [CrossRef]
- Joshi, A.; Vishnu, G.K.A.; Dhruv, D.; Kurpad, V.; Pandya, H.J. Morphology-Tuned Electrochemical Immunosensing of a Breast Cancer Biomarker Using Hierarchical Palladium Nanostructured Interfaces. ACS Omega 2022, 7, 34177–34189. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Syahruni, S.; Gaffar, S.; Wyantuti, S.; Yusuf, M.; Subroto, T. An Electrochemical Aptasensor for the Detection of HER2 as a Breast Cancer Biomarker Based on Gold Nanoparticles-Aptamer Bioconjugates. Indones. J. Chem. 2021, 21, 1526–1536. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Feng, Y.-G.; Wang, A.-J.; Mei, L.-P.; Yuan, P.-X.; Luo, X.; Feng, J.-J. A Facile Ratiometric Electrochemical Strategy for Ultrasensitive Monitoring HER2 Using Polydopamine-Grafted-Ferrocene/Reduced Graphene Oxide, Au@Ag Nanoshuttles and Hollow Ni@PtNi Yolk-Shell Nanocages. Sensors Actuators B Chem. 2021, 331, 129460. [Google Scholar] [CrossRef]
- Dervisevic, M.; Alba, M.; Adams, T.E.; Prieto-Simon, B.; Voelcker, N.H. Electrochemical Immunosensor for Breast Cancer Biomarker Detection Using High-Density Silicon Microneedle Array. Biosens. Bioelectron. 2021, 192, 113496. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Li, N.; Yang, M.; Xiang, T.; Huo, D.; Qiu, Z.; Yang, L.; Hou, C. An Ultra-Sensitive Dual-Signal Ratiometric Electrochemical Aptasensor Based on Functionalized MOFs for Detection of HER2. Bioelectrochemistry 2022, 148, 108272. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Rauf, S.; Aljedaibi, A.; de Oliveira Filho, J.I.; Beduk, T.; Mani, V.; Alshareef, H.N.; Salama, K.N. Laser-Scribed Graphene Sensor Based on Gold Nanostructures and Molecularly Imprinted Polymers: Application for Her-2 Cancer Biomarker Detection. Sensors Actuators B Chem. 2021, 347, 130556. [Google Scholar] [CrossRef]
- Wignarajah, S.; Chianella, I.; Tothill, I.E. Development of Electrochemical Immunosensors for HER-1 and HER-2 Analysis in Serum for Breast Cancer Patients. Biosensors 2023, 13, 355. [Google Scholar] [CrossRef]
- Jalil, O.; Pandey, C.M.; Kumar, D. Highly Sensitive Electrochemical Detection of Cancer Biomarker Based on Anti-EpCAM Conjugated Molybdenum Disulfide Grafted Reduced Graphene Oxide Nanohybrid. Bioelectrochemistry 2021, 138, 107733. [Google Scholar] [CrossRef]
- Akhtartavan, S.; Karimi, M.; Sattarahmady, N.; Heli, H. An Electrochemical Signal-on Apta-Cyto-Sensor for Quantitation of Circulating Human MDA-MB-231 Breast Cancer Cells by Transduction of Electro-Deposited Non-Spherical Nanoparticles of Gold. J. Pharm. Biomed. Anal. 2020, 178, 112948. [Google Scholar] [CrossRef]
- Han, R.; Li, Y.; Chen, M.; Li, W.; Ding, C.; Luo, X. Antifouling Electrochemical Biosensor Based on the Designed Functional Peptide and the Electrodeposited Conducting Polymer for CTC Analysis in Human Blood. Anal. Chem. 2022, 94, 2204–2211. [Google Scholar] [CrossRef]
- Sadeghi, M.; Kashanian, S.; Naghib, S.M.; Askari, E.; Haghiralsadat, F.; Tofighi, D. A Highly Sensitive Nanobiosensor Based on Aptamer-Conjugated Graphene-Decorated Rhodium Nanoparticles for Detection of HER2-Positive Circulating Tumor Cells. Nanotechnol. Rev. 2022, 11, 793–810. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, F.; Wu, X.; Liu, Y.; Chen, A. Recognition and Sensitive Detection of CTCs Using a Controllable Label-Free Electrochemical Cytosensor. Microchim. Acta 2020, 187, 487. [Google Scholar] [CrossRef]
- Zeng, Y.; Qu, X.; Nie, B.; Mu, Z.; Li, C.; Li, G. An Electrochemical Biosensor Based on Electroactive Peptide Nanoprobes for the Sensitive Analysis of Tumor Cells. Biosens. Bioelectron. 2022, 215, 114564. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, Z.; Naghib, S.M.; Askari, E.; Molaabasi, F.; Sadr, A.; Zare, Y.; Afsharpad, M.; Rhee, K.Y. A Rapid Nanobiosensing Platform Based on Herceptin-Conjugated Graphene for Ultrasensitive Detection of Circulating Tumor Cells in Early Breast Cancer. Nanotechnol. Rev. 2021, 10, 744–753. [Google Scholar] [CrossRef]
- Vajhadin, F.; Mazloum-Ardakani, M.; Shahidi, M.; Moshtaghioun, S.M.; Haghiralsadat, F.; Ebadi, A.; Amini, A. MXene-Based Cytosensor for the Detection of HER2-Positive Cancer Cells Using CoFe2O4@Ag Magnetic Nanohybrids Conjugated to the HB5 Aptamer. Biosens. Bioelectron. 2022, 195, 113626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Bai, D.; Yu, H.; Fu, Y.; Song, L.; Wu, Y.; Chen, K.; Li, J.; Yang, Y.; Chen, H.; et al. Detection of Rare CTCs by Electrochemical Biosensor Built on Quaternary PdPtCuRu Nanospheres with Mesoporous Architectures. Talanta 2023, 253, 123955. [Google Scholar] [CrossRef]
- Yan, Y.; Guo, Q.; Wang, F.; Adhikari, R.; Zhu, Z.; Zhang, H.; Zhou, W.; Yu, H.; Li, J.; Zhang, J. Cell-Free DNA: Hope and Potential Application in Cancer. Front. Cell Dev. Biol. 2021, 9, 639233. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.-P.; Zhao, R.-J.; Feng, Z.; Wang, M.-Y.; Ma, R.-N.; Jia, W.-L.; Shang, L.; Zhang, W.; Xue, Q.-W.; Wang, H.-S. Ultrasensitive Electrochemical Detection of Circulating Tumor DNA by Hollow Polymeric Nanospheres and Dual Enzyme Assisted Target Amplification Strategy. Sens. Actuators B Chem. 2022, 350, 130849. [Google Scholar] [CrossRef]
- Zhao, H.; Niu, Z.; Chen, K.; Chen, L.; Wang, Z.; Lan, M.; Shi, J.; Huang, W. A Novel Sandwich-Type Electrochemical Biosensor Enabling Sensitive Detection of Circulating Tumor DNA. Microchem. J. 2021, 171, 106783. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, H.; Wang, Z.; Lan, M. A Novel Signal Amplification Label Based on AuPt Alloy Nanoparticles Supported by High-Active Carbon for the Electrochemical Detection of Circulating Tumor DNA. Anal. Chim. Acta 2021, 1169, 338628. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, Y.; Huang, G.; Situ, B.; Ye, X.; Tao, M.; Huang, Y.; Li, B.; Jiang, X.; Wang, Q.; et al. An Enzyme-Free Amplification Strategy for Sensitive Assay of Circulating Tumor DNA Based on Wheel-like Catalytic Hairpin Assembly and Frame Hybridization Chain Reaction. Sens. Actuators B Chem. 2021, 338, 129857. [Google Scholar] [CrossRef]
- Bryzgunova, O.; Konoshenko, M.; Zaporozhchenko, I.; Yakovlev, A.; Laktionov, P. Isolation of Cell-Free MiRNA from Biological Fluids: Influencing Factors and Methods. Diagnostics 2021, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- Pimalai, D.; Putnin, T.; Waiwinya, W.; Chotsuwan, C.; Aroonyadet, N.; Japrung, D. Development of Electrochemical Biosensors for Simultaneous Multiplex Detection of MicroRNA for Breast Cancer Screening. Microchim. Acta 2021, 188, 329. [Google Scholar] [CrossRef]
- Bharti, A.; Mittal, S.; Rana, S.; Dahiya, D.; Agnihotri, N.; Prabhakar, N. Electrochemical Biosensor for MiRNA-21 Based on Gold-Platinum Bimetallic Nanoparticles Coated 3-Aminopropyltriethoxy Silane. Anal. Biochem. 2020, 609, 113908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, N.; Ma, W.; Yang, M.; Hou, C.; Luo, X.; Huo, D. Ultrasensitive Detection of MicroRNA-21 by Using Specific Interaction of Antimonene with RNA as Electrochemical Biosensor. Bioelectrochemistry 2021, 142, 107890. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Situ, B.; Wu, Y.; Luo, S.; Zheng, L.; Qiu, Y. Rapid Electrochemical Biosensor for Sensitive Profiling of Exosomal MicroRNA Based on Multifunctional DNA Tetrahedron Assisted Catalytic Hairpin Assembly. Biosens. Bioelectron. 2021, 183, 113205. [Google Scholar] [CrossRef]
- Liu, L.; Deng, D.; Wu, D.; Hou, W.; Wang, L.; Li, N.; Sun, Z. Duplex-Specific Nuclease-Based Electrochemical Biosensor for the Detection of MicroRNAs by Conversion of Homogeneous Assay into Surface-Tethered Electrochemical Analysis. Anal. Chim. Acta 2021, 1149, 338199. [Google Scholar] [CrossRef] [PubMed]
- Farshchi, F.; Saadati, A.; Fathi, N.; Hasanzadeh, M.; Samiei, M. Flexible Paper-Based Label-Free Electrochemical Biosensor for the Monitoring of MiRNA-21 Using Core–Shell Ag@Au/GQD Nano-Ink: A New Platform for the Accurate and Rapid Analysis by Low Cost Lab-on-Paper Technology. Anal. Methods 2021, 13, 1286–1294. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, X.; Chang, Y.; Xu, S.; Yuan, R.; Chai, Y. Co-Catalytic Fc/HGQs/Fe3O4 Nanocomposite Mediated Enzyme-Free Electrochemical Biosensor for Ultrasensitive Detection of MicroRNA. Chem. Commun. 2021, 57, 5179–5182. [Google Scholar] [CrossRef] [PubMed]
- Pothipor, C.; Aroonyadet, N.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A Highly Sensitive Electrochemical MicroRNA-21 Biosensor Based on Intercalating Methylene Blue Signal Amplification and a Highly Dispersed Gold Nanoparticles/Graphene/Polypyrrole Composite. Analyst 2021, 146, 2679–2688. [Google Scholar] [CrossRef]
- Zayani, R.; Rabti, A.; Ben Aoun, S.; Raouafi, N. Fluorescent and Electrochemical Bimodal Bioplatform for Femtomolar Detection of MicroRNAs in Blood Sera. Sens. Actuators B Chem. 2021, 327, 128950. [Google Scholar] [CrossRef]
- Kim, H.Y.; Song, J.; Park, H.G.; Kang, T. Electrochemical Detection of Zeptomolar MiRNA Using an RNA-Triggered Cu2+ Reduction Method. Sens. Actuators B Chem. 2022, 360, 131666. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Yang, M.; Hou, C.; Huo, D. An Ultrasensitive Electrochemical Biosensor for Simultaneously Detect MicroRNA-21 and MicroRNA-155 Based on Specific Interaction of Antimonide Quantum Dot with RNA. Microchem. J. 2023, 185, 108173. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, J.; Zhang, Y.; Oderinde, O.; Li, C.; Duan, L.; Wang, Y.; Cui, J. Bipedal DNAzyme Walker Triggered Dual-Amplification Electrochemical Platform for Ultrasensitive Ratiometric Biosensing of MicroRNA-21. Biosens. Bioelectron. 2023, 220, 114879. [Google Scholar] [CrossRef] [PubMed]
- Khodadoust, A.; Nasirizadeh, N.; Seyfati, S.M.; Taheri, R.A.; Ghanei, M.; Bagheri, H. High-Performance Strategy for the Construction of Electrochemical Biosensor for Simultaneous Detection of MiRNA-141 and MiRNA-21 as Lung Cancer Biomarkers. Talanta 2023, 252, 123863. [Google Scholar] [CrossRef]
- Zhao, J.; He, C.; Wu, W.; Yang, H.; Dong, J.; Wen, L.; Hu, Z.; Yang, M.; Hou, C.; Huo, D. MXene-MoS2 Heterostructure Collaborated with Catalyzed Hairpin Assembly for Label-Free Electrochemical Detection of MicroRNA-21. Talanta 2022, 237, 122927. [Google Scholar] [CrossRef]
- Yokoi, A.; Ochiya, T. Exosomes and Extracellular Vesicles: Rethinking the Essential Values in Cancer Biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Wang, F.; Zhang, Y.; Wang, H.; Liu, Y. Ti3C2 MXene Mediated Prussian Blue in Situ Hybridization and Electrochemical Signal Amplification for the Detection of Exosomes. Talanta 2021, 224, 121879. [Google Scholar] [CrossRef]
- Moura, S.L.; Pallarès-Rusiñol, A.; Sappia, L.; Martí, M.; Pividori, M.I. The Activity of Alkaline Phosphatase in Breast Cancer Exosomes Simplifies the Biosensing Design. Biosens. Bioelectron. 2022, 198, 113826. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Cha, B.S.; Lee, E.S.; Park, K.S. Dual Rolling Circle Amplification-Enabled Ultrasensitive Multiplex Detection of Exosome Biomarkers Using Electrochemical Aptasensors. Anal. Chim. Acta 2022, 1205, 339762. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Liu, X.; Xie, Y.; Chen, M.; Zheng, C.; Zhong, H.; Li, M. Integrated SERS-Vertical Flow Biosensor Enabling Multiplexed Quantitative Profiling of Serological Exosomal Proteins in Patients for Accurate Breast Cancer Subtyping. ACS Nano 2023, 17, 4077–4088. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.; Zhang, Y.; Zhao, J.; Wang, Y.; Xu, Z. Dual-Aptamer-Assisted Ratiometric SERS Biosensor for Ultrasensitive and Precise Identification of Breast Cancer Exosomes. ACS Sens. 2023, 8, 875–883. [Google Scholar] [CrossRef]
- Shen, B.; Li, L.; Liu, C.; Li, X.; Li, X.; Cheng, X.; Wu, H.; Yang, T.; Cheng, W.; Ding, S. Mesoporous Nanozyme-Enhanced DNA Tetrahedron Electrochemiluminescent Biosensor with Three-Dimensional Walking Nanomotor-Mediated CRISPR/Cas12a for Ultrasensitive Detection of Exosomal MicroRNA. Anal. Chem. 2023, 95, 4486–4495. [Google Scholar] [CrossRef] [PubMed]
- Yildizhan, Y.; Driessens, K.; Tsao, H.S.K.; Boiy, R.; Thomas, D.; Geukens, N.; Hendrix, A.; Lammertyn, J.; Spasic, D. Detection of Breast Cancer-Specific Extracellular Vesicles with Fiber-Optic SPR Biosensor. Int. J. Mol. Sci. 2023, 24, 3764. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, X.; Zhu, H.; Lu, Y.; Song, H.; Niu, J.; Chen, H. Cancer Cell Membrane Functionalized Gold Nanoparticles: Natural Receptor Tenascin-C as Biomimetic Probe for Sensitive Detection of Circulating Exosomes. Sens. Actuators B Chem. 2022, 372, 132673. [Google Scholar] [CrossRef]
- Pammi Guru, K.T.; Praween, N.; Basu, P.K. Investigating the Electric Field Lysis of Exosomes Immobilized on the Screen-Printed Electrode and Electrochemical Sensing of the Lysed-Exosome-Derived Protein. Biosensors 2023, 13, 323. [Google Scholar] [CrossRef]
- Fan, Z.; Weng, Q.; Li, Y.; Zeng, T.; Wang, J.; Zhang, H.; Yu, H.; Dong, Y.; Zhao, X.; Li, J. Accurate and Rapid Quantification of PD-L1 Positive Exosomes by a Triple-Helix Molecular Probe. Anal. Chim. Acta 2023, 1251, 340984. [Google Scholar] [CrossRef]
- Pallares-Rusiñol, A.; Moura, S.L.; Martí, M.; Pividori, M.I. Electrochemical Genosensing of Overexpressed GAPDH Transcripts in Breast Cancer Exosomes. Anal. Chem. 2023, 95, 2487–2495. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, M.; Ma, C.; Liu, Y.; Lv, M.; Zhang, Z.; Gao, H.; Zhang, K. Highly Effective Detection of Exosomal MiRNAs in Plasma Using Liposome-Mediated Transfection CRISPR/Cas13a. ACS Sens. 2023, 8, 565–575. [Google Scholar] [CrossRef]
- Zhang, D.; Qiao, L.; Xu, S.; Peng, L.; Yang, Y.; Zhang, P.; Song, Z.; Chen, J.; Zhang, C.-H. Accurate Identification of Exosomes Based on Proximity-Induced Autonomous Assembly of DNAzyme Wires. Sens. Actuators B Chem. 2023, 383, 133581. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Zhang, X.; Wang, S.; Xia, Z.; Guo, X.; Zhao, Y.; Wang, P.; Wang, X.-H. Microstructured Optical Fiber-Enhanced Light-Matter Interaction Enables Highly Sensitive Exosome-Based Liquid Biopsy of Breast Cancer. Anal. Chem. 2023, 95, 1095–1105. [Google Scholar] [CrossRef]
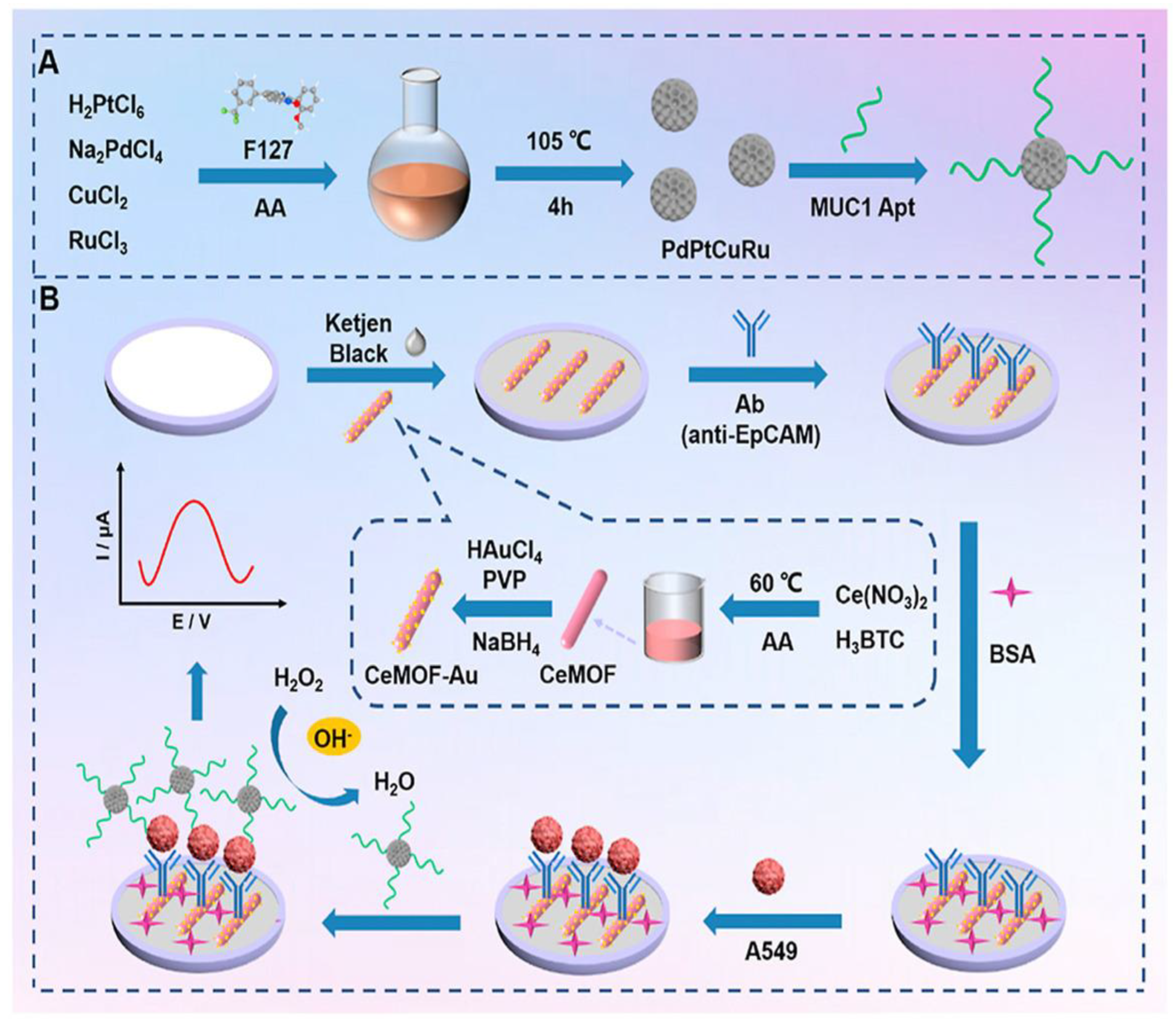
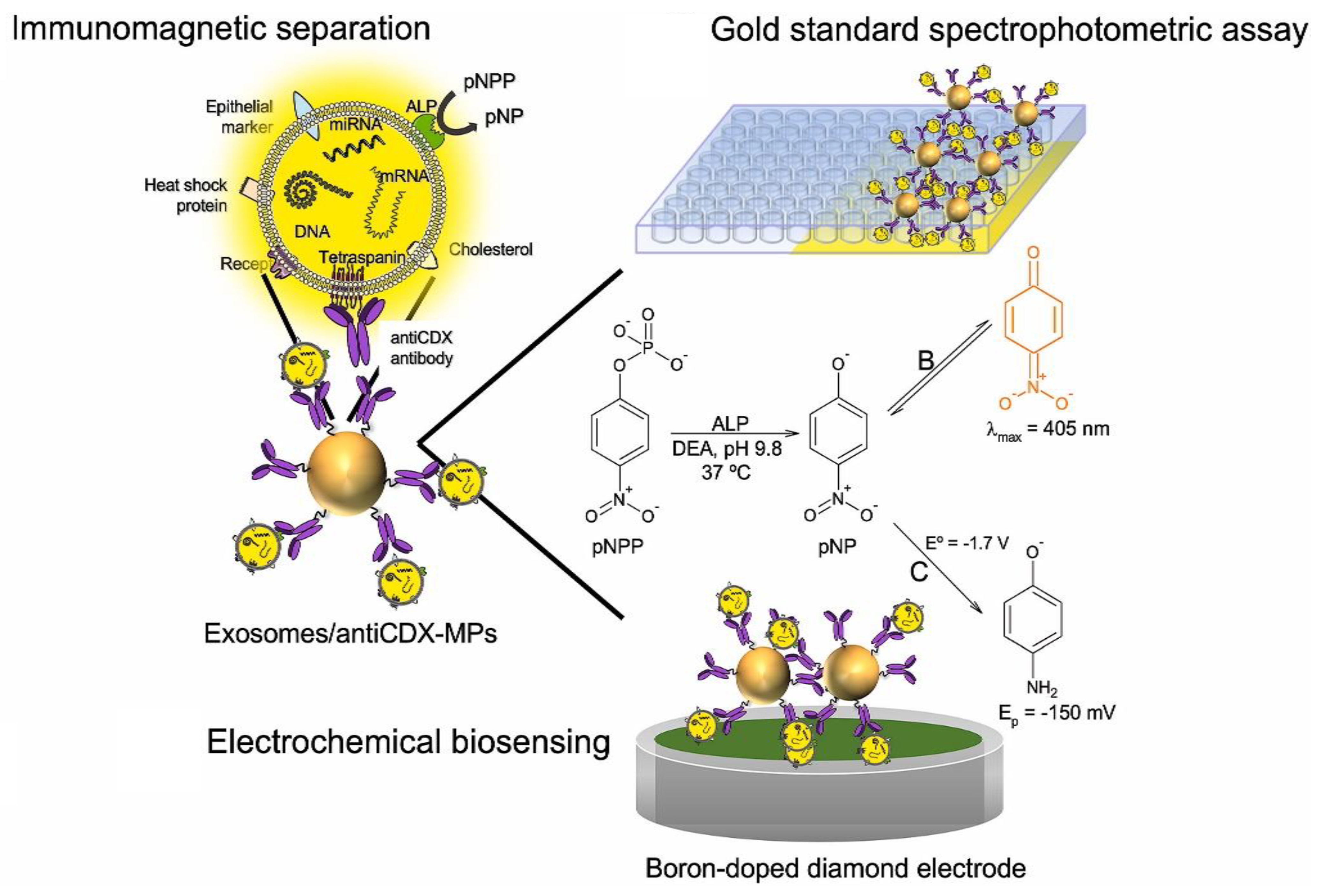
| Biosensing Method | Oncomarker | LOD | Linear Range | Sample Type | Refs. |
|---|---|---|---|---|---|
| SERS | MUC1, HER2, and CEA | N/A | N/A | Exosome-spiked serum | [161] |
| SERS | EpCAM and HER2 | 1.5 × 102 particles/mL | 2.7 × 102 to 2.7 × 108 particles/mL | MCF-7 cell-derived exosomes | [162] |
| ECL | Mir-155 | 273.20 aM | 1.0 fM to 1.0 nM | Serum | [163] |
| FO-SPR | HER2 and EpCAM | HER2: 7 × 108 particles/mL and EpCAM: 1.1 × 108 particles/mL | N/A | Plasma | [164] |
| SPR | TNC | 18.1 particles/mL−1 | 3 × 104∼3 × 107 particles mL−1 | Circulating exosomes in serum | [165] |
| EIS | HER2 | 10 pg | 0.1 ng to 1 µg | Serum | [166] |
| Fluorescence | PD-L1 | 880 particles μL−1 | 0.0001.0 and 0.2.0 particles μL−1 | Plasma | [167] |
| Electrochemical | EpCAM | 4 × 102 exosomes μL–1 | N/A | Serum | [168] |
| (MFS)-CRISPR assay | miR-21 | 1.2 × 103 particles/mL | 104–108 particles/mL | Plasma | [169] |
| Fluorescence | CD63 | 2.5 × 103 particles/μL | N/A | MCF-7 cell exosomes in FBS | [170] |
| Fluorescence | MUC1 | 7.56 particles/μL | 0−400 particles/μL | Plasma | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghi, M.; Sadeghi, S.; Naghib, S.M.; Garshasbi, H.R. A Comprehensive Review on Electrochemical Nano Biosensors for Precise Detection of Blood-Based Oncomarkers in Breast Cancer. Biosensors 2023, 13, 481. https://doi.org/10.3390/bios13040481
Sadeghi M, Sadeghi S, Naghib SM, Garshasbi HR. A Comprehensive Review on Electrochemical Nano Biosensors for Precise Detection of Blood-Based Oncomarkers in Breast Cancer. Biosensors. 2023; 13(4):481. https://doi.org/10.3390/bios13040481
Chicago/Turabian StyleSadeghi, Mahdi, Somayeh Sadeghi, Seyed Morteza Naghib, and Hamid Reza Garshasbi. 2023. "A Comprehensive Review on Electrochemical Nano Biosensors for Precise Detection of Blood-Based Oncomarkers in Breast Cancer" Biosensors 13, no. 4: 481. https://doi.org/10.3390/bios13040481
APA StyleSadeghi, M., Sadeghi, S., Naghib, S. M., & Garshasbi, H. R. (2023). A Comprehensive Review on Electrochemical Nano Biosensors for Precise Detection of Blood-Based Oncomarkers in Breast Cancer. Biosensors, 13(4), 481. https://doi.org/10.3390/bios13040481






