Fluorescent Nanocomposite Hydrogels Based on Conjugated Polymer Nanoparticles as Platforms for Alkaline Phosphatase Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
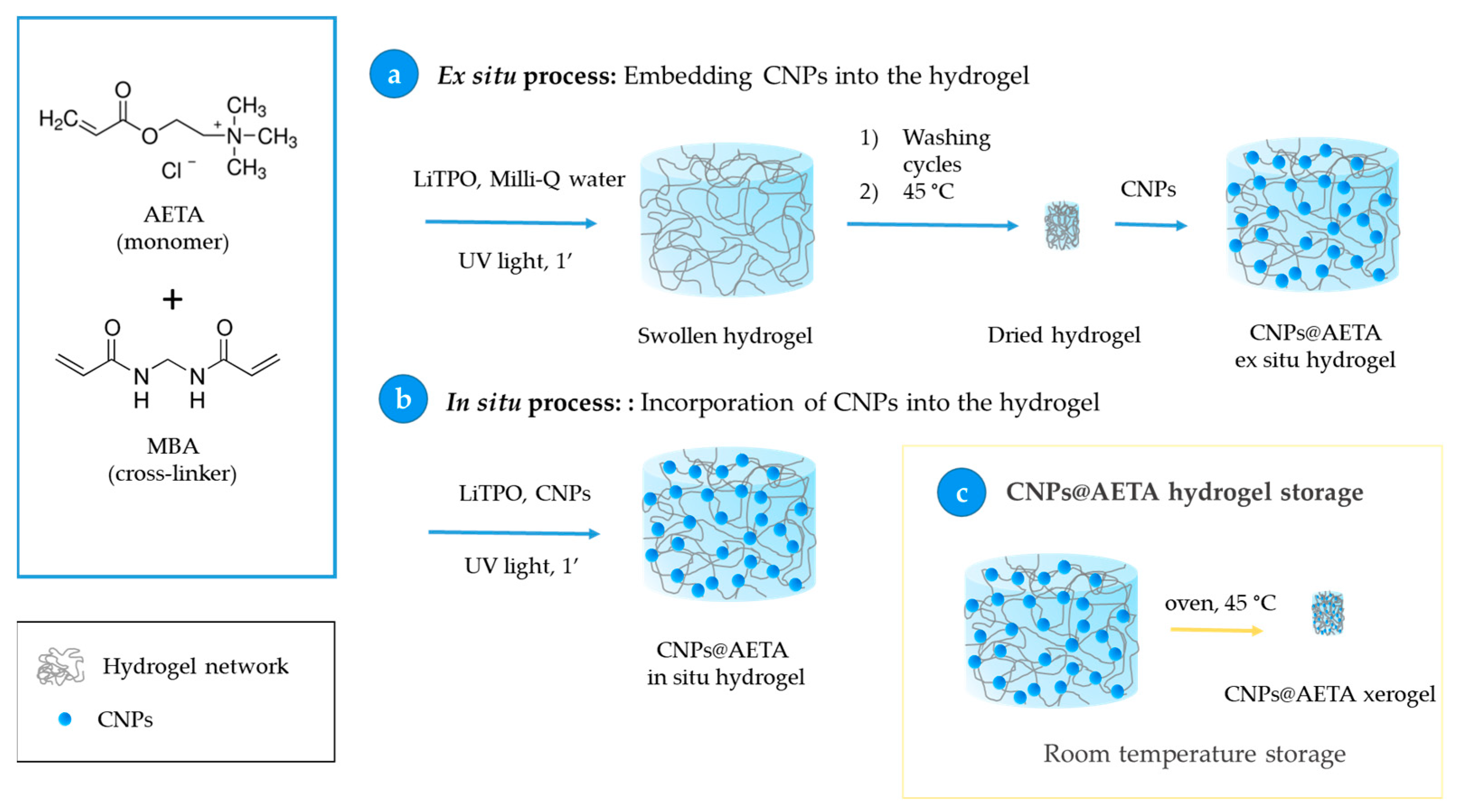
2.2. Obtention of AETA Hydrogels
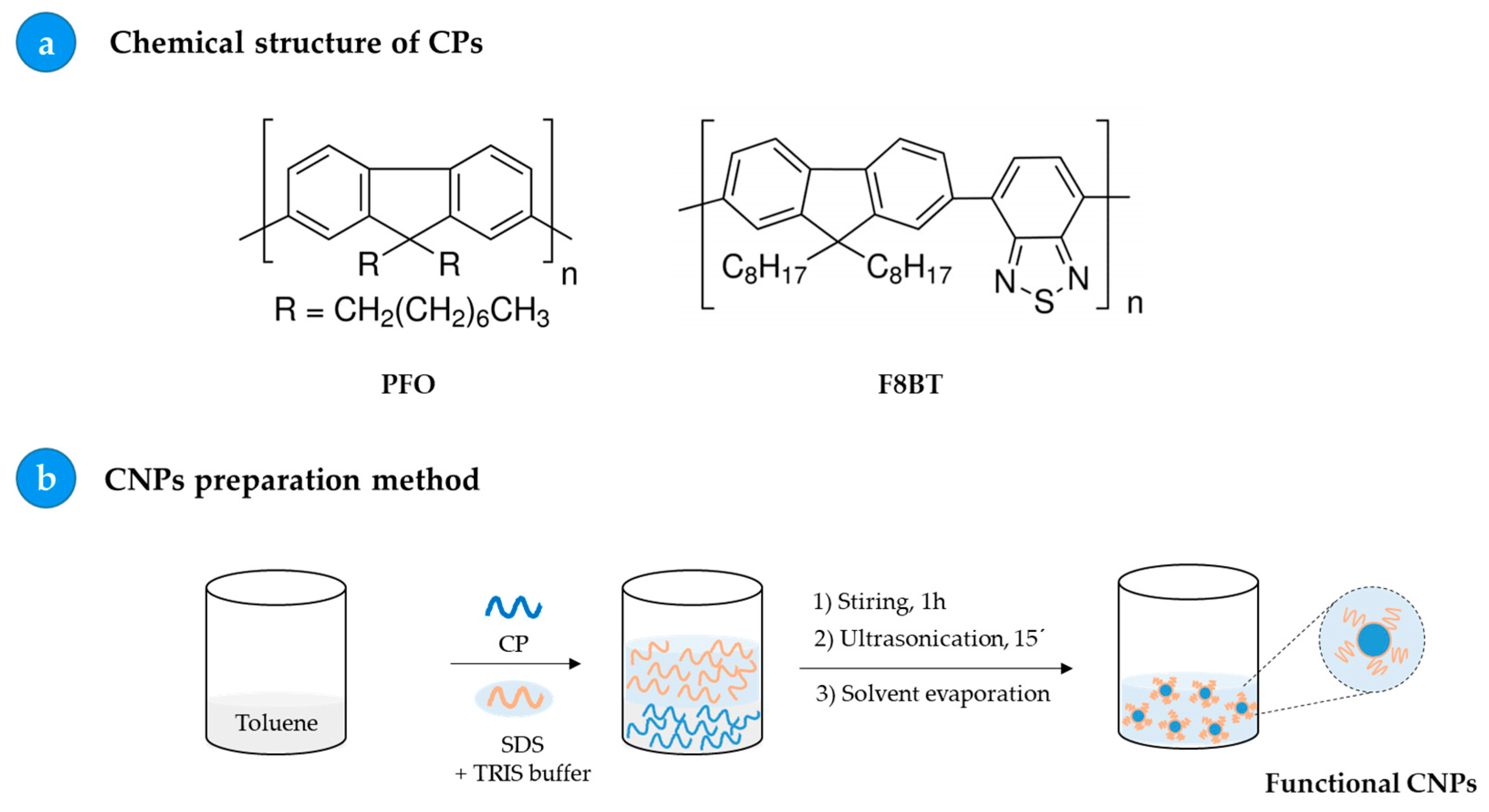
2.3. Preparation of Fluorescent Nanoparticles: PFO_CNPs and F8BT_CNPs
2.4. Obtention of PFO@AETA and F8BT@AETA Hydrogels
- Ex situ process: Embedment of the CNPs into the hydrogel. In this process, 0.07 g of hydrogel prepared by the above method was immersed in 1.8 mL of a 6 µM solution of already prepared CNPs for 24 h, to ensure that all the solution was completely absorbed, and then stored at 4 °C. This step allows the total loading of the fluorescent nanoparticles from the CNPs solution into the hydrogel network (Scheme 2a).
- In situ process: Incorporation of CNPs into the hydrogel. Nanoparticles of PFO and F8BT were incorporated before the polymerization process by adding 5 mL of a solution of CNPs 6 µM to the AETA-MBA-LiTPO mixture. After the irradiation with UV light, fluorescent nanocomposite hydrogels were obtained (Scheme 2b).
2.5. Absorbance Measurements
2.6. Steady-State Fluorescence Measurements
2.7. Time-Resolved Fluorescence Measurements
2.8. Swelling Measurements
2.9. Particle Size and Zeta Potential
2.10. Scanning Electron Microscope
3. Results and Discussion
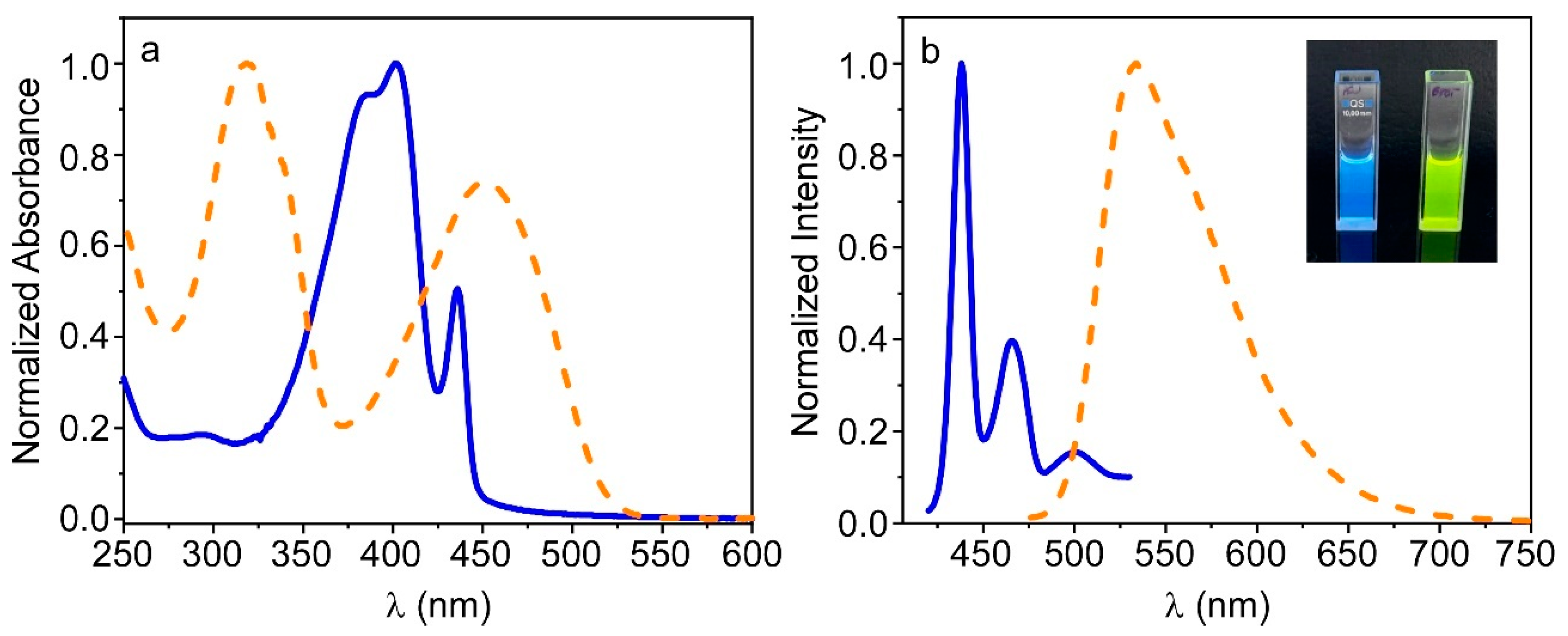
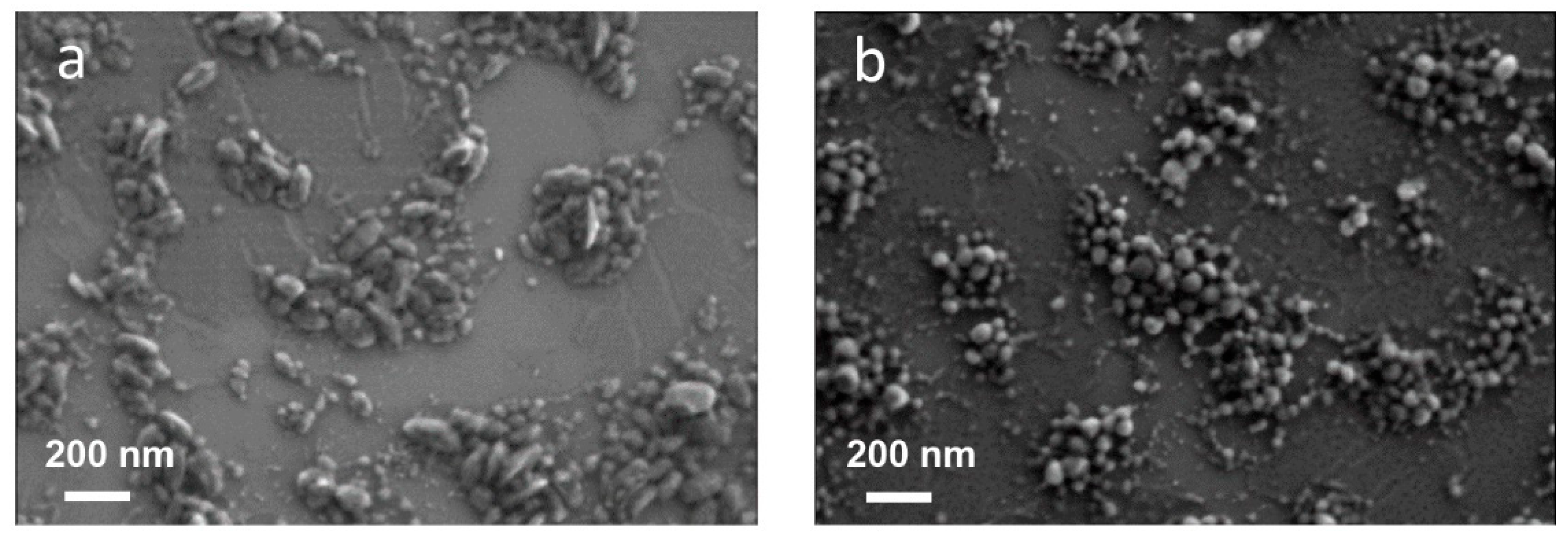
3.1. Preparation and Characterization of PFO_CNPs and F8BT_CNPs
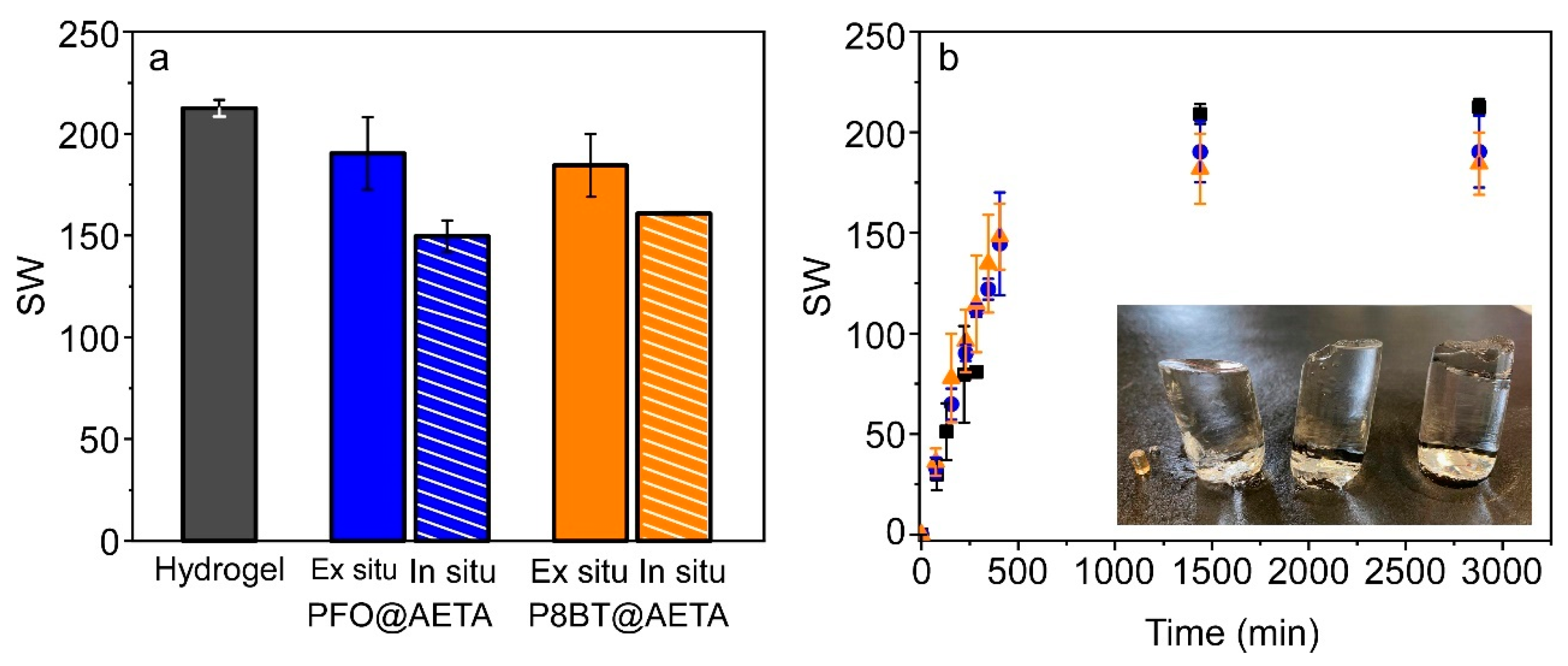
3.2. Preparation and Swelling Behavior of PFO@AETA and F8BT@AETA Hydrogels
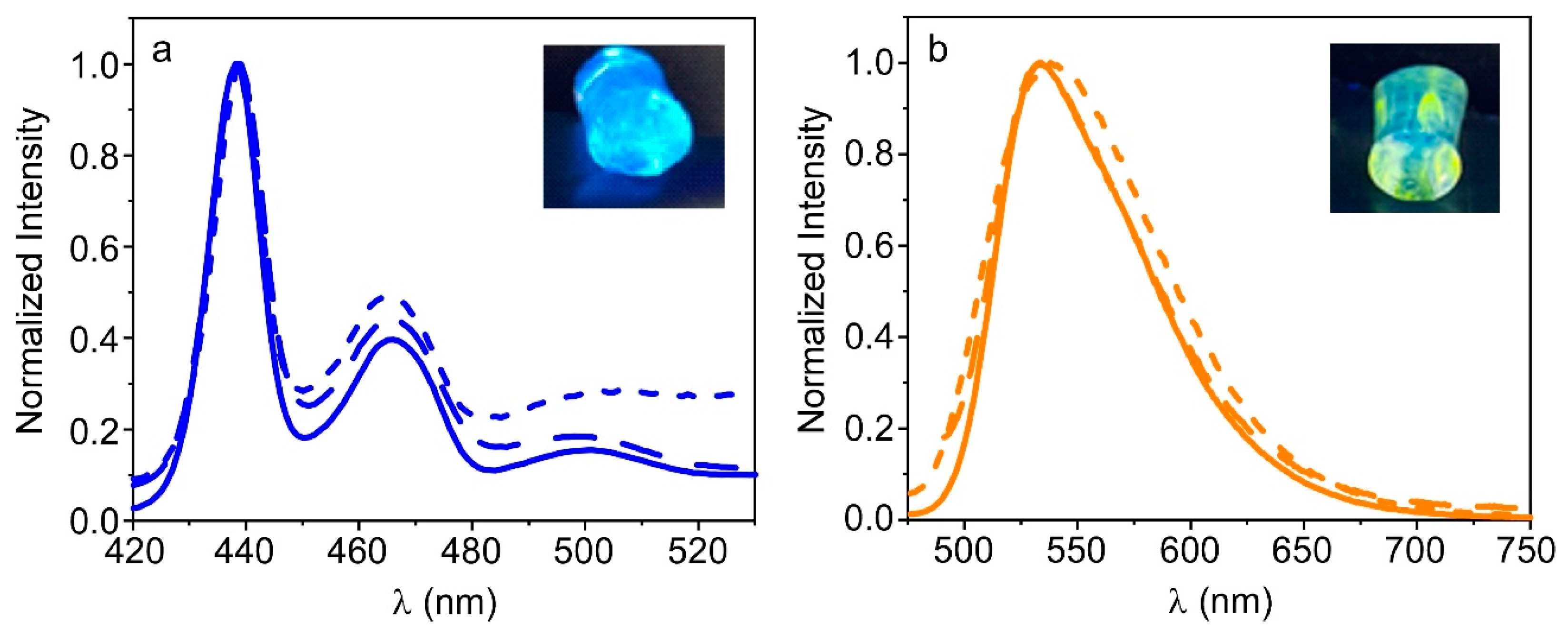
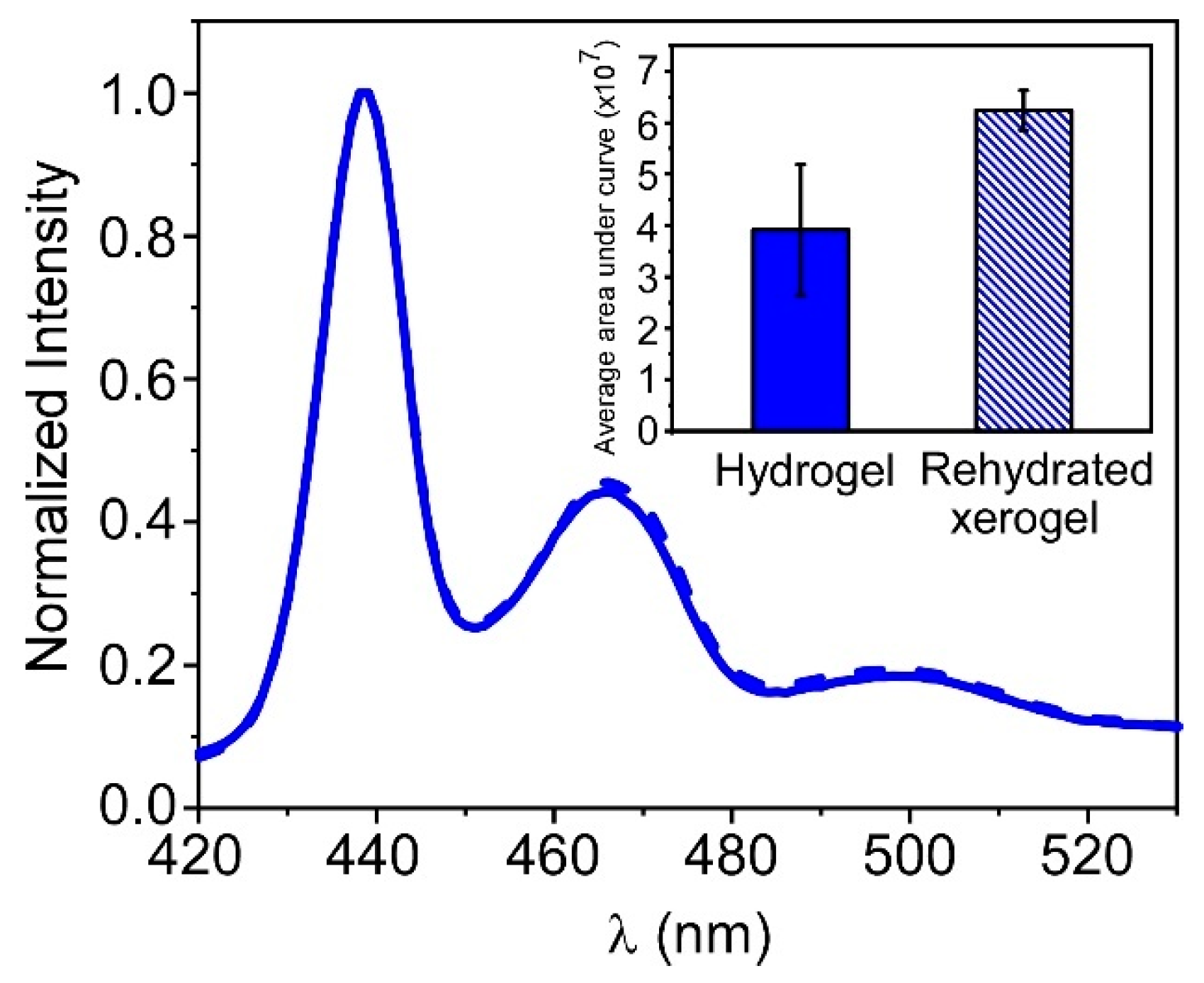
3.3. Fluorescence Properties of PFO@AETA and F8BT@AETA Hydrogels
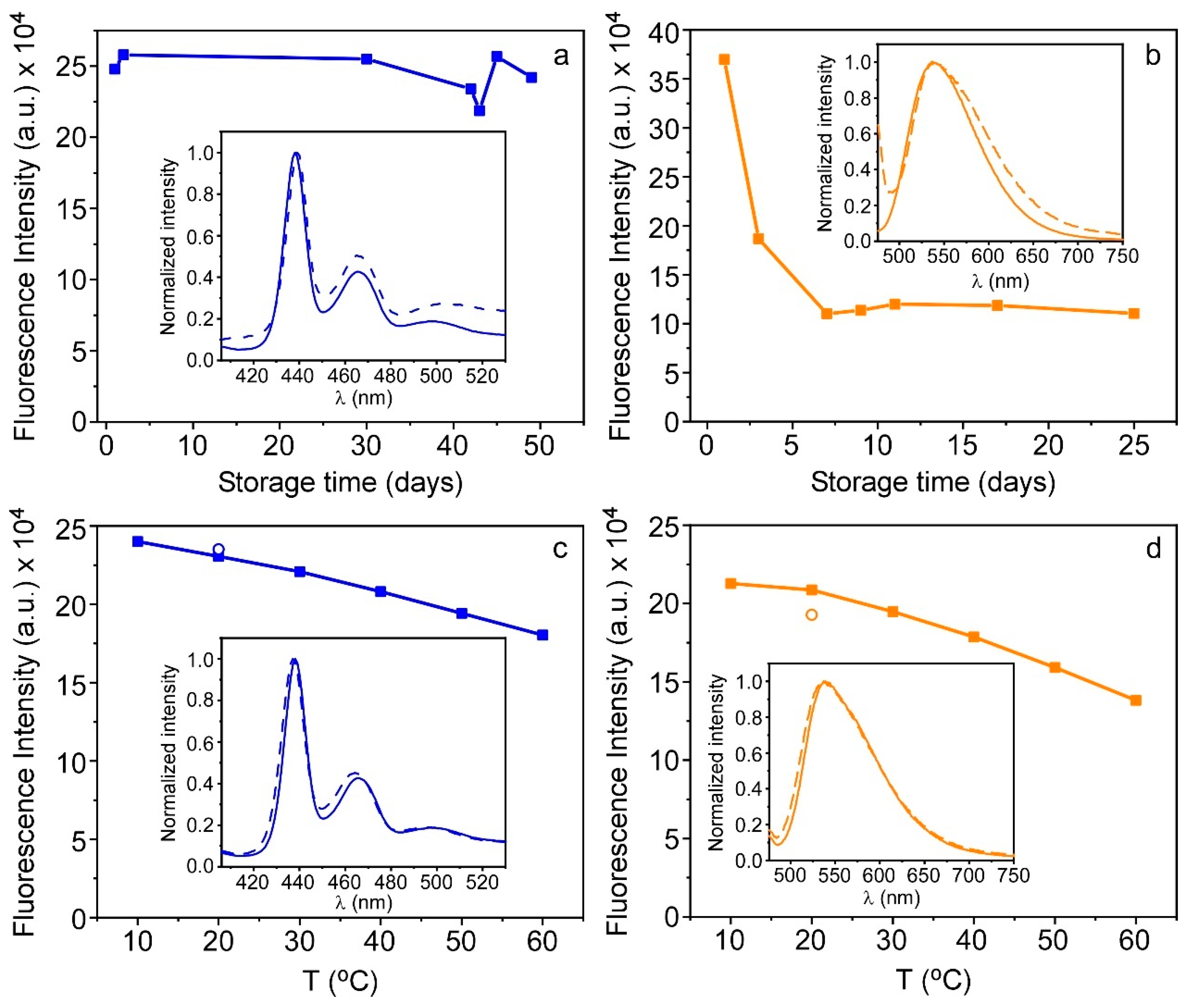
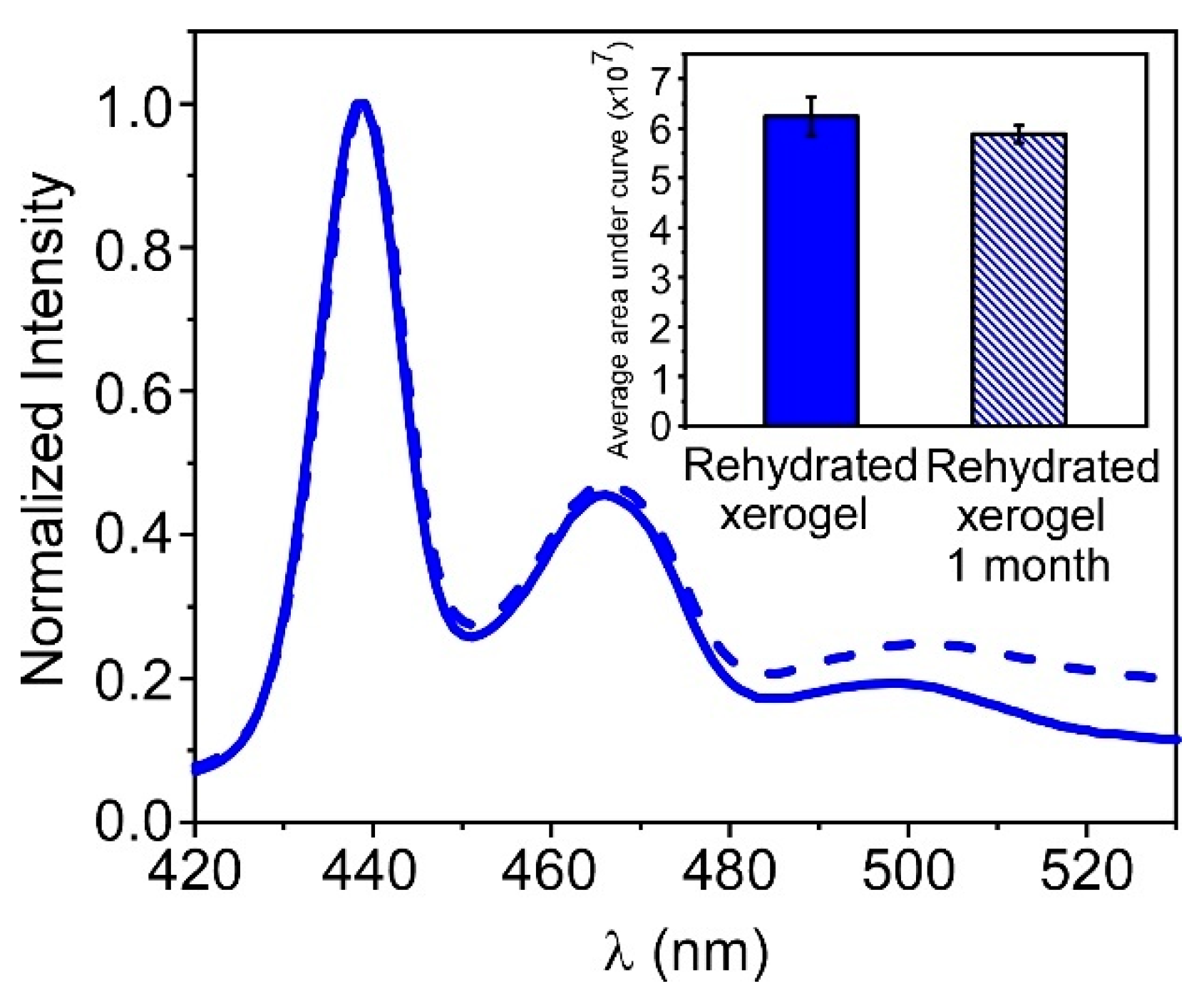
3.4. Storage of PFO@AETA as Xerogels
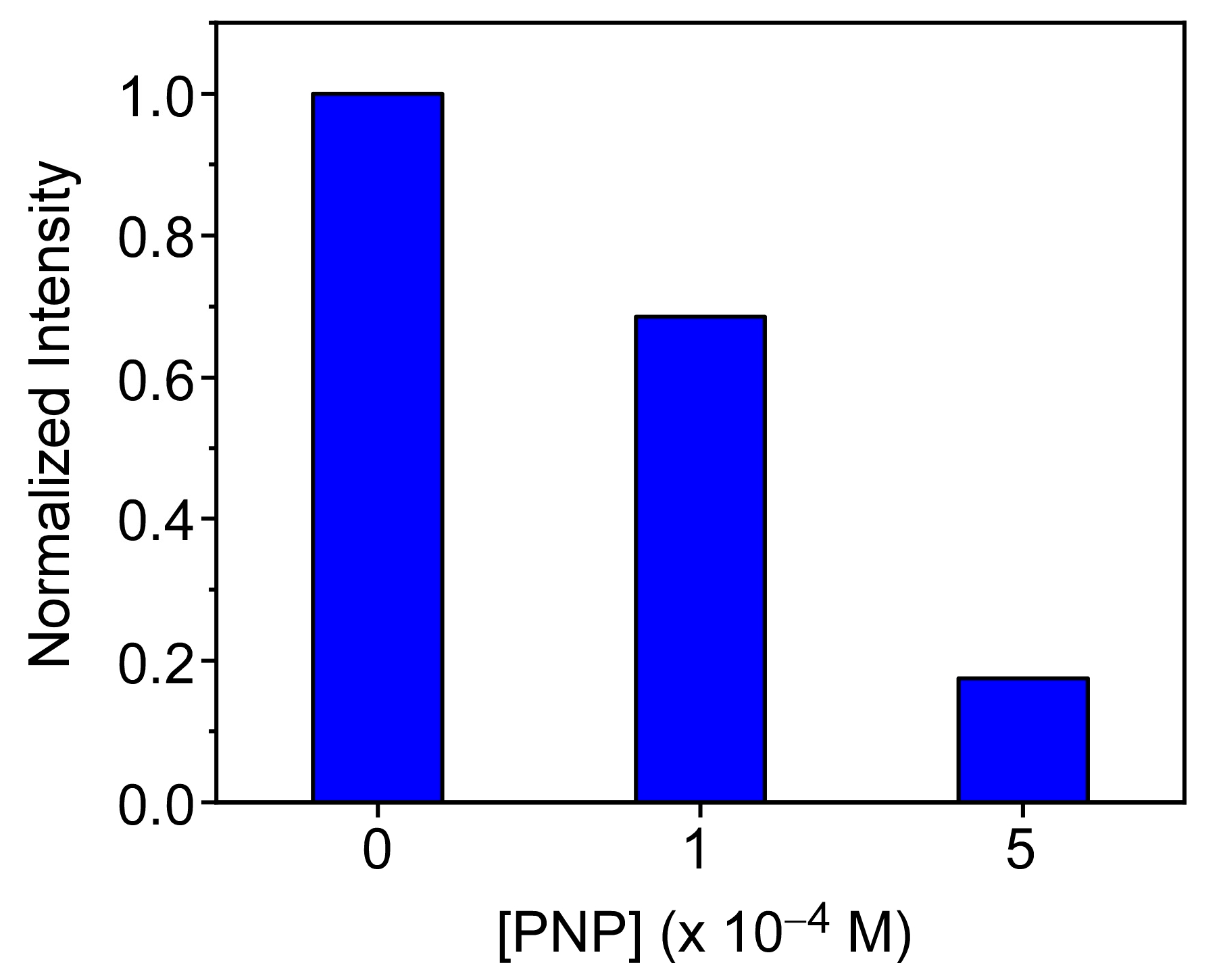
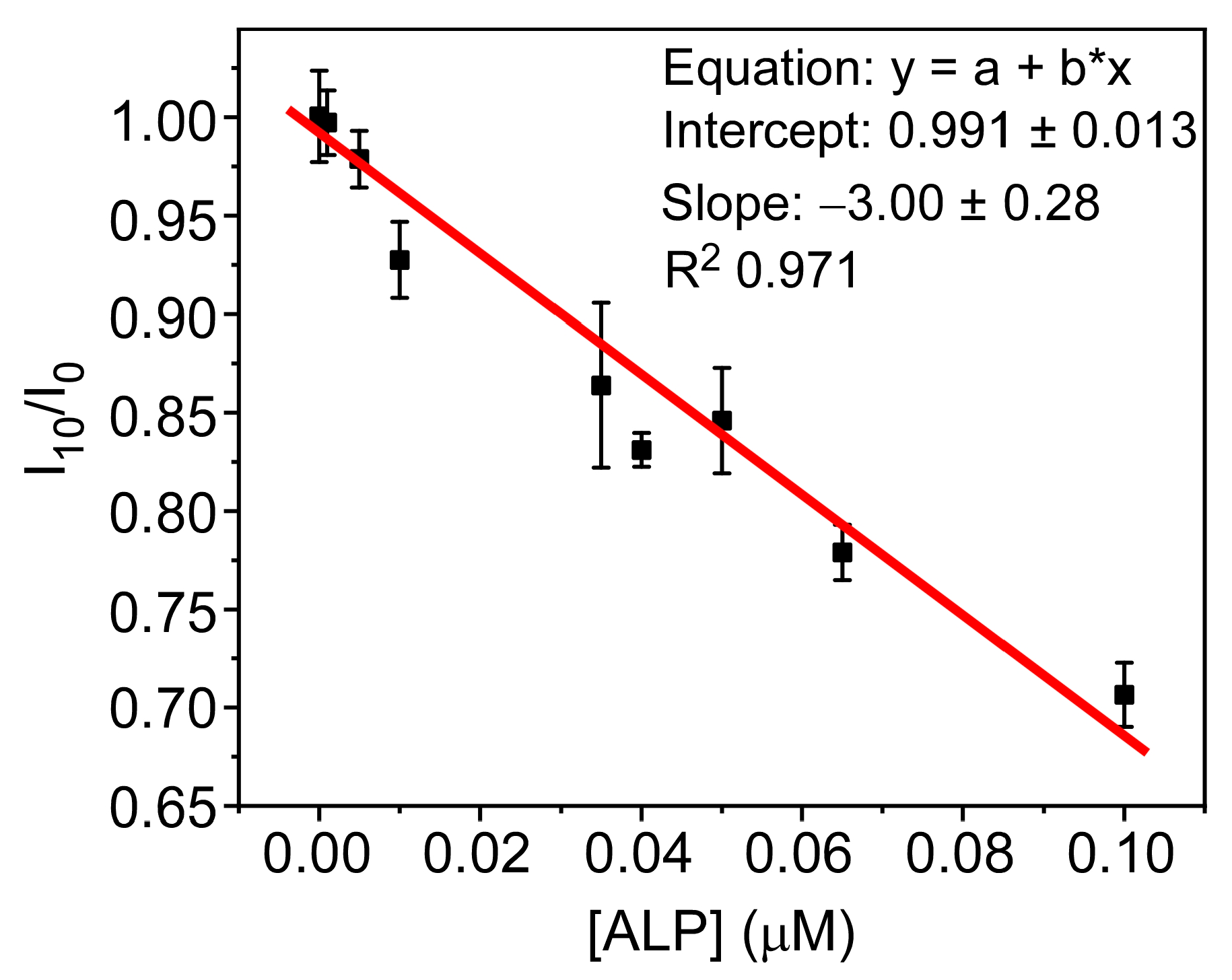
3.5. PFO@AETA as a Sensing Platform
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Barbero, C.A.; Martínez, M.V.; Acevedo, D.F.; Molina, M.A.; Rivarola, C.R. Cross-Linked Polymeric Gels and Nanocomposites: New Materials and Phenomena Enabling Technological Applications. Macromol 2022, 2, 440–475. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Azady, M.A.R.; Ahmed, S.; Islam, M.S. A review on polymer nanocomposite hydrogel preparation, characterization, and applications. Eur. J. Chem. 2021, 12, 11. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T. Nanocomposite Hydrogels: A Unique Organic–Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-swelling Properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar] [CrossRef]
- Li, Y.; Young, D.J.; Loh, X.J. Fluorescent gels: A review of synthesis, properties, applications and challenges. Mater. Chem. Front. 2019, 3, 1489–1502. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, e2100062. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Li, Z.; Lu, W.; Liu, H.; Zhang, J.; Chen, T.; Tang, B.Z. Multicolor Fluorescent Polymeric Hydrogels. Angew. Chem. Int. Ed. Engl. 2021, 60, 8608–8624. [Google Scholar] [CrossRef]
- Chang, C.; Peng, J.; Zhang, L.; Pang, D.-W. Strongly fluorescent hydrogels with quantum dots embedded in cellulose matrices. J. Mater. Chem. 2009, 19, 7771–7776. [Google Scholar] [CrossRef]
- Qasemi, S.; Ghaemy, M. Highly sensitive and strongly fluorescent gum tragacanth based superabsorbent hydrogel as a new biosensor for glucose optical detection. J. Mater. Chem. C 2020, 8, 4148–4156. [Google Scholar] [CrossRef]
- Chen, M.; Grazon, C.; Sensharma, P.; Nguyen, T.T.; Feng, Y.; Chern, M.; Baer, R.C.; Varongchayakul, N.; Cook, K.; Lecommandoux, S.; et al. Hydrogel-Embedded Quantum Dot-Transcription Factor Sensors for Quantitative Progesterone Detection. ACS Appl. Mater. Interfaces 2020, 12, 43513–43521. [Google Scholar] [CrossRef]
- Martín, C.; Martín-Pacheco, A.; Naranjo, A.; Criado, A.; Merino, S.; Díez-Barra, E.; Herrero, M.A.; Vázquez, E. Graphene hybrid materials? The role of graphene materials in the final structure of hydrogels. Nanoscale 2019, 11, 4822–4830. [Google Scholar] [CrossRef]
- Yan, X.; Rahman, S.; Rostami, M.; Tabasi, Z.A.; Khan, F.; Alodhayb, A.; Zhang, Y. Carbon Quantum Dot-Incorporated Chitosan Hydrogel for Selective Sensing of Hg2+ Ions: Synthesis, Characterization, and Density Functional Theory Calculation. ACS Omega 2021, 6, 23504–23514. [Google Scholar] [CrossRef]
- Shao, J.; Yu, Q.; Wang, S.; Hu, Y.; Guo, Z.; Kang, K.; Ji, X. Poly(vinyl alcohol)–Carbon Nanodots Fluorescent Hydrogel with Superior Mechanical Properties and Sensitive to Detection of Iron(III) Ions. Macromol. Mater. Eng. 2019, 304, 1900326. [Google Scholar] [CrossRef]
- Martín-Pacheco, A.; Del Río Castillo, A.E.; Martín, C.; Herrero, M.A.; Merino, S.; García Fierro, J.L.; Díez-Barra, E.; Vázquez, E. Graphene Quantum Dot–Aerogel: From Nanoscopic to Macroscopic Fluorescent Materials. Sensing Polyaromatic Compounds in Water. ACS Appl. Mater. Interfaces 2018, 10, 18192–18201. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Mayr, J.; Eritja, R.; Díaz, D.D. Biodegradable liposome-encapsulated hydrogels for biomedical applications: A marriage of convenience. Biomater. Sci. 2016, 4, 555–574. [Google Scholar] [CrossRef]
- Gao, D.; Hu, D.; Liu, X.; Zhang, X.; Yuan, Z.; Sheng, Z.; Zheng, H. Recent Advances in Conjugated Polymer Nanoparticles for NIR-II Imaging and Therapy. ACS Appl. Polym. Mater. 2020, 2, 4241–4257. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional nanoparticles through π-conjugated polymer self-assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- Koralli, P.; Nega, A.; Vagiaki, L.; Pavlou, A.; Siskos, M.; Dimitrakopoulou-Strauss, A.; Gregoriou, V.; Chochos, C. New Conjugated Polymer Nanoparticles with High Photoluminescence Quantum Yields for Far-red and Near Infrared Fluorescence Bioimaging. Mater. Chem. Front. 2020, 4, 2357–2369. [Google Scholar] [CrossRef]
- Chen, X.; Hussain, S.; Abbas, A.; Hao, Y.; Malik, A.H.; Tian, X.; Song, H.; Gao, R. Conjugated polymer nanoparticles and their nanohybrids as smart photoluminescent and photoresponsive material for biosensing, imaging, and theranostics. Mikrochim. Acta 2022, 189, 83. [Google Scholar] [CrossRef]
- Alacid, Y.; Martínez-Tomé, M.J.; Mateo, C.R. Reusable Fluorescent Nanobiosensor Integrated in a Multiwell Plate for Screening and Quantification of Antidiabetic Drugs. ACS Appl. Mater. Interfaces 2021, 13, 25624–25634. [Google Scholar] [CrossRef]
- Aguado, R.; Santos, A.; Vallejos, S.; Valente, A.J.M. Paper-Based Probes with Visual Response to Vapors from Nitroaromatic Explosives: Polyfluorenes and Tertiary Amines. Molecules 2022, 27, 2900. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hussain, S.; Chen, X.; Hao, Y.; Zhang, P.; Gao, R. Fabrication of conjugated polymer encapsulated fluorescent hybrid micelles for augmented, highly selective and step-wise detection of nitroaromatic pollutants and hepatobiliary biomarker. Sens. Actuators B Chem. 2023, 377, 133081. [Google Scholar] [CrossRef]
- Ji, X.; Yao, Y.; Li, J.; Yan, X.; Huang, F. A Supramolecular Cross-Linked Conjugated Polymer Network for Multiple Fluorescent Sensing. J. Am. Chem. Soc. 2013, 135, 74–77. [Google Scholar] [CrossRef]
- Namgung, H.; Jo, S.; Lee, T.S. Fluorescence Modulation of Conjugated Polymer Nanoparticles Embedded in Poly(N-Isopropylacrylamide) Hydrogel. Polymers 2021, 13, 4315. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Camacho, M.; Martínez-Tomé, M.J.; Mira, A.; Mallavia, R.; Mateo, C.R. Formation of Multicolor Nanogels Based on Cationic Polyfluorenes and Poly(methyl vinyl ether-alt-maleic monoethyl ester): Potential Use as pH-Responsive Fluorescent Drug Carriers. Int. J. Mol. Sci. 2021, 22, 9607. [Google Scholar] [CrossRef] [PubMed]
- Alacid, Y.; Martínez-Tomé, M.J.; Esquembre, R.; Herrero, M.A.; Mateo, C.R. Portable Alkaline Phosphatase–Hydrogel Platform: From Enzyme Characterization to Phosphate Sensing. Int. J. Mol. Sci. 2023, 24, 2672. [Google Scholar] [PubMed]
- Alacid, Y.; Quintero Jaime, A.F.; Martínez-Tomé, M.J.; Mateo, C.R.; Montilla, F. Disposable Electrochemical Biosensor Based on the Inhibition of Alkaline Phosphatase Encapsulated in Acrylamide Hydrogels. Biosensors 2022, 12, 698. [Google Scholar] [CrossRef]
- Qu, F.; Meng, L.; Zi, Y.; You, J. Ratiometric detection of alkaline phosphatase based on aggregation-induced emission enhancement. Anal. Bioanal. Chem. 2019, 411, 7431–7440. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Guo, W.; Wang, Y.; Hua, Q.; Tang, F.; Luan, F.; Tian, C.; Zhuang, X.; Zhao, L. Selective Detection of Alkaline Phosphatase Activity in Environmental Water Samples by Copper Nanoclusters Doped Lanthanide Coordination Polymer Nanocomposites as the Ratiometric Fluorescent Probe. Biosensors 2022, 12, 372. [Google Scholar] [CrossRef]
- He, J.; Jiang, X.; Ling, P.; Sun, J.; Gao, F. Ratiometric Sensing for Alkaline Phosphatase Based on Two Independent Signals from in Situ Formed Nanohybrids of Semiconducting Polymer Nanoparticles and MnO2 Nanosheets. ACS Omega 2019, 4, 8282–8289. [Google Scholar] [CrossRef]
- Kahveci, Z.; Martínez-Tomé, M.J.; Mallavia, R.; Mateo, C.R. Fluorescent Biosensor for Phosphate Determination Based on Immobilized Polyfluorene-Liposomal Nanoparticles Coupled with Alkaline Phosphatase. ACS Appl. Mater. Interfaces 2017, 9, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Pecher, J.; Mecking, S. Nanoparticles of Conjugated Polymers. Chem. Rev. 2010, 110, 6260–6279. [Google Scholar] [CrossRef] [PubMed]
- Urbano, L.; Clifton, L.; Ku, H.K.; Kendall-Troughton, H.; Vandera, K.-K.A.; Matarese, B.F.E.; Abelha, T.; Li, P.; Desai, T.; Dreiss, C.A.; et al. Influence of the Surfactant Structure on Photoluminescent π-Conjugated Polymer Nanoparticles: Interfacial Properties and Protein Binding. Langmuir 2018, 34, 6125–6137. [Google Scholar] [CrossRef]
- Eggimann, H.J.; Le Roux, F.; Herz, L.M. How β-Phase Content Moderates Chain Conjugation and Energy Transfer in Polyfluorene Films. J. Phys. Chem. 2019, 10, 1729–1736. [Google Scholar] [CrossRef]
- Wu, C.; McNeill, J. Swelling-Controlled Polymer Phase and Fluorescence Properties of Polyfluorene Nanoparticles. Langmuir 2008, 24, 5855–5861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, R.; Jia, Y. Tuning the formation of β-phase poly(9,9-di-n-octylfluorenyl-2,7-diyl) via nano-confinement and polystyrene blending for improved photocatalysis. Chem. Phys. Mater. 2022, 1, 219–226. [Google Scholar] [CrossRef]
- Cao, A.; Tang, Y.; Liu, Y. Novel Fluorescent Biosensor for α-Glucosidase Inhibitor Screening Based on Cationic Conjugated Polymers. ACS Appl. Mater. Interfaces 2012, 4, 3773–3778. [Google Scholar] [CrossRef]
- Astier, Y.; Bartlett, P.N. The measurement of alkaline phosphatase at nanomolar concentration within 70 s using a disposable microelectrochemical transistor. Bioelectrochemistry 2004, 64, 53–59. [Google Scholar] [CrossRef]
- National Library of Medicine. ALP—Blood Test. Available online: https://medlineplus.gov/ency/article/003470.htm (accessed on 14 February 2023).
- Shaban, S.M.; Jo, S.B.; Hafez, E.; Cho, J.H.; Kim, D.-H. A comprehensive overview on alkaline phosphatase targeting and reporting assays. Coord. Chem. Rev. 2022, 465, 214567. [Google Scholar] [CrossRef]
- Martínez-Crego, B.; Romero, J.; Alcoverro, T. The use of surface alkaline phosphatase activity in the seagrass Posidonia oceanica as a biomarker of eutrophication. Mar. Ecol. 2006, 27, 381–387. [Google Scholar] [CrossRef]
- Bañeras, L.; Ros-Ponsatí, M.; Cristina, X.P.; Garcia-Gil, J.L.; Borrego, C.M. Phosphorus deficiency and kinetics of alkaline phosphatase in isolates and natural populations of phototrophic sulphur bacteria. FEMS Microbiol. Ecol. 2010, 73, 243–253. [Google Scholar] [CrossRef] [PubMed]
| Sample | d ± SD (nm) | PDI ± SD | ZP ± SD (mV) |
|---|---|---|---|
| PFO_CNPs | 76.6 ± 0,5 | 0.203 ± 0.005 | −45 ± 1 |
| F8BT_CNPs | 65.7 ± 0,9 | 0.155 ± 0.010 | −62 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alacid, Y.; Esquembre, R.; Montilla, F.; Martínez-Tomé, M.J.; Mateo, C.R. Fluorescent Nanocomposite Hydrogels Based on Conjugated Polymer Nanoparticles as Platforms for Alkaline Phosphatase Detection. Biosensors 2023, 13, 408. https://doi.org/10.3390/bios13030408
Alacid Y, Esquembre R, Montilla F, Martínez-Tomé MJ, Mateo CR. Fluorescent Nanocomposite Hydrogels Based on Conjugated Polymer Nanoparticles as Platforms for Alkaline Phosphatase Detection. Biosensors. 2023; 13(3):408. https://doi.org/10.3390/bios13030408
Chicago/Turabian StyleAlacid, Yolanda, Rocío Esquembre, Francisco Montilla, María José Martínez-Tomé, and C. Reyes Mateo. 2023. "Fluorescent Nanocomposite Hydrogels Based on Conjugated Polymer Nanoparticles as Platforms for Alkaline Phosphatase Detection" Biosensors 13, no. 3: 408. https://doi.org/10.3390/bios13030408
APA StyleAlacid, Y., Esquembre, R., Montilla, F., Martínez-Tomé, M. J., & Mateo, C. R. (2023). Fluorescent Nanocomposite Hydrogels Based on Conjugated Polymer Nanoparticles as Platforms for Alkaline Phosphatase Detection. Biosensors, 13(3), 408. https://doi.org/10.3390/bios13030408





