Photoacoustic Spectral Analysis for Evaluating the Aggressiveness of Prostate Cancer Labeled by Methylene Blue Polyacrylamide Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
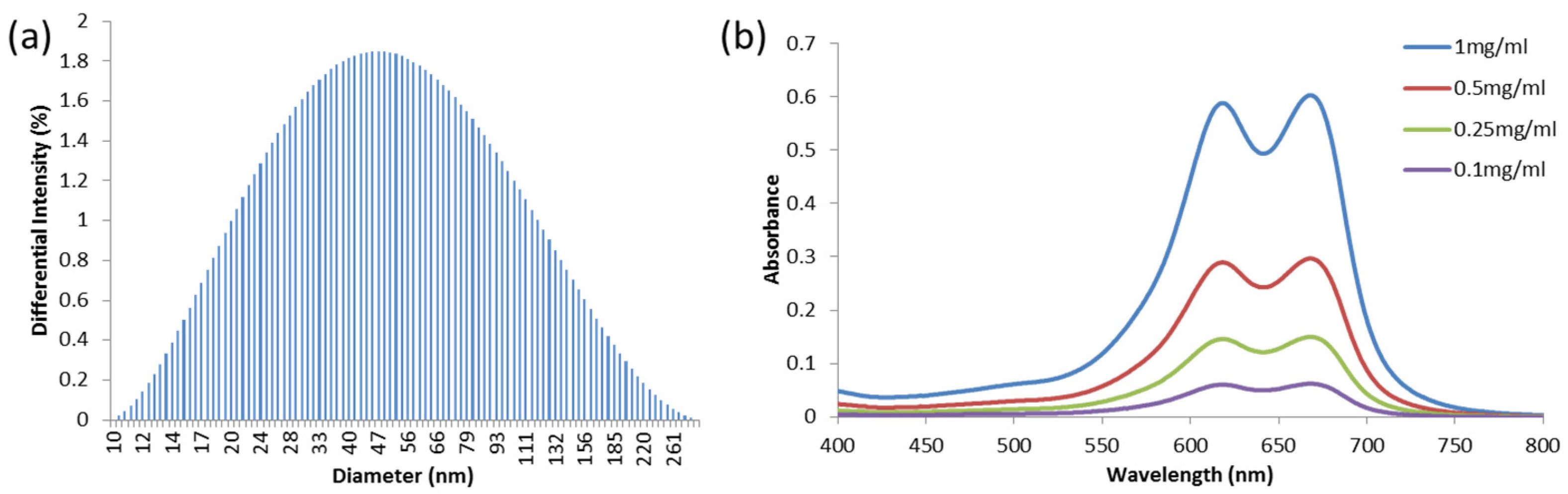
2.1. Preparation of MB-PAA NPs
2.2. Animal Model
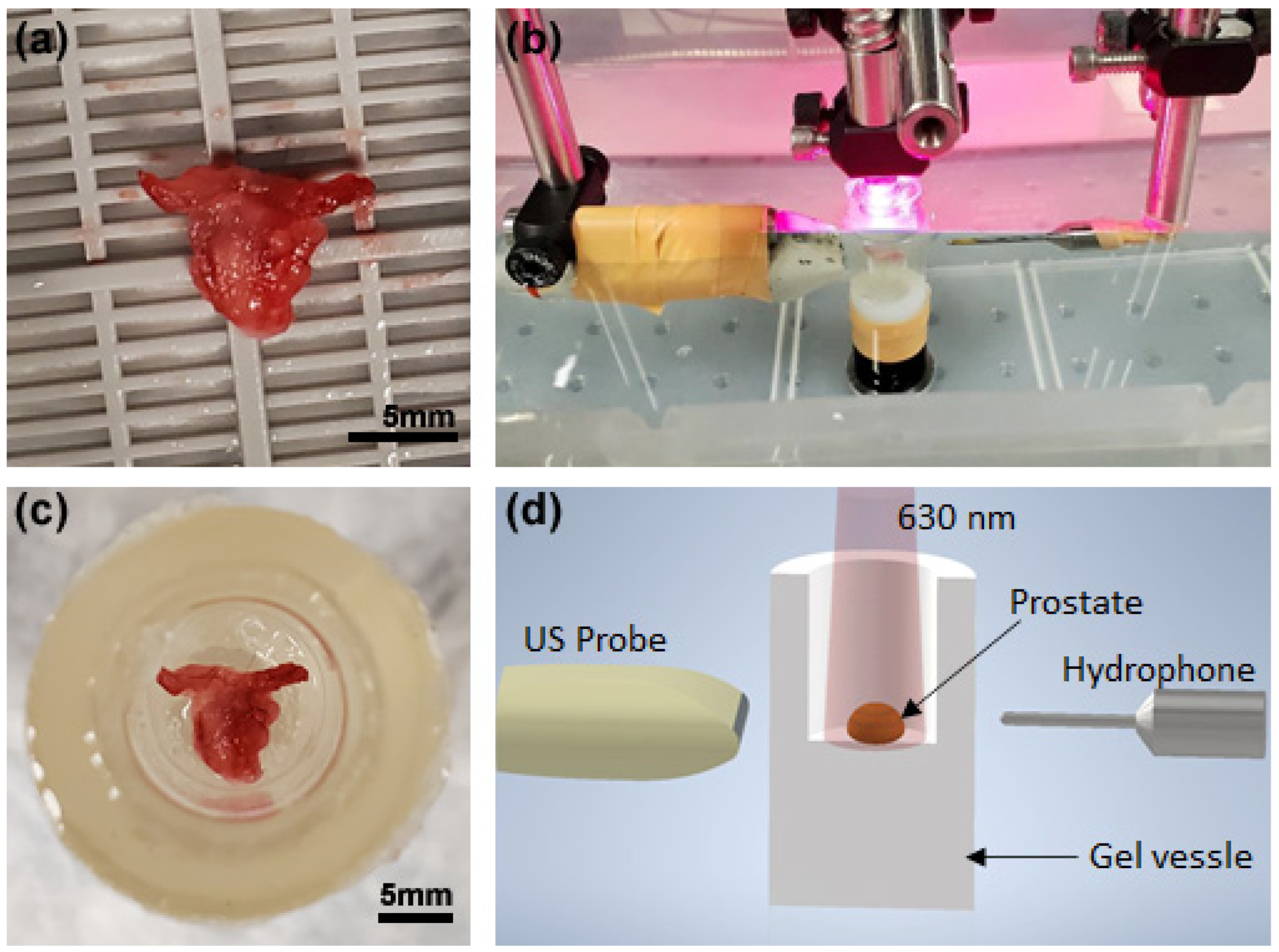
2.3. Experimental Procedure
2.4. Photoacoustic Spectral Analysis (PASA)
2.5. Histology
3. Results
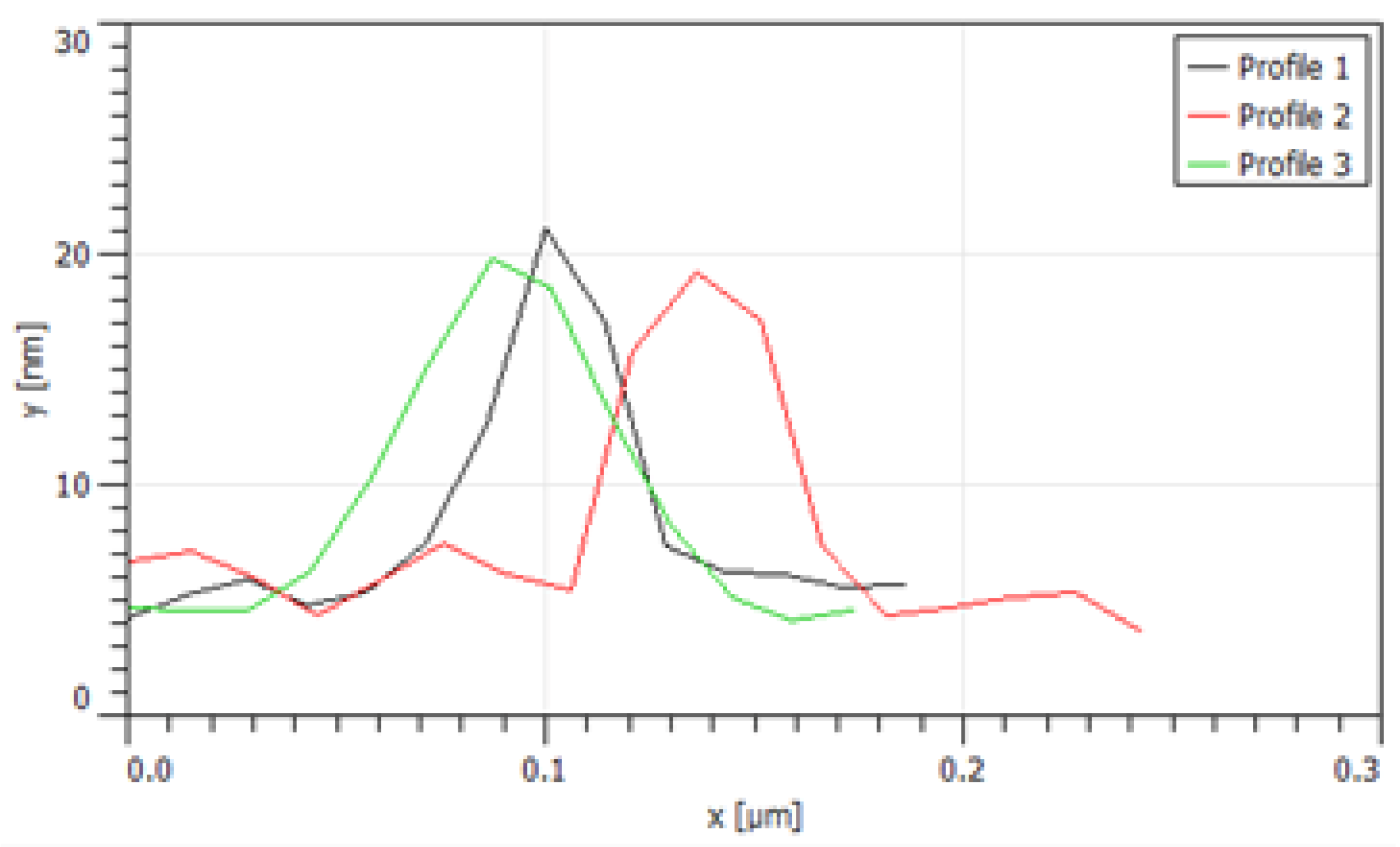
3.1. PCa Microarchitectures Labeled by MB PAA NPs
3.2. PA-US Dual Imaging of Prostate
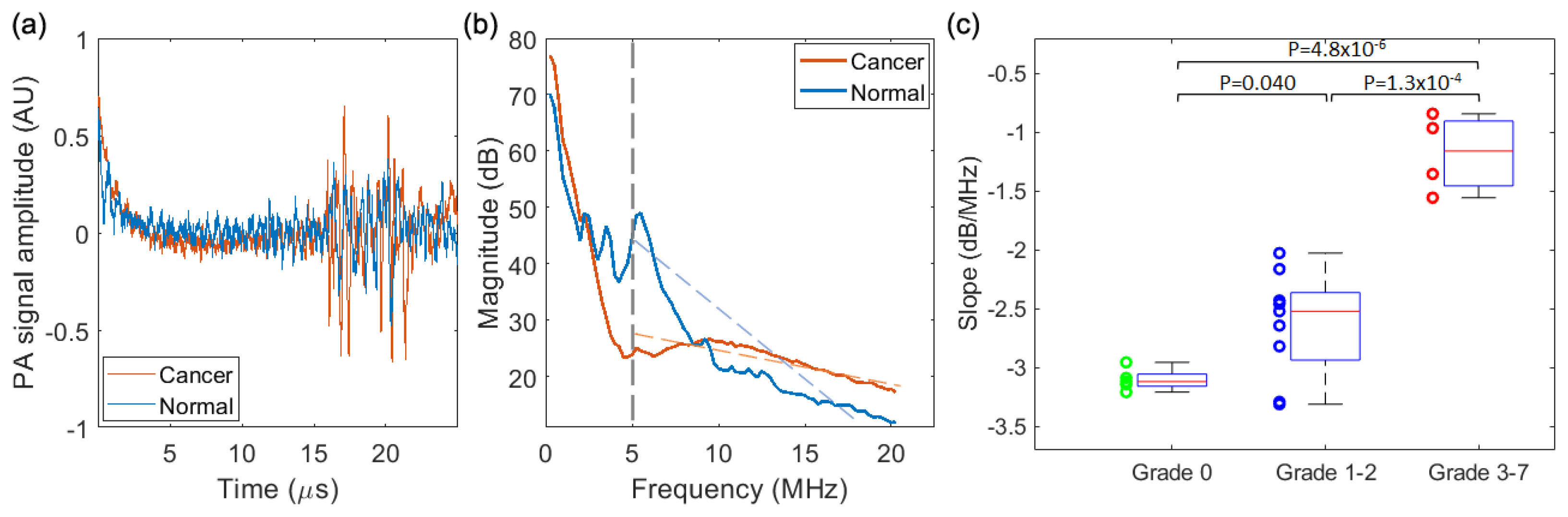
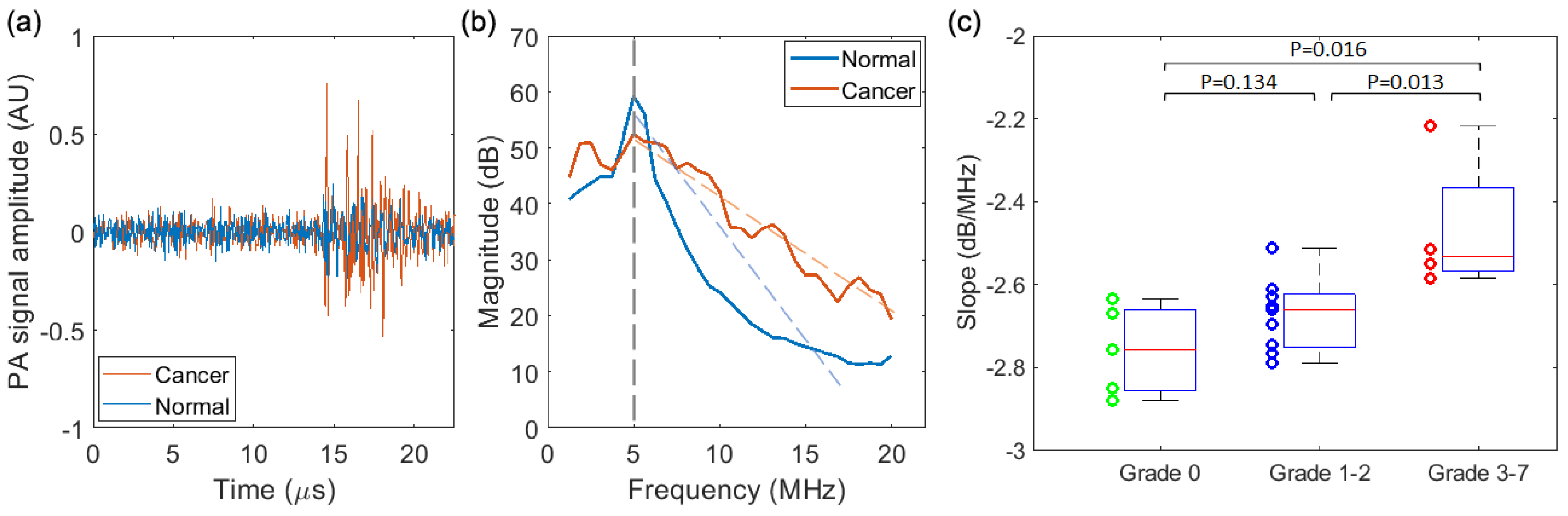
3.3. PASA of the Signals from Prostate
3.3.1. PASA of the Results from the Needle Hydrophone
3.3.2. PASA of the Results from the PA-US Imaging System
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ghani, K.R.; Dundas, D.; Patel, U. Bleeding after transrectal ultrasonography-guided prostate biopsy: A study of 7-day morbidity after a six-, eight- and 12-core biopsy protocol. BJU Int. 2004, 94, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Gleason, D.F. Histologic grading of prostate cancer: A perspective. Hum. Pathol. 1992, 23, 273–279. [Google Scholar] [CrossRef]
- Taira, A.V.; Merrick, G.S.; Galbreath, R.W.; Andreini, H.; Taubenslag, W.; Curtis, R.; Butler, W.M.; Adamovich, E.; Wallner, K.E. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2010, 13, 71–77. [Google Scholar] [CrossRef]
- Rabbani, F.; Stroumbakis, N.; Kava, B.R.; Cookson, M.S.; Fair, W.R. Incidence and clinical significance of false-negative sextant prostate biopsies. J. Urol. 1998, 159, 1247–1250. [Google Scholar] [CrossRef]
- Merrick, G.S.; Gutman, S.; Andreini, H.; Taubenslag, W.; Lindert, D.L.; Curtis, R.; Adamovich, E.; Anderson, R.; Allen, Z.; Butler, W.; et al. Prostate Cancer Distribution in Patients Diagnosed by Transperineal Template-Guided Saturation Biopsy. Eur. Urol. 2007, 52, 715–724. [Google Scholar] [CrossRef]
- Xu, M.H.; Wang, L.H.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L.V. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef]
- Wang, L.V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 2009, 3, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xu, G.; Yu, Y.; Zhou, Y.; Carson, P.L.; Wang, X.; Liu, X. Real-time photoacoustic and ultrasound dual-modality imaging system facilitated with graphics processing unit and code parallel optimization. J. Biomed. Opt. 2013, 18, 086001. [Google Scholar] [CrossRef]
- Jo, J.; Lee, C.H.; Folz, J.; Tan, J.W.Y.; Wang, X.; Kopelman, R. In Vivo Photoacoustic Lifetime Based Oxygen Imaging with Tumor Targeted G2 Polyacrylamide Nanosonophores. ACS Nano 2019, 13, 14024–14032. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Lee, C.H.; Kopelman, R.; Wang, X. In vivo quantitative imaging of tumor pH by nanosonophore assisted multispectral photoacoustic imaging. Nat. Commun. 2017, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.-K.; Maslov, K.; Shung, K.K.; Zhou, Q.; Wang, L.V. In vivo label-free photoacoustic microscopy of cell nuclei by excitation of DNA and RNA. Opt. Lett. 2010, 35, 4139–4141. [Google Scholar] [CrossRef]
- Jo, J.; Siddiqui, J.; Zhu, Y.; Ni, L.; Kothapalli, S.; Tomlins, S.A.; Wei, J.T.; Keller, E.T.; Udager, A.M.; Wang, X.; et al. Photoacoustic spectral analysis at ultraviolet wavelengths for characterizing the Gleason grades of prostate cancer. Opt. Lett. 2020, 45, 6042–6045. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, S.-R.; Sonn, G.A.; Choe, J.W.; Nikoozadeh, A.; Bhuyan, A.; Park, K.K.; Cristman, P.; Fan, R.; Moini, A.; Lee, B.C.; et al. Simultaneous transrectal ultrasound and photoacoustic human prostate imaging. Sci. Transl. Med. 2019, 11, eaav2169. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Hah, H.J.; Kim, G.; Lee, Y.-E.K.; Qin, M.; Ratani, T.S.; Fotiadis, P.; Miller, A.; Kochi, A.; Gao, D.; et al. Hydrogel nanoparticles with covalently linked coomassie blue for brain tumor delineation visible to the surgeon. Small 2012, 8, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.Y.; Lee, C.H.; Kopelman, R.; Wang, X. Transient Triplet Differential (TTD) Method for Background Free Photoacoustic Imaging. Sci. Rep. 2018, 8, 9290. [Google Scholar] [CrossRef]
- Yoon, H.K.; Lou, X.; Chen, Y.-C.; Koo Lee, Y.-E.; Yoon, E.; Kopelman, R. Nanophotosensitizers Engineered to Generate a Tunable Mix of Reactive Oxygen Species, for Optimizing Photodynamic Therapy, Using a Microfluidic Device. Chem. Mater. 2014, 26, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, A.A.; Foster, B.A.; Allison, J.P.; Greenberg, N.M.; Kwon, E.D. The TRAMP Mouse as a Model for Prostate Cancer. In Current Protocols in Immunology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Berman-Booty, L.D.; Sargeant, A.M.; Rosol, T.J.; Rengel, R.C.; Clinton, S.K.; Chen, C.S.; Kulp, S.K. A review of the existing grading schemes and a proposal for a modified grading scheme for prostatic lesions in TRAMP mice. Toxicol. Pathol. 2012, 40, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Wang, S.; Marko, A.; Joshi, P.; Ethirajan, M.; Chen, Y.; Yao, R.; Sajjad, M.; Kopelman, R.; Pandey, R.K. Polyacrylamide-Based Biocompatible Nanoplatform Enhances the Tumor Uptake, PET/fluorescence Imaging and Anticancer Activity of a Chlorophyll Analog. Theranostics 2014, 4, 614–628. [Google Scholar] [CrossRef]
- Patterson, M.P.; Riley, C.B.; Kolios, M.C.; Whelan, W.M. Optoacoustic characterization of prostate cancer in an in vivo transgenic murine model. J. Biomed. Opt. 2014, 19, 056008. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, J.; Salfi, E.; Folz, J.; Udager, A.M.; Keller, E.; Kopelman, R.; Kothapalli, S.-R.; Xu, G.; Wang, X. Photoacoustic Spectral Analysis for Evaluating the Aggressiveness of Prostate Cancer Labeled by Methylene Blue Polyacrylamide Nanoparticles. Biosensors 2023, 13, 403. https://doi.org/10.3390/bios13030403
Jo J, Salfi E, Folz J, Udager AM, Keller E, Kopelman R, Kothapalli S-R, Xu G, Wang X. Photoacoustic Spectral Analysis for Evaluating the Aggressiveness of Prostate Cancer Labeled by Methylene Blue Polyacrylamide Nanoparticles. Biosensors. 2023; 13(3):403. https://doi.org/10.3390/bios13030403
Chicago/Turabian StyleJo, Janggun, Eamon Salfi, Jeff Folz, Aaron M. Udager, Evan Keller, Raoul Kopelman, Sri-Rajasekhar Kothapalli, Guan Xu, and Xueding Wang. 2023. "Photoacoustic Spectral Analysis for Evaluating the Aggressiveness of Prostate Cancer Labeled by Methylene Blue Polyacrylamide Nanoparticles" Biosensors 13, no. 3: 403. https://doi.org/10.3390/bios13030403
APA StyleJo, J., Salfi, E., Folz, J., Udager, A. M., Keller, E., Kopelman, R., Kothapalli, S.-R., Xu, G., & Wang, X. (2023). Photoacoustic Spectral Analysis for Evaluating the Aggressiveness of Prostate Cancer Labeled by Methylene Blue Polyacrylamide Nanoparticles. Biosensors, 13(3), 403. https://doi.org/10.3390/bios13030403





