Recent Progress of Biomaterials-Based Epidermal Electronics for Healthcare Monitoring and Human–Machine Interaction
Abstract
1. Introduction
2. Biomaterials-Based Epidermal Electronics
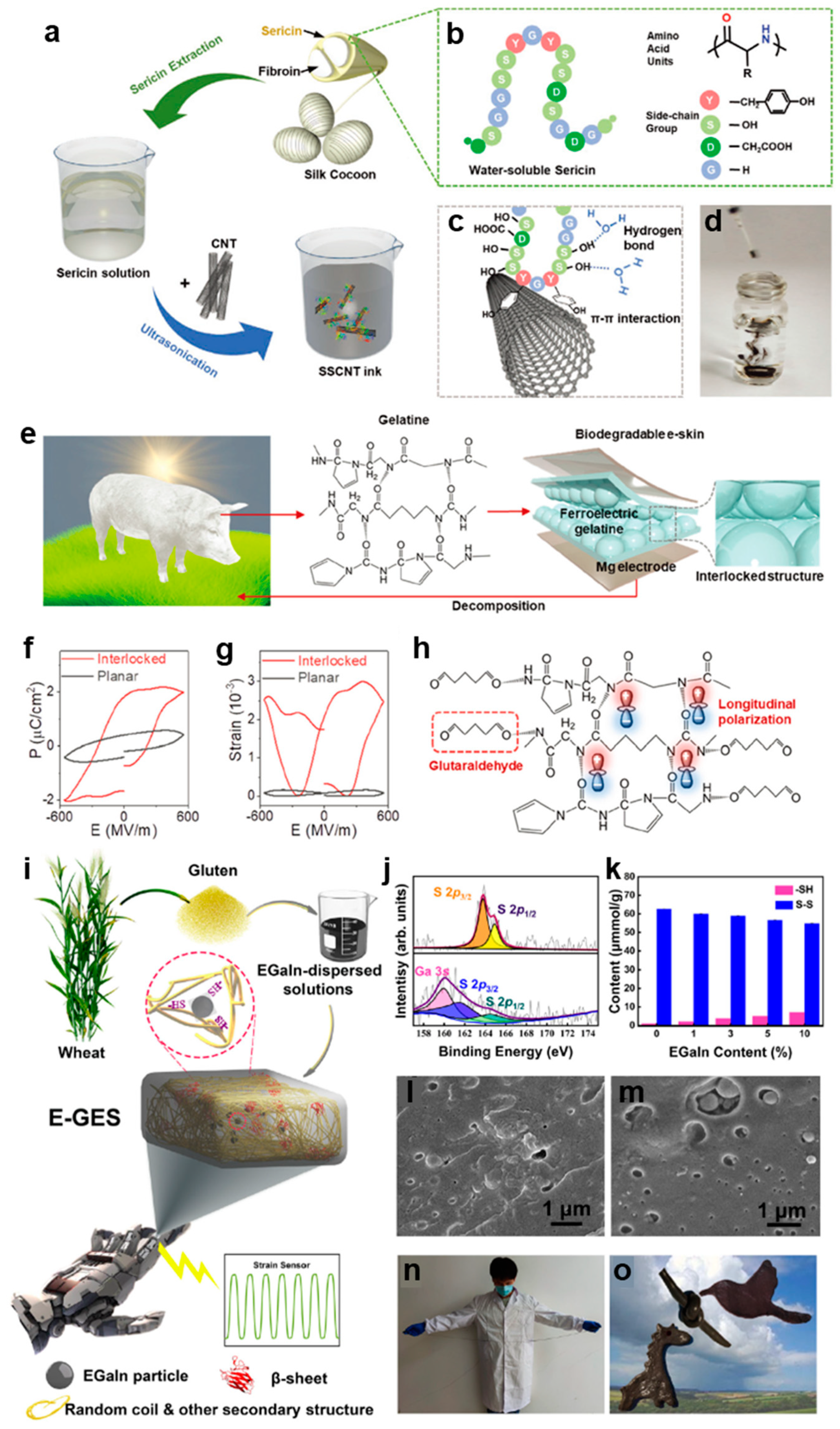
2.1. Protein Based Epidermal Electronics
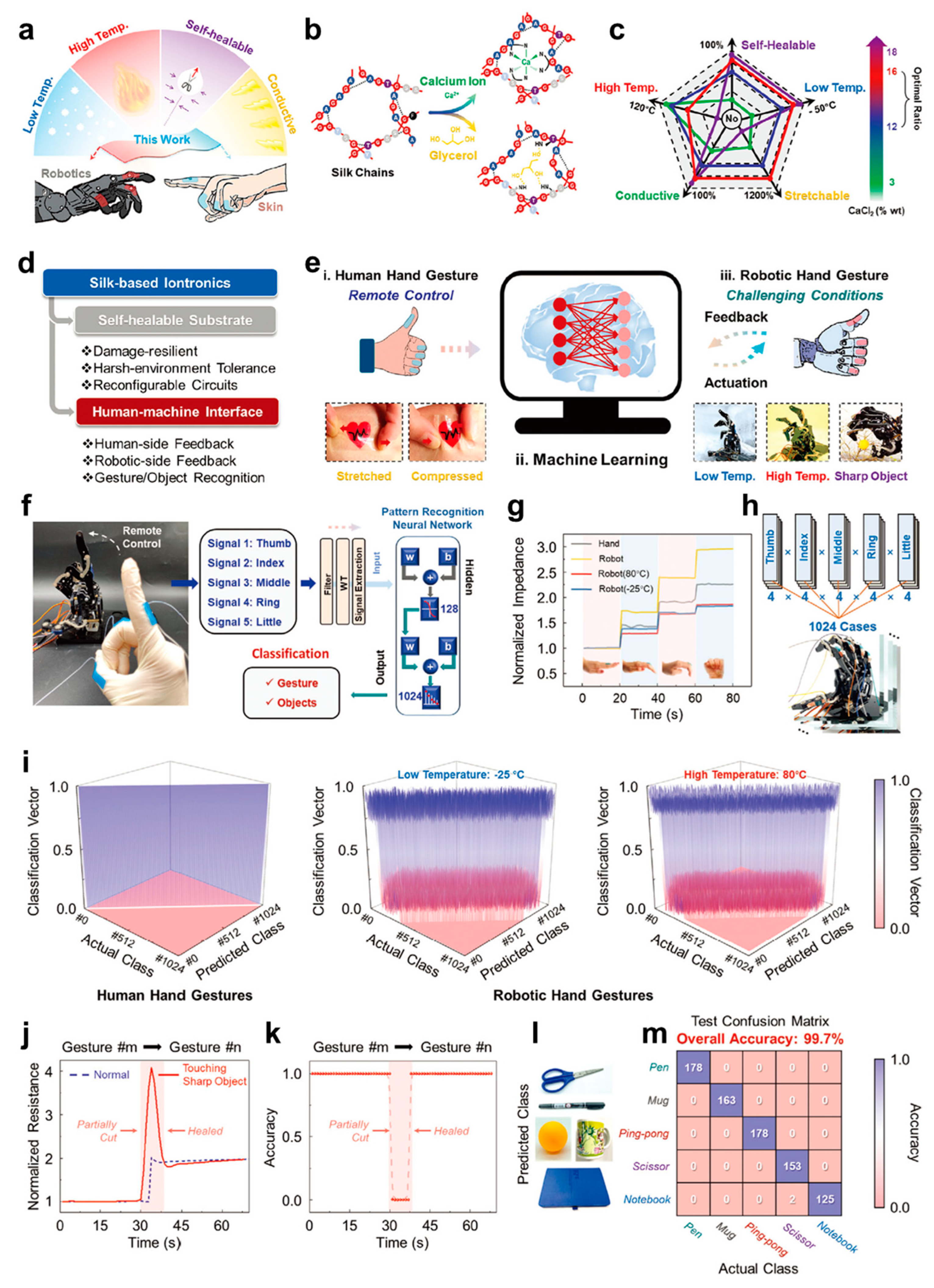
2.1.1. Silk Protein
2.1.2. Gelatin
2.1.3. Gluten Protein
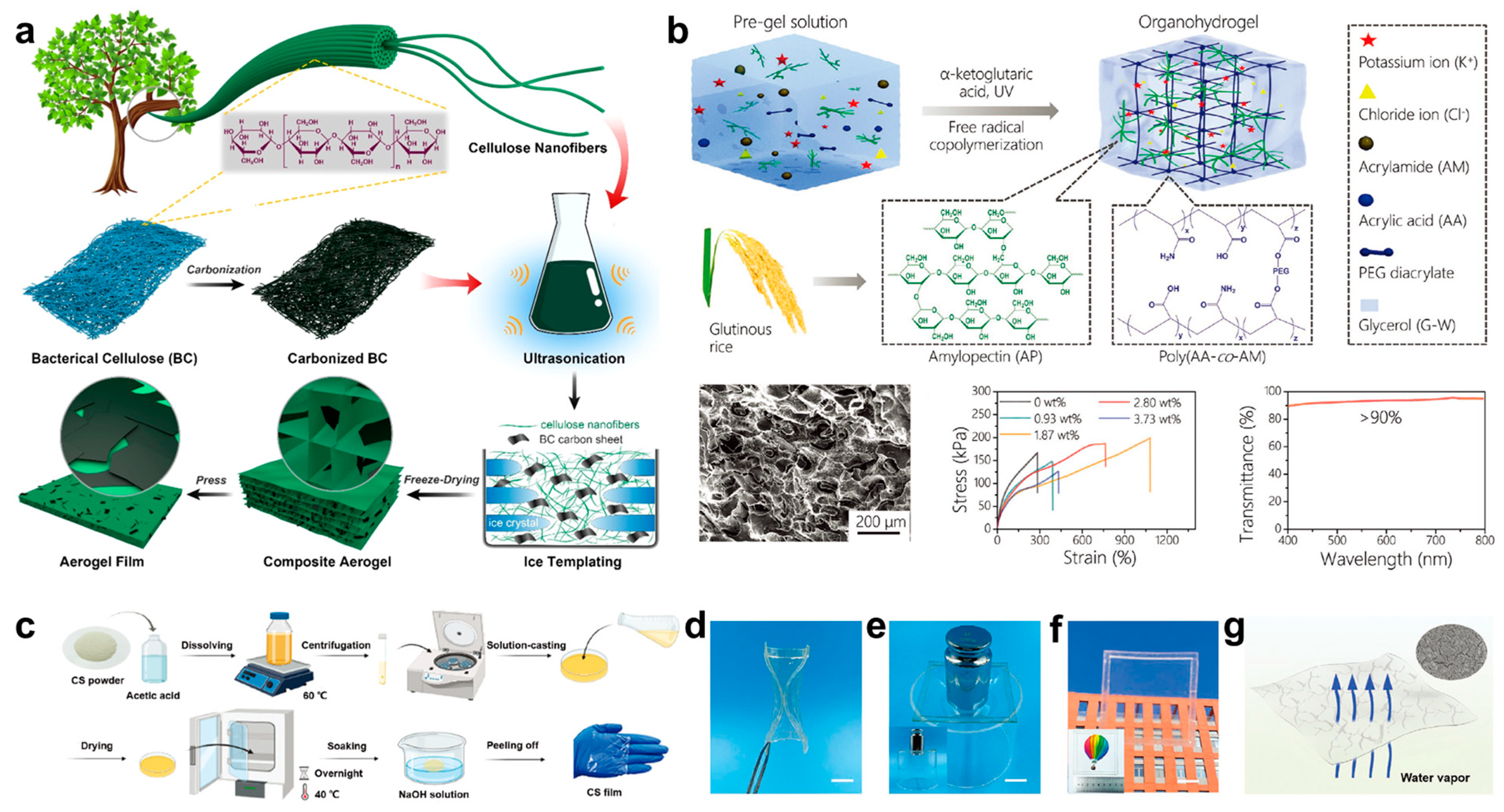
2.2. Polysaccharide Based Epidermal Electronics
2.2.1. Cellulose
2.2.2. Starch
2.2.3. Chitosan
3. Applications
3.1. Health Monitoring
3.1.1. ECG
3.1.2. EMG
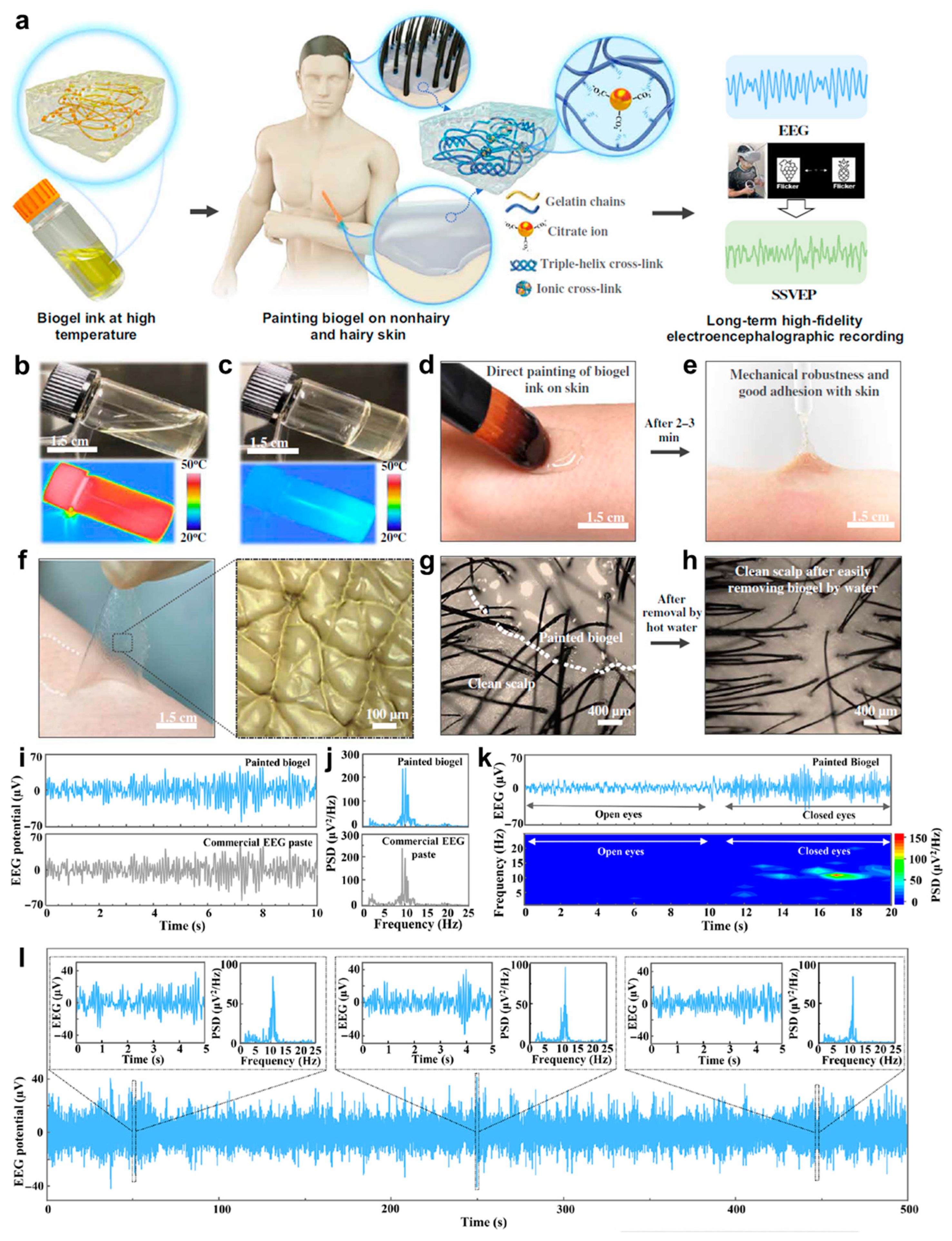
3.1.3. EEG
3.2. Human–Machine Interactions
3.2.1. Robot Control
3.2.2. Personal Device Control
3.2.3. Virtual Reality
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, R.; Wang, D.; Zhao, S.; Lou, Z.; Shen, G. Wearable Sensors-Enabled Human-Machine Interaction Systems: From Design to Application. Adv. Funct. Mater. 2020, 31, 2008936. [Google Scholar] [CrossRef]
- Hong, Y.J.; Jeong, H.; Cho, K.W.; Lu, N.; Kim, D.H. Wearable and Implantable Devices for Cardiovascular Healthcare: From Monitoring to Therapy Based on Flexible and Stretchable Electronics. Adv. Funct. Mater. 2019, 29, 1808247. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, H.; Chen, Z.; Wang, Z.; Jiang, L.; Lu, G.; Li, X.; Chen, R.; Jin, J.; Kang, H.; et al. Stretchable Nanolayered Thermoelectric Energy Harvester on Complex and Dynamic Surfaces. Nano Lett. 2020, 20, 4445–4453. [Google Scholar] [CrossRef]
- Park, Y.G.; Min, H.; Kim, H.; Zhexembekova, A.; Lee, C.Y.; Park, J.U. Three-Dimensional, High-Resolution Printing of Carbon Nanotube/Liquid Metal Composites with Mechanical and Electrical Reinforcement. Nano Lett. 2019, 19, 4866–4872. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yao, S.; Wang, H.; Du, Q.; Ma, Y.; Zhu, Y. Gas-Permeable, Ultrathin, Stretchable Epidermal Electronics with Porous Electrodes. ACS Nano 2020, 14, 5798–5805. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, P.; Zhang, F.; Li, P.; Huang, W.; Li, C.; Han, N.; Mu, S.; Zhou, H.; Mao, Y. Intrinsically Stretchable Polymer Semiconductor Based Electronic Skin for Multiple Perceptions of Force, Temperature, and Visible Light. Nano Res. 2023, 16, 1196–1204. [Google Scholar] [CrossRef]
- Wong, T.H.; Liu, Y.; Li, J.; Yao, K.; Liu, S.; Yu, C.K.; Huang, X.; Wu, M.; Park, W.; Zhou, J.; et al. Triboelectric Nanogenerator Tattoos Enabled by Epidermal Electronic Technologies. Adv. Funct. Mater. 2021, 32, 2111269. [Google Scholar] [CrossRef]
- Huang, Q.; Zheng, Z. Pathway to Developing Permeable Electronics. ACS Nano 2022, 16, 15537–15544. [Google Scholar] [CrossRef]
- Ye, G.; Qiu, J.; Fang, X.; Yu, T.; Xie, Y.; Zhao, Y.; Yan, D.; He, C.; Liu, N. A Lamellibranchia-inspired epidermal electrode for electrophysiology. Mater. Horiz. 2021, 8, 1047–1057. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; He, P.; Liu, K.; Ni, Y.; Chen, L.; Ouyang, X.; Huang, L.; Wang, H.; Xu, S. A bionic tactile plastic hydrogel-based electronic skin constructed by a nerve-like nanonetwork combining stretchable, compliant, and self-healing properties. Chem. Eng. J. 2020, 379, 122271. [Google Scholar] [CrossRef]
- Hanif, A.; Bag, A.; Zabeeb, A.; Moon, D.B.; Kumar, S.; Shrivastava, S.; Lee, N.E. A Skin-Inspired Substrate with Spaghetti-Like Multi-Nanofiber Network of Stiff and Elastic Components for Stretchable Electronics. Adv. Funct. Mater. 2020, 30, 2003540. [Google Scholar] [CrossRef]
- Luo, Y.; Li, W.; Lin, Q.; Zhang, F.; He, K.; Yang, D.; Loh, X.J.; Chen, X. A Morphable Ionic Electrode Based on Thermogel for Non-Invasive Hairy Plant Electrophysiology. Adv. Mater. 2021, 33, 2007848. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cheng, Y.F.; Yue, Y.; Chen, Y.; Gao, H.; Li, L.; Cai, B.; Liu, W.J.; Wang, Z.Y.; Guo, H.Z.; et al. High-performance flexible pressure sensor with a self-healing function for tactile feedback. Adv. Sci. 2022, 9, 2200507. [Google Scholar] [CrossRef] [PubMed]
- Leng, Z.W.; Zhu, P.C.; Wang, X.C.; Wang, Y.F.; Li, P.S.; Huang, W.; Li, B.C.; Jin, R.; Han, N.N.; Wu, J.; et al. Sebum-Membrane-Inspired Protein-Based Bioprotonic Hydrogel for Artificial Skin and Human-Machine Merging Interface. Adv. Funct. Mater. 2023, 2211056. [Google Scholar] [CrossRef]
- Dinh Xuan, H.; Timothy, B.; Park, H.Y.; Lam, T.N.; Kim, D.; Go, Y.; Kim, J.; Lee, Y.; Ahn, S.I.; Jin, S.H.; et al. Super Stretchable and Durable Electroluminescent Devices Based on Double-Network Ionogels. Adv. Mater. 2021, 33, 2008849. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Lee, H.; Yi, C.; Lee, J.; Won, C.; Oh, S.; Jekal, J.; Kwon, C.; Lee, S.; Song, J.; et al. Ultrastretchable Helical Conductive Fibers Using Percolated Ag Nanoparticle Networks Encapsulated by Elastic Polymers with High Durability in Omnidirectional Deformations for Wearable Electronics. Adv. Funct. Mater. 2020, 30, 1910026. [Google Scholar] [CrossRef]
- Zeng, J.; Dong, L.; Sha, W.; Wei, L.; Guo, X. Highly stretchable, compressible and arbitrarily deformable all-hydrogel soft supercapacitors. Chem. Eng. J. 2020, 383, 123098. [Google Scholar] [CrossRef]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Riyajan, S.A.; Sasithornsonti, Y.; Phinyocheep, P. Green natural rubber-g-modified starch for controlling urea release. Carbohydr. Polym. 2012, 89, 251–258. [Google Scholar] [CrossRef]
- Zou, W.; Yu, L.; Liu, X.; Chen, L.; Zhang, X.; Qiao, D.; Zhang, R. Effects of amylose/amylopectin ratio on starch-based superabsorbent polymers. Carbohydr. Polym. 2012, 87, 1583–1588. [Google Scholar] [CrossRef]
- Miyamoto, A.; Lee, S.; Cooray, N.F.; Lee, S.; Mori, M.; Matsuhisa, N.; Jin, H.; Yoda, L.; Yokota, T.; Itoh, A.; et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017, 12, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; McCay, R.N.; Goswami, S.; Xu, Y.; Zhang, C.; Ling, Y.; Lin, J.; Yan, Z. Gas-Permeable, Multifunctional On-Skin Electronics Based on Laser-Induced Porous Graphene and Sugar-Templated Elastomer Sponges. Adv. Mater. 2018, 30, 1804327. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.J.; Li, X.; Kuang, S.Y.; Zhang, L.; Chen, Y.H.; Liu, L.; Zhang, K.; Ma, S.W.; Liang, F.; Wu, T.; et al. Highly Robust, Transparent, and Breathable Epidermal Electrode. ACS Nano 2018, 12, 9326–9332. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Song, A.Y.; Wu, P.; Hsu, P.C.; Peng, Y.; Chen, J.; Liu, C.; Catrysse, P.B.; Liu, Y.; Yang, A.; et al. Warming up human body by nanoporous metallized polyethylene textile. Nat. Commun. 2017, 8, 496. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Lee, S.P.; Huang, I.; Li, W.; Wang, S.; Su, C.J.; Jeang, W.J.; Huang, T.; Mehta, S.; Rogers, J.A.; et al. Sweat-activated biocompatible batteries for epidermal electronic and microfluidic systems. Nat. Electron. 2020, 3, 554–562. [Google Scholar] [CrossRef]
- Reeder, J.T.; Xie, Z.; Yang, Q.; Seo, M.; Yan, Y.; Deng, Y.J.; Jinkins, K.R.; Krishnan, S.R.; Liu, C.; Rogers, J.A.; et al. Soft, bioresorbable coolers for reversible conduction block of peripheral nerves. Science 2022, 377, 109–115. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Huo, Z.H.; Wang, X.D.; Han, X.; Wu, W.Q.; Wan, B.S.; Wang, H.; Zhai, J.Y.; Tao, J.; Wang, Z.L.; et al. High precision epidermal radio frequency antenna via nanofiber network for wireless stretchable multifunction electronics. Nat. Commun. 2020, 3, 5629. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, X.; Wang, C.; Zhang, L.; Li, X.S.; Lee, S.; Huang, Z.L.; Chen, R.; Chen, R.; Xu, S.; et al. Stretchable ultrasonic transducer array for three-dimensional imaging on complex surfaces. Sci. Adv. 2018, 4, eaar3979. [Google Scholar] [CrossRef]
- Shyu, T.C.; Damasceno, P.F.; Dodd, P.M.; Lamoureux, A.; Xu, L.Z.; Shlian, M.; Shtein, M.; Glotaer, S.C.; Kotov, N.A. A kirigami approach to engineering elasticity in nanocomposites through patterned defects. Nat. Mater. 2015, 14, 785–789. [Google Scholar] [CrossRef]
- Fan, J.A.; Yeo, W.H.; Su, Y.W.; Hattori, Y.; Lee, W.; Jung, S.Y.; Zhang, Y.H.; Liu, Z.J.; Cheng, H.Y.; Rogers, J.A.; et al. Fractal design concepts for stretchable electronics. Nat. Commun. 2014, 5, 3266. [Google Scholar] [CrossRef]
- Wang, X.D.; Dong, L.; Zhang, H.L.; Yu, R.; Pan, C.F.; Wang, Z.L. Recent Progress in Electronic Skin. Adv. Sci. 2015, 2, 1500169. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Tee, B.C.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef] [PubMed]
- Tee, B.C.K.; Chortos, A.; Dunn, R.R.; Schwartz, G.; Eason, E.; Bao, Z. Tunable Flexible Pressure Sensors using Microstructured Elastomer Geometries for Intuitive Electronics. Adv. Funct. Mater. 2014, 24, 5427–5434. [Google Scholar] [CrossRef]
- Mannsfeld, S.C.; Tee, B.C.; Stoltenberg, R.M.; Chen, C.V.; Barman, S.; Muir, B.V.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.L.; Shim, M.B.; Lee, B.S.; Jeon, S.; Ko, D.S.; Kang, T.H.; Bae, J.; Lee, S.H.; Byun, K.E.; Im, J.; et al. Highly stretchable resistive pressure sensors using a conductive elastomeric composite on a micropyramid array. Adv. Mater. 2014, 26, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.R.; Lin, L.; Zhu, G.; Wu, W.; Zhang, R.; Wang, Z.L. Transparent triboelectric nanogenerators and self-powered pressure sensors based on micropatterned plastic films. Nano Lett. 2012, 12, 3109–3114. [Google Scholar] [CrossRef]
- Xing, X.; Chen, M.; Gong, Y.; Lv, Z.; Han, S.T.; Zhou, Y. Building memory devices from biocomposite electronic materials. Sci. Technol. Adv. Mater. 2020, 21, 100–121. [Google Scholar] [CrossRef]
- Li, X.; Ding, C.; Li, X.; Yang, H.; Liu, S.; Wang, X.; Zhang, L.; Sun, Q.; Liu, X.; Chen, J. Electronic biopolymers: From molecular engineering to functional devices. Chem. Eng. J. 2020, 397, 125499. [Google Scholar] [CrossRef]
- Rehman, M.M.; ur Rehman, H.M.M.; Kim, W.Y.; Sherazi, S.S.H.; Rao, M.W.; Khan, M.; Muhammad, Z. Biomaterial-Based Nonvolatile Resistive Memory Devices toward Ecofriendliness and Biocompatibility. ACS Appl. Electron. Mater. 2021, 3, 2832–2861. [Google Scholar] [CrossRef]
- Lalzawmliana, V.; Anand, A.; Mukherjee, P.; Chaudhuri, S.; Kundu, B.; Nandi, S.K.; Thakur, N.L. Marine organisms as a source of natural matrix for bone tissue engineering. Ceram. Int. 2019, 45, 1469–1481. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. Organ engineering—Combining stem cells, biomaterials, and bioreactors to produce bioengineered organs for transplantation. Bioessays 2013, 35, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Fontana, G.; Gershlak, J.; Adamski, M.; Lee, J.S.; Matsumoto, S.; Le, H.D.; Binder, B.; Wirth, J.; Gaudette, G.; Murphy, W.L. Biofunctionalized Plants as Diverse Biomaterials for Human Cell Culture. Adv. Healthc. Mater. 2017, 6, 1601225. [Google Scholar] [CrossRef] [PubMed]
- Insuasti-Cruz, E.; Suarez-Jaramillo, V.; Mena Urresta, K.A.; Pila-Varela, K.O.; Fiallos-Ayala, X.; Dahoumane, S.A.; Alexis, F. Natural Biomaterials from Biodiversity for Healthcare Applications. Adv. Healthc. Mater. 2022, 11, 2101389. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, G.; Guo, T.; Zhou, Y.N.; Wu, Y.A. Biomemristors as the next generation bioelectronics. Nano Energy 2020, 75, 104938. [Google Scholar] [CrossRef]
- Wang, C.; Yokota, T.; Someya, T. Natural Biopolymer-Based Biocompatible Conductors for Stretchable Bioelectronics. Chem. Rev. 2021, 121, 2109–2146. [Google Scholar] [CrossRef]
- Hu, X.; Ricci, S.; Naranjo, S.; Hill, Z.; Gawason, P. Protein and Polysaccharide-Based Electroactive and Conductive Materials for Biomedical Applications. Molecules 2021, 26, 4499. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Taghizadeh, M.; Yazdi, M.K.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Stadler, F.J.; Lee, H.; Saeb, M.R.; Mozafari, M. Mussel-inspired biomaterials: From chemistry to clinic. Bioeng. Transl. Med. 2022, 7, 10385. [Google Scholar] [CrossRef]
- Wang, H.; Meng, F.; Zhu, B.; Leow, W.R.; Liu, Y.; Chen, X. Resistive Switching Memory Devices Based on Proteins. Adv. Mater. 2015, 27, 7670–7676. [Google Scholar] [CrossRef] [PubMed]
- Chorsi, M.T.; Curry, E.J.; Chorsi, H.T.; Das, R.; Baroody, J.; Purohit, P.K.; Ilies, H.; Nguyen, T.D. Piezoelectric Biomaterials for Sensors and Actuators. Adv. Mater. 2019, 31, 1802084. [Google Scholar] [CrossRef]
- Mao, S.; Sun, B.; Zhou, G.; Guo, T.; Wang, J.; Zhao, Y. Applications of biomemristors in next generation wearable electronics. Nanoscale Horiz. 2022, 7, 822–848. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Wu, N.; Ye, Y.; Li, S.; Huang, H.; Chen, L.; Bi, H.; Sun, L. Highly Stretchable Starch Hydrogel Wearable Patch for Electrooculographic Signal Detection and Human–Machine Interaction. Small Struct. 2021, 2, 2100105. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, T.; Han, L.; Zhu, M.; Cheng, Z.; Li, D.; Ren, F.; Wang, K.; Lu, X. Conductive Cellulose Bio-Nanosheets Assembled Biostable Hydrogel for Reliable Bioelectronics. Adv. Funct. Mater. 2021, 31, 2010465. [Google Scholar] [CrossRef]
- Jo, M.; Min, K.; Roy, B.; Kim, S.; Lee, S.; Park, J.-Y.; Kim, S. Protein-Based Electronic Skin Akin to Biological Tissues. ACS Nano 2018, 12, 5637–5645. [Google Scholar] [CrossRef]
- Hou, J.; Xie, Y.; Ji, A.; Cao, A.; Fang, Y.; Shi, E. Carbon-Nanotube-Wrapped Spider Silks for Directed Cardiomyocyte Growth and Electrophysiological Detection. ACS Appl. Mater. Interfaces 2018, 10, 6793–6798. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Zhang, M.; Jian, M.; Zhang, Y. An All-Silk-Derived Dual-Mode E-skin for Simultaneous Temperature–Pressure Detection. ACS Appl. Mater. Interfaces 2017, 9, 39484–39492. [Google Scholar] [CrossRef]
- Cui, C.; Fu, Q.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Recent Progress in Natural Biopolymers Conductive Hydrogels for Flexible Wearable Sensors and Energy Devices: Materials, Structures, and Performance. ACS Appl. Bio Mater. 2021, 4, 85–121. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, C.; Ying, Y.; Ping, J. Biotriboelectric Nanogenerators: Materials, Structures, and Applications. Adv. Energy Mater. 2020, 10, 2002001. [Google Scholar] [CrossRef]
- Pang, B.; Jiang, G.; Zhou, J.; Zhu, Y.; Cheng, W.; Zhao, D.; Wang, K.; Xu, G.; Yu, H. Molecular-Scale Design of Cellulose-Based Functional Materials for Flexible Electronic Devices. Adv. Electron. Mater. 2020, 7, 2000944. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; Lou, Z.; Jiang, K.; Shen, G. Plant-Based Modular Building Blocks for “Green” Electronic Skins. Adv. Funct. Mater. 2018, 28, 1804510. [Google Scholar] [CrossRef]
- Lan, L.; Ping, J.; Xiong, J.; Ying, Y. Sustainable Natural Bio-Origin Materials for Future Flexible Devices. Adv. Sci. 2022, 9, 2200560. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Zhou, Y.; Han, S.-T.; Roy, V.A.L. From biomaterial-based data storage to bio-inspired artificial synapse. Mater. Today 2018, 21, 537–552. [Google Scholar] [CrossRef]
- Pradhan, S.; Brooks, A.K.; Yadavalli, V.K. Nature-derived materials for the fabrication of functional biodevices. Mater. Today Bio 2020, 7, 100065. [Google Scholar] [CrossRef]
- Hang, Y.; Zhang, Y.; Jin, Y.; Shao, H.; Hu, X. Preparation of regenerated silk fibroin/silk sericin fibers by coaxial electrospinning. Int. J. Biol. Macromol. 2012, 51, 980–986. [Google Scholar] [CrossRef]
- Gogurla, N.; Kim, Y.; Cho, S.; Kim, J.; Kim, S. Multifunctional and Ultrathin Electronic Tattoo for On-Skin Diagnostic and Therapeutic Applications. Adv. Mater. 2021, 33, 2008308. [Google Scholar] [CrossRef]
- Liang, X.; Li, H.; Dou, J.; Wang, Q.; He, W.; Wang, C.; Li, D.; Lin, J.M.; Zhang, Y. Stable and Biocompatible Carbon Nanotube Ink Mediated by Silk Protein for Printed Electronics. Adv. Mater. 2020, 32, 2000165. [Google Scholar] [CrossRef] [PubMed]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron’ko, N.G. Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef]
- Ohyabu, Y.; Hatayama, H.; Yunoki, S. Evaluation of gelatin hydrogel as a potential carrier for cell transportation. J. Biosci. Bioeng. 2014, 118, 112–115. [Google Scholar] [CrossRef]
- Irimia-Vladu, M. “Green” electronics: Biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev. 2014, 43, 588–610. [Google Scholar] [CrossRef]
- Garlapati, V.K. E-waste in India and developed countries: Management, recycling, business and biotechnological initiatives. Renew. Sustain. Energy Rev. 2016, 54, 874–881. [Google Scholar] [CrossRef]
- Bhutta, M.K.S.; Omar, A.; Yang, X. Electronic Waste: A Growing Concern in Today’s Environment. Econ. Res. Int. 2011, 2011, 474230. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Park, J.; Na, S.; Kim, M.P.; Ko, H. A Fully Biodegradable Ferroelectric Skin Sensor from Edible Porcine Skin Gelatine. Adv. Sci. 2021, 8, 2005010. [Google Scholar] [CrossRef]
- Wang, Q.; Jian, M.; Wang, C.; Zhang, Y. Carbonized Silk Nanofiber Membrane for Transparent and Sensitive Electronic Skin. Adv. Funct. Mater. 2017, 27, 1605657. [Google Scholar] [CrossRef]
- Chen, G.; Matsuhisa, N.; Liu, Z.; Qi, D.; Cai, P.; Jiang, Y.; Wan, C.; Cui, Y.; Leow, W.R.; Liu, Z.; et al. Plasticizing Silk Protein for On-Skin Stretchable Electrodes. Adv. Mater. 2018, 30, 1800129. [Google Scholar] [CrossRef]
- Day, L.; Augustin, M.A.; Batey, I.L.; Wrigley, C.W. Wheat-gluten uses and industry needs. Trends Food Sci. Technol. 2006, 17, 82–90. [Google Scholar] [CrossRef]
- Chen, B.; Cao, Y.; Li, Q.; Yan, Z.; Liu, R.; Zhao, Y.; Zhang, X.; Wu, M.; Qin, Y.; Sun, C.; et al. Liquid metal-tailored gluten network for protein-based e-skin. Nat. Commun. 2022, 13, 1206. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-Based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Jones, D.M. Structure and Some Reactions of Cellulose. Adv. Carbohydr. Chem. 1964, 19, 219–246. [Google Scholar]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2013, 21, 1–30. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Y.; Li, D.; Xu, Y.; Xu, F. Flexible and Sensitivity-Adjustable Pressure Sensors Based on Carbonized Bacterial Nanocellulose/Wood-Derived Cellulose Nanofibril Composite Aerogels. ACS Appl. Mater. Interfaces 2021, 13, 8754–8763. [Google Scholar] [CrossRef] [PubMed]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y.; Huang, T.; Gong, B.; Yu, W.W. A combined action of amylose and amylopectin fine molecular structures in determining the starch pasting and retrogradation property. Int. J. Biol. Macromol. 2020, 164, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Man, J.; Huang, J.; Liu, Q.; Wei, W.; Wei, C. Relationship between structure and functional properties of normal rice starches with different amylose contents. Carbohydr. Polym. 2015, 125, 35–44. [Google Scholar] [CrossRef]
- Rojas-Bringas, P.M.; De-la-Torre, G.E.; Torres, F.G. Influence of the source of starch and plasticizers on the environmental burden of starch-Brazil nut fiber biocomposite production: A life cycle assessment approach. Sci. Total Environ. 2021, 769, 144869. [Google Scholar] [CrossRef]
- Pachuau, L.; Dutta, R.S.; Roy, P.K.; Kalita, P.; Lalhlenmawia, H. Physicochemical and disintegrant properties of glutinous rice starch of Mizoram, India. Int. J. Biol. Macromol. 2017, 95, 1298–1304. [Google Scholar] [CrossRef]
- Wang, H.; Xu, K.; Ma, Y.; Liang, Y.; Zhang, H.; Chen, L. Impact of ultrasonication on the aggregation structure and physicochemical characteristics of sweet potato starch. Ultrason. Sonochem. 2020, 63, 104868. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, D.; Jin, Z.; Xu, X.; Chen, H. Effect of repeated heat-moisture treatments on digestibility, physicochemical and structural properties of sweet potato starch. Food Hydrocoll. 2016, 54, 202–210. [Google Scholar] [CrossRef]
- Tian, S.; Xing, Y.; Long, Y.; Guo, H.; Xu, S.; Ma, Y.; Wen, C.; Li, Q.; Liu, X.; Zhang, L.; et al. A Degradable-Renewable Ionic Skin Based on Edible Glutinous Rice Gel. ACS Appl. Mater. Interfaces 2022, 14, 5122–5133. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gao, Z.; Li, X.; Gao, G. An amylopectin-enabled skin-mounted hydrogel wearable sensor. J. Mater. Chem. B 2021, 9, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lai, J.; Zheng, B.; Jin, X.; Zhao, G.; Liu, H.; Chen, W.; Ma, A.; Li, X.; Wu, Y. From Glutinous-Rice-Inspired Adhesive Organohydrogels to Flexible Electronic Devices toward Wearable Sensing, Power Supply, and Energy Storage. Adv. Funct. Mater. 2021, 32, 2108423. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Z.; Sun, J.; Yan, Y.; Bu, M.; Huo, Y.; Li, Y.F.; Hu, N. Recent Advances in Natural Functional Biopolymers and Their Applications of Electronic Skins and Flexible Strain Sensors. Polymers 2021, 13, 813. [Google Scholar] [CrossRef]
- Ramadhan, Z.R.; Han, J.W.; Hong, J.; Park, S.B.; Kim, J.H.; Wibowo, A.F.; Prameswati, A.; Kim, S.Y.; Lee, J.; Kim, S.; et al. Conductive PEDOT:PSS on surface-functionalized chitosan biopolymers for stretchable skin-like electronics. Org. Electron. 2021, 94, 106165. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Kim, J.-N.; Lee, J.; Go, T.W.; Rajabi-Abhari, A.; Mahato, M.; Park, J.Y.; Lee, H.; Oh, I.-K. Skin-attachable and biofriendly chitosan-diatom triboelectric nanogenerator. Nano Energy 2020, 75, 104904. [Google Scholar] [CrossRef]
- Peng, X.; Dong, K.; Zhang, Y.; Wang, L.; Wei, C.; Lv, T.; Wang, Z.L.; Wu, Z. Sweat-Permeable, Biodegradable, Transparent and Self-powered Chitosan-Based Electronic Skin with Ultrathin Elastic Gold Nanofibers. Adv. Funct. Mater. 2022, 32, 2112241. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Lignin-based hydrogels: A review of preparation, properties, and application. Int. J. Biol. Macromol. 2019, 135, 1006–1019. [Google Scholar] [CrossRef]
- Xiu, H.; Zhao, H.; Dai, L.; Li, J.; Wang, Z.; Cui, Y.; Bai, Y.; Zheng, X.; Li, J. Robust and adhesive lignin hybrid hydrogel as an ultrasensitive sensor. Int. J. Biol. Macromol. 2022, 213, 226–233. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Hu, H.; Huang, Z.; Yang, M.; Chen, D.; Huang, K.; Huang, A.; Qin, X.; Feng, Z. Effect of mechanical activation on structure changes and reactivity in further chemical modification of lignin. Int. J. Biol. Macromol. 2016, 91, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Thombare, N.; Kumar, S.; Kumari, U.; Sakare, P.; Yogi, R.K.; Prasad, N.; Sharma, K.K. Shellac as a multifunctional biopolymer: A review on properties, applications and future potential. Int. J. Biol. Macromol. 2022, 215, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Poulin, A.; Aeby, X.; Siqueira, G.; Nyström, G. Versatile carbon-loaded shellac ink for disposable printed electronics. Sci. Rep. 2021, 11, 23784. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.C.Y.; Zheng, Y.; Luo, N.; Ding, X.; Zhang, Y.T. Chapter 7.2—Wearing Sensors Inside and Outside of the Human Body for the Early Detection of Diseases. In Wearable Sensors; Academic Press: Cambridge, MA, USA, 2014; pp. 543–562. [Google Scholar]
- Ha, M.; Lim, S.; Ko, H. Wearable and flexible sensors for user-interactive health-monitoring devices. J. Mater. Chem. B 2018, 6, 4043–4064. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, S.; Lonjaret, T.; Crisp, D.; Badier, J.M.; Malliaras, G.G.; Ismailova, E. Direct patterning of organic conductors on knitted textiles for long-term electrocardiography. Sci. Rep. 2015, 5, 15003. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Oh, J.Y.; Bao, Z. Second Skin Enabled by Advanced Electronics. Adv. Sci. 2019, 6, 1900186. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Kim, Y.S.; Kwon, S.; Mahmood, M.; Lim, H.R.; Park, S.W.; Kang, S.O.; Choi, J.J.; Herbert, R.; Jang, Y.C.; et al. All-printed nanomembrane wireless bioelectronics using a biocompatible solderable graphene for multimodal human-machine interfaces. Nat. Commun. 2020, 11, 3450. [Google Scholar] [CrossRef]
- Zarei, M.; Lee, G.; Lee, S.G.; Cho, K. Advances in Biodegradable Electronic Skin: Material Progress and Recent Applications in Sensing, Robotics, and Human-Machine Interfaces. Adv. Mater. 2022, 35, 2203193. [Google Scholar] [CrossRef]
- Sun, Q.Q.; Qian, B.B.; Uto, K.; Chen, J.Z.; Liu, X.Y.; Minari, T. Functional biomaterials towards flexible electronics and sensors. Biosens. Bioelectron. 2018, 119, 237–251. [Google Scholar] [CrossRef]
- Cha, G.D.; Kang, D.; Lee, J.; Kim, D.H. Bioresorbable Electronic Implants: History, Materials, Fabrication, Devices, and Clinical Applications. Adv. Healthc. Mater. 2018, 119, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Noseworthy, P.A.; Attia, Z.I.; Friedman, P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 2021, 18, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.H.; Ribeiro, M.H.; Paixao, G.M.M.; Oliveira, D.M.; Gomes, P.R.; Canazart, J.A.; Ferreira, M.P.S.; Andersson, C.R.; Macfarlane, P.W.; Meira, W., Jr.; et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat. Commun. 2020, 11, 1760. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Friedman, R.A.; Kligfield, P.; Levine, B.D.; Viskin, S.; Chaitman, B.R.; Okin, P.M.; Saul, J.P.; Salberg, L.; Van Hare, G.F.; et al. Assessment of the 12-lead electrocardiogram as a screening test for detection of cardiovascular disease in healthy general populations of young people (12–25 years of age): A scientific statement from the American Heart Association and the American College of Cardiology. J. Am. Coll. Cardiol. 2014, 64, 1479–1514. [Google Scholar]
- Ahmed, A.; Bain, S.; Prottoy, Z.H.; Morsada, Z.; Islam, M.T.; Hossain, M.M.; Shkir, M. Silk-Templated Nanomaterial Interfaces for Wearables and Bioelectronics: Advances and Prospects. ACS Mater. Lett. 2021, 4, 68–86. [Google Scholar] [CrossRef]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.K.; Jung, J. Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef]
- Wang, Q.; Ling, S.; Liang, X.; Wang, H.; Lu, H.; Zhang, Y. Self-Healable Multifunctional Electronic Tattoos Based on Silk and Graphene. Adv. Funct. Mater. 2019, 29, 1808695. [Google Scholar] [CrossRef]
- Hogrel, J.Y. Clinical applications of surface electromyography in neuromuscular disorders. Neurophysiol. Clin. 2005, 35, 59–71. [Google Scholar] [CrossRef]
- Campanari, M.L.; Bourefis, A.R.; Kabashi, E. Diagnostic Challenge and Neuromuscular Junction Contribution to ALS Pathogenesis. Front. Neurol. 2019, 10, 68. [Google Scholar] [CrossRef]
- Reaz, M.B.I.; Hussain, M.S.; Mohd-Yasin, F. Techniques of EMG signal analysis: Detection, processing, classification and applications. Biol. Proced. Online 2006, 8, 11–35. [Google Scholar] [CrossRef]
- Suvinen, T.I.; Kemppainen, P. Review of clinical EMG studies related to muscle and occlusal factors in healthy and TMD subjects. J. Oral Rehabil. 2007, 34, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Ye, G.; Zhao, Y.; Zhang, Y.; Hou, X.; Liu, N. An All-in-One, Bioderived, Air-Permeable, and Sweat-Stable MXene Epidermal Electrode for Muscle Theranostics. ACS Nano 2022, 16, 17168–17178. [Google Scholar] [CrossRef] [PubMed]
- Freismuth, D.; TaheriNejad, N. On the Treatment and Diagnosis of Attention Deficit Hyperactivity Disorder with EEG Assistance. Electronics 2022, 11, 606. [Google Scholar] [CrossRef]
- Reuber, M.; Fernandez, G.; Bauer, J.; Singh, D.D.; Elger, C.E. Interictal EEG abnormalities in patients with psychogenic nonepileptic seizures. Epilepsia 2002, 43, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.J.; Teruel, M.A.; Maté, A.; Trujillo, J. Diagnosis and prognosis of mental disorders by means of EEG and deep learning: A systematic mapping study. Artif. Intell. Rev. 2021, 55, 1209–1251. [Google Scholar] [CrossRef]
- Pedrosa, P.; Fiedler, P.; Schinaia, L.; Vasconcelos, B.; Martins, A.C.; Amaral, M.H.; Comani, S.; Haueisen, J.; Fonseca, C. Alginate-based hydrogels as an alternative to electrolytic gels for rapid EEG monitoring and easy cleaning procedures. Sens. Actuators B Chem. 2017, 247, 273–283. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Deng, L.; Wang, B. Acquisition technology research of EEG and related physiological signals under +Gz acceleration. Ir. J. Med. Sci. 2014, 183, 187–197. [Google Scholar] [CrossRef]
- Peng, H.-L.; Jing-Quan, L.; Tian, H.-C.; Dong, Y.-Z.; Yang, B.; Chen, X.; Yang, C.-S. A novel passive electrode based on porous Ti for EEG recording. Sens. Actuators B Chem. 2016, 226, 349–356. [Google Scholar] [CrossRef]
- Liao, L.D.; Wang, I.J.; Chen, S.F.; Chang, J.Y.; Lin, C.T. Design, fabrication and experimental validation of a novel dry-contact sensor for measuring electroencephalography signals without skin preparation. Sensors 2011, 11, 5819–5834. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, H.Y.; Wang, B.H.; Miyata, H.; Wang, Y.; Nayeem, M.O.G.; Kim, J.J.; Lee, S.; Yokota, T.; Onodera, H.; et al. On-skin paintable biogel for long-term high-fidelity electroencephalogram recording. Sci. Adv. 2022, 8, abo1396. [Google Scholar] [CrossRef]
- Seo, J.W.; Kim, H.J.; Kim, K.H.; Choi, S.Q.; Lee, H.J. Calcium-Modified Silk as a Biocompatible and Strong Adhesive for Epidermal Electronics. Adv. Funct. Mater. 2018, 28, 1800802. [Google Scholar] [CrossRef]
- Li, Q.S.; Chen, G.; Cui, Y.J.; Ji, S.B.; Liu, Z.Y.; Wan, C.J.; Liu, Y.P.; Lu, Y.H.; Wang, C.X.; Chen, X.D.; et al. Highly Thermal-Wet Comfortable and Conformal Silk-Based Electrodes for On-Skin Sensors with Sweat Tolerance. ACS Nano 2021, 15, 9955–9966. [Google Scholar] [CrossRef]
- Meng, L.; Fu, Q.J.; Hao, S.W.; Xu, F.; Yang, J. Self-adhesive, biodegradable silk-based dry electrodes for epidermal electrophysiological monitoring. Chem. Eng. J. 2022, 427, 131999. [Google Scholar] [CrossRef]
- Cheng, J.; You, L.; Cai, X.; Yang, J.; Chen, H.; Shi, X.; Wu, J.; Wang, J.; Xiong, C.; Wang, S. Fermentation-Inspired Gelatin Hydrogels with a Controllable Supermacroporous Structure and High Ductility for Wearable Flexible Sensors. ACS Appl. Mater. Interfaces 2022, 14, 26338–26349. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.T.; Wang, Y.Y.; Meng, Q.Y.; Lan, Z.Y.; Liu, C.C.; Zhou, Z.; Sun, Q.F.; Shen, X.P. Conductive Cellulose-Drived Carbon Nanofibrous Membranes with Superior Softness for High-Resolution Pressure Sensing and Electrophysiology Monitoring. ACS Appl. Mater. Interfaces 2023, 15, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.P.; Zhu, M.J.; Li, H.F.; Dou, J.X.; Jian, M.Q.; Xia, K.L.; Li, S.; Zhang, Y.Y. Hydrophilic Breathable, and Washable Graphene Decorated Textile Assisted by Silk Sericin for Integrated Multimodal Smart Wearables. Adv. Funct. Mater. 2022, 32, 2200162. [Google Scholar] [CrossRef]
- Liu, H.T.; Wei, W.; Zhang, L.; Xiao, J.L.; Pan, J.; Wu, Q.; Ma, S.Q.; Dong, H.; Yu, L.T.; Ouyang, H.W.; et al. Shape-Engineerable Silk Fibroin Papers for Ideal Substrate Alternatives of Plastic Electronics. Adv. Funct. Mater. 2021, 31, 2104088. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yun, Y.J.; Jeong, J.; Kim, C.Y.; Müller, K.R.; Lee, S.W. Leaf-inspired homeostatic cellulose biosensors. Sci. Adv. 2021, 7, eabe7432. [Google Scholar] [CrossRef]
- Song, Y.J.; Li, P.H.; Li, M.J.; Li, H.J.; Li, C.P.; Sun, D.Z.; Yang, B.H. Fabrication of chitosan/Au-TiO2 nanotube-based dry electrodes for electroencephalography recording. Mater. Sci. Eng. C 2017, 79, 740–747. [Google Scholar] [CrossRef]
- Kang, B.C.; Ha, T.J. Noninvasive electroencephalogram sensors based on all-solution-processed trapezoidal electrode array. Appl. Phys. Lett. 2022, 120, 213301. [Google Scholar] [CrossRef]
- Heng, W.; Solomon, S.; Gao, W. Flexible Electronics and Devices as Human-Machine Interfaces for Medical Robotics. Adv. Mater. 2022, 34, 2107902. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, L.; Zhao, L.; Wang, D.; Xu, H.; Wang, K.; Han, W. A Flexible Humidity Sensor Based on Natural Biocompatible Silk Fibroin Films. Adv. Mater. Technol. 2020, 6, 2001053. [Google Scholar] [CrossRef]
- Liu, H.; Xu, D.; Hu, B.; Jiang, J.; Li, M.; Zhao, D.; Zhai, W. Eco-friendly biogenic hydrogel for wearable skin-like iontronics. J. Mater. Chem. A 2021, 9, 4692–4699. [Google Scholar] [CrossRef]
- Han, W.B.; Ko, G.-J.; Jang, T.-M.; Hwang, S.-W. Materials, Devices, and Applications for Wearable and Implantable Electronics. ACS Appl. Electron. Mater. 2021, 3, 485–503. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Tao, T.H. Recent Progress in Bio-Integrated Intelligent Sensing System. Adv. Intell. Syst. 2022, 4, 2100280. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Chen, Y.; Han, X.; Jiang, F. Cellulose Nanofibrils Enhanced, Strong, Stretchable, Freezing-Tolerant Ionic Conductive Organohydrogel for Multi-Functional Sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Wang, M.; Yan, Z.; Wang, T.; Cai, P.; Gao, S.; Zeng, Y.; Wan, C.; Wang, H.; Pan, L.; Yu, J.; et al. Gesture recognition using a bioinspired learning architecture that integrates visual data with somatosensory data from stretchable sensors. Nat. Electron. 2020, 3, 563–570. [Google Scholar] [CrossRef]
- Wang, M.; Wang, T.; Luo, Y.; He, K.; Pan, L.; Li, Z.; Cui, Z.; Liu, Z.; Tu, J.; Chen, X. Fusing Stretchable Sensing Technology with Machine Learning for Human–Machine Interfaces. Adv. Funct. Mater. 2021, 31, 2008807. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Zhang, Y.; Zhou, Z.; Qin, N.; Tao, T.H. Robotic Manipulation under Harsh Conditions Using Self-Healing Silk-Based Iontronics. Adv. Sci. 2022, 9, 2102596. [Google Scholar] [CrossRef]
- Gogurla, N.; Roy, B.; Park, J.-Y.; Kim, S. Skin-contact actuated single-electrode protein triboelectric nanogenerator and strain sensor for biomechanical energy harvesting and motion sensing. Nano Energy 2019, 62, 674–681. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, B.; Wang, H.; Wu, Y.; Cao, H.; He, L.; Li, C.; Luo, X.; Li, X.; Mao, Y. 3D printed triboelectric nanogenerator as self-powered human-machine interactive sensor for breathing-based language expression. Nano Res. 2022, 15, 7460–7467. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Liang, X.; Wang, H.; Lu, H.; Zhu, M.; Wang, H.; Zhang, M.; Qiu, X.; Song, Y.; et al. Humidity-sensitive chemoelectric flexible sensors based on metal-air redox reaction for health management. Nat. Commun. 2022, 13, 5416. [Google Scholar] [CrossRef] [PubMed]
- Bordegoni, M.; Ferrise, F. Designing interaction with consumer products in a multisensory virtual reality environment. Virtual Phys. Prototyp. 2013, 8, 51–64. [Google Scholar] [CrossRef]
- Goulding, J.; Nadim, W.; Petridis, P.; Alshawi, M. Construction industry offsite production: A virtual reality interactive training environment prototype. Adv. Eng. Inform. 2012, 26, 103–116. [Google Scholar] [CrossRef]
- Kohli, V.; Tripathi, U.; Chamola, V.; Rout, B.K.; Kanhere, S.S. A review on Virtual Reality and Augmented Reality use-cases of Brain Computer Interface based applications for smart cities. Microprocess. Microsyst. 2022, 88, 104392. [Google Scholar] [CrossRef]
- Shen, S.; Yi, J.; Sun, Z.D.; Guo, Z.H.; He, T.; Ma, L.Y.; Li, H.M.; Fu, J.J.; Lee, C.K.; Wang, Z.L. Human Machine Interface with Wearable Electronics Using Biodegradable Triboelectric Films for Calligraphy Practice and Correction. Nano-Micro Lett. 2022, 14, 225. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Dai, F.; Zhao, J.; Li, S.; Shi, Y.; Li, W.; Geng, L.; Ye, M.; Chen, X.; et al. Wearable five-finger keyboardless input system based on silk fibroin electronic skin. Nano Energy 2022, 103, 107764. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, N.; Tang, Y.; Wang, M.; Chao, M.; Liang, E. A paper triboelectric nanogenerator for self-powered electronic systems. Nanoscale 2017, 9, 14499–14505. [Google Scholar] [CrossRef]
- Zhang, N.; Qin, C.; Feng, T.; Li, J.; Yang, Z.; Sun, X.; Liang, E.; Mao, Y. Non-contact cylindrical rotating triboelectric nanogenerator for harvesting kinetic energy from hydraulics. Nano Res. 2020, 13, 1903–1907. [Google Scholar] [CrossRef]




| Physiological Signal | Functional Materials | Included Biomaterials | Signal to Noise Ratio (SNR) | Interfacial Impedance | Ref. |
|---|---|---|---|---|---|
| ECG | Ca2+-modified silk | Silk fibroin | n/a | 1.5 kΩ (106 Hz) | [132] |
| ECG | PEDOT:PSS/glycerol-plasticized porous silk fiber | Silk | n/a | ~5 kΩ (103 Hz) | [133] |
| ECG | Ppy@AM-SF/CNC electrode | Silk fibroin | n/a | ~1 kΩ (103 Hz) | [134] |
| ECG | RGO/Gelatin/AgNWs | Gelatin | n/a | ~15 kΩ (102 Hz) | [135] |
| EMG | MXene/Cellulose electrode | Cellulose | 36 dB | ~1 kΩ (105 Hz) | [123] |
| EMG | SiO2/carbon nanofibrils | Cellulose | 28 dB | n/a | [136] |
| EMG | Hexamethylene diisocyanate cross-linked sericin-graphene textile | Silk sericin | n/a | ~2 kΩ (104 Hz) | [137] |
| EMG | Silk/Ca2+/FA/Au | Silk fibroin | n/a | ~1 kΩ (104 Hz) | [138] |
| EEG | Gelatin/Citrate ion | Gelatin | n/a | 6.95 kΩ (103 Hz) | [131] |
| EEG | Mesoporous cellulose membrane/NaCl | Cellulose membrane | n/a | 6.64 kΩ(103 Hz) | [139] |
| EEG | Chitosan/Au-TiO2 | Chitosan | n/a | 5 kΩ (103 Hz) | [140] |
| EEG | Single-wall carbon nanotube/gelatin | Gelatin | 14.81 dB | 1.12 kΩ (105 Hz) | [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, N.; Yao, X.; Wang, Y.; Huang, W.; Niu, M.; Zhu, P.; Mao, Y. Recent Progress of Biomaterials-Based Epidermal Electronics for Healthcare Monitoring and Human–Machine Interaction. Biosensors 2023, 13, 393. https://doi.org/10.3390/bios13030393
Han N, Yao X, Wang Y, Huang W, Niu M, Zhu P, Mao Y. Recent Progress of Biomaterials-Based Epidermal Electronics for Healthcare Monitoring and Human–Machine Interaction. Biosensors. 2023; 13(3):393. https://doi.org/10.3390/bios13030393
Chicago/Turabian StyleHan, Ningning, Xin Yao, Yifan Wang, Wenhao Huang, Mengjuan Niu, Pengcheng Zhu, and Yanchao Mao. 2023. "Recent Progress of Biomaterials-Based Epidermal Electronics for Healthcare Monitoring and Human–Machine Interaction" Biosensors 13, no. 3: 393. https://doi.org/10.3390/bios13030393
APA StyleHan, N., Yao, X., Wang, Y., Huang, W., Niu, M., Zhu, P., & Mao, Y. (2023). Recent Progress of Biomaterials-Based Epidermal Electronics for Healthcare Monitoring and Human–Machine Interaction. Biosensors, 13(3), 393. https://doi.org/10.3390/bios13030393







