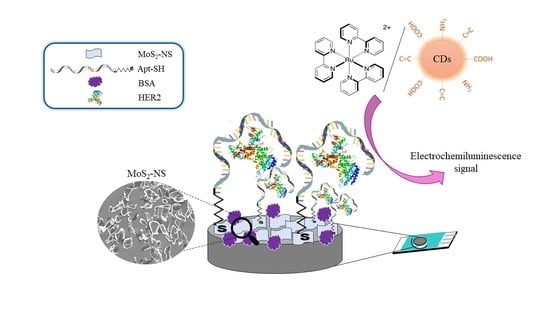
MoS2-Carbon Nanodots as a New Electrochemiluminescence Platform for Breast Cancer Biomarker Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Procedures
2.3.1. Synthesis of Carbon Nanodots (CDs)
2.3.2. Synthesis of Molybdenum Disulfide Nanosheets (MoS2-NS)
2.3.3. Thiolated Aptamer, BSA and HER2 Solutions Preparation
2.3.4. ECL Signal of [Ru(bpy)3]2+/CD system
2.3.5. Biosensor Electrochemical Characterization
2.3.6. HER2 Aptasensor Development
- Molybdenum Disulfide Nanosheet Electrode Modification
- Immobilization of Thiolated Aptamer on Molybdenum Disulfide Modifed Cspes
- Blocking with BSA
- Incubation with HER2 Protein
- Electrochemiluminescence Detection
- HER2 Detection in Spiked Human Serum Samples
3. Results and Discussion
3.1. Synthesis and Characterization of Nanomaterials: CDs and MoS2-NS
3.2. ECL Signal of [Ru(bpy)3]2+/CD system
3.3. Aptasensor Development and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Houssein, E.H.; Emam, M.M.; Ali, A.A.; Suganthan, P.N. Deep and Machine Learning Techniques for Medical Imaging-Based Breast Cancer: A Comprehensive Review. Expert Syst. Appl. 2021, 167, 114161. [Google Scholar] [CrossRef]
- Iacoviello, L.; Bonaccio, M.; de Gaetano, G.; Donati, M.B. Epidemiology of Breast Cancer, a Paradigm of the “Common Soil” Hypothesis. Semin. Cancer Biol. 2021, 72, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Cáncer. Available online: https://www.who.int/es/news-room/fact-sheets/detail/cancer (accessed on 22 July 2022).
- Zielonke, N.; Kregting, L.M.; Heijnsdijk, E.A.M.; Veerus, P.; Heinävaara, S.; McKee, M.; de Kok, I.M.C.M.; de Koning, H.J.; van Ravesteyn, N.T.; Gredinger, G.; et al. The Potential of Breast Cancer Screening in Europe. Int. J. Cancer 2021, 148, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Orfanou, I.M.; Argyros, O.; Papapetropoulos, A.; Tseleni-Balafouta, S.; Vougas, K.; Tamvakopoulos, C. Discovery and Pharmacological Evaluation of STEAP4 as a Novel Target for HER2 Overexpressing Breast Cancer. Front. Oncol. 2021, 11, 908. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Li, Y.; Zhao, Q.; Niu, Z. Amplification of the Human Epidermal Growth Factor Receptor 2 (HER2) Gene Is Associated with a Microsatellite Stable Status in Chinese Gastric Cancer Patients. J. Gastrointest. Oncol. 2021, 12, 377–387. [Google Scholar] [CrossRef]
- Lv, H.; Yan, M.; Jiang, Z. Recent Advances in the Treatment of Hormone Receptor-Positive/Human Epidermal Growth Factor 2-Positive Advanced Breast Cancer. Ther. Adv. Med. Oncol. 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Meenakshi, A.; Suresh Kumar, R.; Siva Kumar, N. ELISA for Quantitation of Serum C-ErbB-2 Oncoprotein in Breast Cancer Patients. J. Immunoass. Immunochem. 2002, 23, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Shamshirian, A.; Aref, A.R.; Yip, G.W.; Ebrahimi Warkiani, M.; Heydari, K.; Razavi Bazaz, S.; Hamzehgardeshi, Z.; Shamshirian, D.; Moosazadeh, M.; Alizadeh-Navaei, R. Diagnostic Value of Serum HER2 Levels in Breast Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2020, 20, 1049. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O. Enzyme-Linked Immunosorbent Assay (ELISA); SpringerBriefs in Applied Sciences and Technology; Springer: Singapore, 2018; ISBN 978-981-10-6765-5. [Google Scholar]
- Guerrero-Esteban, T.; Gutiérrez-Sánchez, C.; García-Mendiola, T.; Revenga-Parra, M.; Pariente, F.; Lorenzo, E. Bifunctional Carbon Nanodots for Highly Sensitive HER2 Determination Based on Electrochemiluminescence. Sens. Actuators B Chem. 2021, 343, 130096. [Google Scholar] [CrossRef]
- Sadeghi, M.; Kashanian, S.; Naghib, S.M.; Arkan, E. A High-Performance Electrochemical Aptasensor Based on Graphene-Decorated Rhodium Nanoparticles to Detect HER2-ECD Oncomarker in Liquid Biopsy. Sci. Rep. 2022, 12, 3299. [Google Scholar] [CrossRef]
- Harahsheh, T.; Makableh, Y.F.; Rawashdeh, I.; Al-Fandi, M. Enhanced Aptasensor Performance for Targeted HER2 Breast Cancer Detection by Using Screen-Printed Electrodes Modified with Au Nanoparticles. Biomed. Microdevices 2021, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; You, M.; Zhang, F.; Wang, Q.; He, P. A Sensitive Electrochemical Aptasensing Platform Based on Exonuclease Recycling Amplification and Host-Guest Recognition for Detection of Breast Cancer Biomarker HER2. Sens. Actuators B Chem. 2018, 258, 796–802. [Google Scholar] [CrossRef]
- Centane, S.; Nyokong, T. Impedimetric Aptasensor for HER2 Biomarker Using Graphene Quantum Dots, Polypyrrole and Cobalt Phthalocyanine Modified Electrodes. Sens. Bio-Sens. Res. 2021, 34, 100467. [Google Scholar] [CrossRef]
- Gu, C.; Guo, C.; Li, Z.; Wang, M.; Zhou, N.; He, L.; Zhang, Z.; Du, M. Bimetallic ZrHf-Based Metal-Organic Framework Embedded with Carbon Dots: Ultra-Sensitive Platform for Early Diagnosis of HER2 and HER2-Overexpressed Living Cancer Cells. Biosens. Bioelectron. 2019, 134, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Mu, Z.; Qing, M.; Zhou, J.; Sun, S.; Bai, L. A Novel Binary Luminophore Based High-Efficient Electrochemiluminescence Biosensor for Ultrasensitive Detection of Human Epidermal Growth Factor Receptor-2. Chem. Eng. J. 2022, 450, 138362. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, L.; Chen, P.; Zheng, J.; Huang, Y.; Liang, J.; Zhong, J.; Huang, Y.; Yu, M.; Guan, B.O. Optical Microfiber with a Gold Nanorods-Black Phosphorous Nanointerface: An Ultrasensitive Biosensor and Nanotherapy Platform. Anal. Chem. 2022, 94, 8058–8065. [Google Scholar] [CrossRef]
- Mishra, S.; Kachhawa, P.; Jain, A.K.; Thakur, R.R.; Chaturvedi, N. High Sensitivity Label-Free Detection of HER2 Using AlGaN/GaN High Electron Mobility Transistor-Based Biosensor. Lab Chip 2022, 22, 4129–4140. [Google Scholar] [CrossRef]
- Pileri, T.; Sinibaldi, A.; Allegretti, M.; Danz, N.; Munzert, P.; Giordani, E.; Occhicone, A.; Sonntag, F.; Giacomini, P.; Michelotti, F. Multiplexed Bio-Assay for a Reliable Detection of HER2 in Human Plasma Using a Combined Label-Free/Fluorescence Biosensing Platform. In Frontiers in Biological Detection: From Nanosensors to Systems XIV; SPIE: Bellingham, WA, USA, 2022; p. PC1197903. [Google Scholar] [CrossRef]
- Zhao, Q.; Xue, J.; Ren, X.; Fan, D.; Kuang, X.; Li, Y.; Wei, Q.; Ju, H. Competitive Electrochemiluminescence Aptasensor Based on the Ru(II) Derivative Utilizing Intramolecular ECL Emission for E2 Detection. Sens. Actuators B Chem. 2021, 348, 130717. [Google Scholar] [CrossRef]
- Ma, X.; Gao, W.; Du, F.; Yuan, F.; Yu, J.; Guan, Y.; Sojic, N.; Xu, G. Rational Design of Electrochemiluminescent Devices. Acc. Chem. Res. 2021, 54, 2936–2945. [Google Scholar] [CrossRef]
- Kitte, S.A.; Bushira, F.A.; Xu, C.; Wang, Y.; Li, H.; Jin, Y. Plasmon-Enhanced Nitrogen Vacancy-Rich Carbon Nitride Electrochemiluminescence Aptasensor for Highly Sensitive Detection of MiRNA. Anal. Chem. 2022, 94, 1406–1414. [Google Scholar] [CrossRef]
- Miao, W. Electrogenerated Chemiluminescence and Its Biorelated Applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef]
- Hu, L.; Wu, Y.; Xu, M.; Gu, W.; Zhu, C. Recent Advances in Co-Reaction Accelerators for Sensitive Electrochemiluminescence Analysis. Chem. Commun. 2020, 56, 10989–10999. [Google Scholar] [CrossRef]
- Miao, W.; Choi, J.P.; Bard, A.J. Electrogenerated Chemiluminescence 69: The Tris(2,2′-Bipyridine)Ruthenium(II), (Ru(Bpy)32+)/Tri-n-Propylamine (TPrA) System Revisited—A New Route Involving TPrA.+ Cation Radicals. J. Am. Chem. Soc. 2002, 124, 14478–14485. [Google Scholar] [CrossRef] [PubMed]
- Kitte, S.A.; Wang, C.; Li, S.; Zholudov, Y.; Qi, L.; Li, J.; Xu, G. Electrogenerated Chemiluminescence of Tris(2,2′-Bipyridine)Ruthenium(II) Using N-(3-Aminopropyl)Diethanolamine as Coreactant. Anal. Bioanal. Chem. 2016, 408, 7059–7065. [Google Scholar] [CrossRef]
- Kitte, S.A.; Bushira, F.A.; Soreta, T.R. A New Anodic Electrochemiluminescence of Tris(2,2′- Bipyridine)Ruthenium(II) with 1-Ethyl-3-(3-Dimethylaminopropyl)Carbodiimide as a Coreactant for Determination of Hydrogen Peroxide. Microchem. J. 2022, 177, 107256. [Google Scholar] [CrossRef]
- Long, Y.M.; Bao, L.; Zhao, J.Y.; Zhang, Z.L.; Pang, D.W. Revealing Carbon Nanodots as Coreactants of the Anodic Electrochemiluminescence of Ru(Bpy)32+. Anal. Chem. 2014, 86, 7224–7228. [Google Scholar] [CrossRef]
- Strauss, V.; Margraf, J.T.; Dirian, K.; Syrgiannis, Z.; Prato, M.; Wessendorf, C.; Hirsch, A.; Clark, T.; Guldi, D.M. Carbon Nanodots: Supramolecular Electron Donor–Acceptor Hybrids Featuring Perylenediimides. Angew. Chem. Int. Ed. 2015, 54, 8292–8297. [Google Scholar] [CrossRef] [PubMed]
- Strauss, V.; Margraf, J.T.; Dolle, C.; Butz, B.; Nacken, T.J.; Walter, J.; Bauer, W.; Peukert, W.; Spiecker, E.; Clark, T.; et al. Carbon Nanodots: Toward a Comprehensive Understanding of Their Photoluminescence. J. Am. Chem. Soc. 2014, 136, 17308–17316. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Liu, K.; Zhou, R.; Shan, C. Multicolor Biomass Based Carbon Nanodots for Bacterial Imaging. Chin. Chem. Lett. 2022, 33, 798–802. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, B. Environmental Applications of 2D Molybdenum Disulfide (MoS2) Nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, P.; Zhou, D.; Sun, Y.; Li, Y.C.; Nguyen, M.A.T.; Terrones, M.; Mallouk, T.E. Fast and Efficient Preparation of Exfoliated 2H MoS2 Nanosheets by Sonication-Assisted Lithium Intercalation and Infrared Laser-Induced 1T to 2H Phase Reversion. Nano Lett. 2015, 15, 5956–5960. [Google Scholar] [CrossRef]
- Chen, X.; McGlynn, C.; McDonald, A.R. Two-Dimensional MoS2 Catalyzed Oxidation of Organic Thiols. Chem. Mater. 2018, 30, 6978–6982. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Emerging Device Applications for Semiconducting Two-Dimensional Transition Metal Dichalcogenides. ACS Nano 2014, 8, 1102–1120. [Google Scholar] [CrossRef]
- Tuxen, A.; Kibsgaard, J.; Gøbel, H.; Lægsgaard, E.; Topsøe, H.; Lauritsen, J.V.; Besenbacher, F. Size Threshold in the Dibenzothiophene Adsorption on MoS2 Nanoclusters. ACS Nano 2010, 4, 4677–4682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Z.; Yang, W.; Su, Q.; Na, C.; Zhang, X.; Zhao, R.; Song, H. Immobilization of Bovine Hemoglobin on Au Nanoparticles/MoS2 Nanosheets—Chitosan Modified Screen-Printed Electrode as Chlorpyrifos Biosensor. Enzym. Microb. Technol. 2022, 154, 109959. [Google Scholar] [CrossRef]
- Lu, K.; Liu, J.; Dai, X.; Zhao, L.; Yang, Y.; Li, H.; Jiang, Y. Construction of a Au@MoS2 Composite Nanosheet Biosensor for the Ultrasensitive Detection of a Neurotransmitter and Understanding of Its Mechanism Based on DFT Calculations. RSC Adv. 2022, 12, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Yarali, E.; Eksin, E.; Torul, H.; Ganguly, A.; Tamer, U.; Papakonstantinou, P.; Erdem, A. Impedimetric Detection of MiRNA Biomarkers Using Paper-Based Electrodes Modified with Bulk Crystals or Nanosheets of Molybdenum Disulfide. Talanta 2022, 241, 123233. [Google Scholar] [CrossRef]
- Zhai, J.; Li, X.; Zhang, J.; Pan, H.; Peng, Q.; Gan, H.; Su, S.; Yuwen, L.; Song, C. SERS/Electrochemical Dual-Mode Biosensor Based on Multi-Functionalized Molybdenum Disulfide Nanosheet Probes and SERS-Active Ag Nanorods Array Electrodes for Reliable Detection of Cancer-Related MiRNA. Sens. Actuators B Chem. 2022, 368, 132245. [Google Scholar] [CrossRef]
- Cui, Z.; Li, D.; Yang, W.; Fan, K.; Liu, H.; Wen, F.; Li, L.; Dong, L.; Wang, G.; Wu, W. An Electrochemical Biosensor Based on Few-Layer MoS2 Nanosheets for Highly Sensitive Detection of Tumor Marker CtDNA. Anal. Methods 2022, 14, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Periñán, E.; García-Mendiola, T.; Enebral-Romero, E.; del Caño, R.; Vera-Hidalgo, M.; Vázquez Sulleiro, M.; Navío, C.; Pariente, F.; Pérez, E.M.; Lorenzo, E. A MoS2 Platform and Thionine-Carbon Nanodots for Sensitive and Selective Detection of Pathogens. Biosens. Bioelectron. 2021, 189, 113375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Duan, J.H.; Song, Y.M.; Ma, J.; Wang, F.D.; Lu, X.; Yang, X.-D. Novel HER2 Aptamer Selectively Delivers Cytotoxic Drug to HER2-Positive Breast Cancer Cells in Vitro. J. Transl. Med. 2012, 10, 148. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A Software for Scanning Probe Microscopy and a Tool for Nanotechnology. Rev. Sci. Instrum. 2007, 78, 13705. [Google Scholar] [CrossRef]
- Gutiérrez-Gálvez, L.; García-Mendiola, T.; Gutiérrez-Sánchez, C.; Guerrero-Esteban, T.; García-Diego, C.; Buendía, I.; García-Bermejo, M.L.; Pariente, F.; Lorenzo, E. Carbon Nanodot–Based Electrogenerated Chemiluminescence Biosensor for MiRNA-21 Detection. Microchim. Acta 2021, 188, 398. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gálvez, L.; del Caño, R.; Menéndez-Luque, I.; García-Nieto, D.; Rodríguez-Peña, M.; Luna, M.; Pineda, T.; Pariente, F.; García-Mendiola, T.; Lorenzo, E. Electrochemiluminescent Nanostructured DNA Biosensor for SARS-CoV-2 Detection. Talanta 2022, 240, 123203. [Google Scholar] [CrossRef]
- Giovanelli, E.; Castellanos-Gomez, A.; Pérez, E.M. Surfactant-Free Polar-to-Nonpolar Phase Transfer of Exfoliated MoS2 Two-Dimensional Colloids. Chempluschem 2017, 82, 732–741. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Batistuti, M.R.; Bachour, B.; Mulato, M. Aptasensor Based on Screen-Printed Electrode for Breast Cancer Detection in Undiluted Human Serum. Bioelectrochemistry 2021, 137, 107586. [Google Scholar] [CrossRef]
- Abrego-Martinez, J.C.; Jafari, M.; Chergui, S.; Pavel, C.; Che, D.; Siaj, M. Aptamer-Based Electrochemical Biosensor for Rapid Detection of SARS-CoV-2: Nanoscale Electrode-Aptamer-SARS-CoV-2 Imaging by Photo-Induced Force Microscopy. Biosens. Bioelectron. 2022, 195, 113595. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Kalaiyarasan, G.; Raju, C.V.; Veerapandian, M.; Kumar, S.S.; Joseph, J. Impact of Aminated Carbon Quantum Dots as a Novel Co-Reactant for Ru(Bpy)32+: Resolving Specific Electrochemiluminescence for Butein Detection. Anal. Bioanal. Chem. 2020, 412, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Sartin, M.M.; Camerel, F.; Ziessel, R.; Bard, A.J. Electrogenerated Chemiluminescence of B8amide: A BODIPY-Based Molecule with Asymmetric ECL Transients. J. Phys. Chem. C 2008, 112, 10833–10841. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, C.; Huang, Z.; Wang, L.; Wang, W.; Bard, A.J. Electrochemistry and Electrogenerated Chemiluminescence of 1,3,5-Tri(Anthracen-10-Yl)-Benzene-Centered Starburst Oligofluorenes. J. Am. Chem. Soc. 2016, 138, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Shi, G.F.; Zhang, J.J.; Zhou, M.; Cao, J.T.; Huang, K.J.; Ren, S.W. A Novel Label-Free Electrochemiluminescence Aptasensor Based on Layered Flowerlike Molybdenum Sulfide–Graphene Nanocomposites as Matrix. Colloids Surf. B Biointerfaces 2014, 122, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhao, G.; Shi, H.; Liu, M.; Li, Z. A Highly Selective Electrochemical Impedance Spectroscopy-Based Aptasensor for Sensitive Detection of Acetamiprid. Biosens. Bioelectron. 2013, 43, 12–18. [Google Scholar] [CrossRef]
- Mehennaoui, S.; Poorahong, S.; Jimenez, G.C.; Siaj, M. Selection of High Affinity Aptamer-Ligand for Dexamethasone and Its Electrochemical Biosensor. Sci. Rep. 2019, 9, 6600. [Google Scholar] [CrossRef]
- Lokich, J.J. Plasma CEA Levels in Small Cell Lung Cancer Correlation with Stage, Distribution of Metastases, and Survival. Cancer 1982, 50, 2154–2156. [Google Scholar] [CrossRef] [PubMed]
- Chun, L.; Kim, S.E.; Cho, M.; Choe, W.S.; Nam, J.; Lee, D.W.; Lee, Y. Electrochemical Detection of HER2 Using Single Stranded DNA Aptamer Modified Gold Nanoparticles Electrode. Sens. Actuators B Chem. 2013, 186, 446–450. [Google Scholar] [CrossRef]









| Nomenclature | Oligonucleotide Sequence |
|---|---|
| Apt-SH | 5′-SH-(CH2)6-AACCGCCCAAATCCCTAAGAGTCTGCACTTGTCA TTTTGTATATGTATTTGGTTTTTGGCTCTCACAGACACACTACACACGCACA-3′ |
| Apt-TAMRA-SH | 5′-SH-(CH2)6-AACCGCCCAAATCCCTAAGAGTCTGCACTTGTCA TTTTGTATATGTATTTGGTTTTTGGCTCTCACAGACACACTACACACGCACA-TAMRA-3′ |
| Interference DNA sequence | AGTGAGTGCGGTTAGACCTGCTAGG |
| Principle | Method | Target Analyte | Linear Range | L.O.D. | Reference |
|---|---|---|---|---|---|
| Reduced graphene oxide nanosheets and rhodium nanoparticles | DPV | Extracellular domain of HER2 | 10.0 to 500.0 ng/mL | 0.667 ng/mL | [14] |
| Au nanoparticles | DPV | HER2 | 0.001 to 100 ng/mL | 0.001 ng/mL | [15] |
| Exonuclease recycling amplification and host–guest recognition | DPV | HER2 | 10 ng/mL to 150 ng/mL | 4.9 ng/ml | [16] |
| Graphene quantum dots, polypyrrole and cobalt phthalocyanine | EIS | HER2 | 1 to 10 ng/mL | 0.00141 ng/mL | [17] |
| Bimetallic ZrHf-based metal-organic framework embedded with carbon dots | EIS | HER2 | 0.001 to 10 ng/mL | 19 fg/mL | [18] |
| A novel binary luminophore (Zr-MOF modified bulk boron carbon oxynitride) | ECL | HER2 | 10 fg/mL to 100 ng/mL | 0.2 fg/mL | [19] |
| CDs and MoS2-NS | ECL | HER2 | 6.08 fg/mL to 13.7 pg/mL | 1.84 fg/mL | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Gálvez, L.; Sulleiro, M.V.; Gutiérrez-Sánchez, C.; García-Nieto, D.; Luna, M.; Pérez, E.M.; García-Mendiola, T.; Lorenzo, E. MoS2-Carbon Nanodots as a New Electrochemiluminescence Platform for Breast Cancer Biomarker Detection. Biosensors 2023, 13, 348. https://doi.org/10.3390/bios13030348
Gutiérrez-Gálvez L, Sulleiro MV, Gutiérrez-Sánchez C, García-Nieto D, Luna M, Pérez EM, García-Mendiola T, Lorenzo E. MoS2-Carbon Nanodots as a New Electrochemiluminescence Platform for Breast Cancer Biomarker Detection. Biosensors. 2023; 13(3):348. https://doi.org/10.3390/bios13030348
Chicago/Turabian StyleGutiérrez-Gálvez, Laura, Manuel Vázquez Sulleiro, Cristina Gutiérrez-Sánchez, Daniel García-Nieto, Mónica Luna, Emilio M. Pérez, Tania García-Mendiola, and Encarnación Lorenzo. 2023. "MoS2-Carbon Nanodots as a New Electrochemiluminescence Platform for Breast Cancer Biomarker Detection" Biosensors 13, no. 3: 348. https://doi.org/10.3390/bios13030348
APA StyleGutiérrez-Gálvez, L., Sulleiro, M. V., Gutiérrez-Sánchez, C., García-Nieto, D., Luna, M., Pérez, E. M., García-Mendiola, T., & Lorenzo, E. (2023). MoS2-Carbon Nanodots as a New Electrochemiluminescence Platform for Breast Cancer Biomarker Detection. Biosensors, 13(3), 348. https://doi.org/10.3390/bios13030348