Reduced Glutathione-Modified Electrode for the Detection of Hydroxyl Free Radicals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
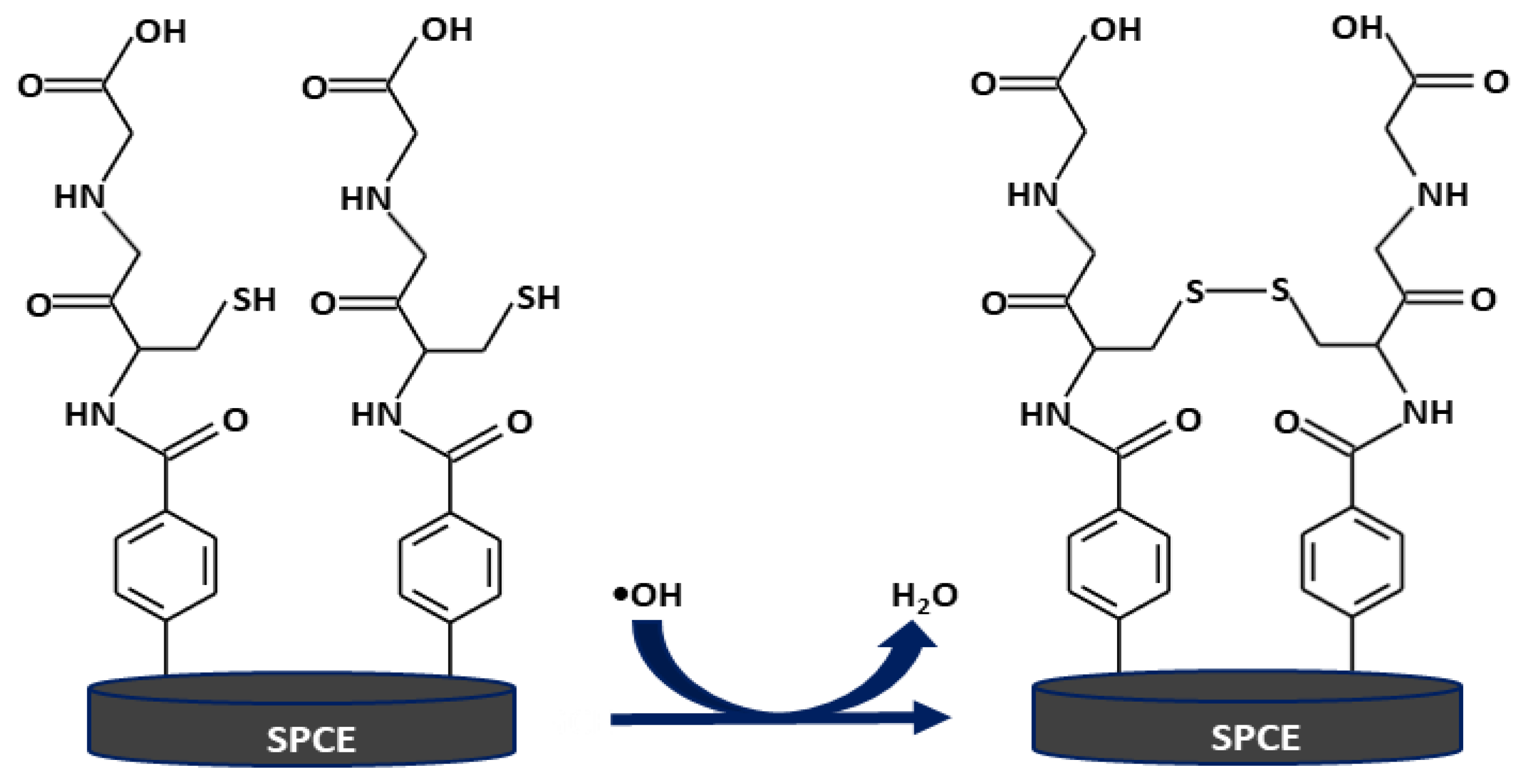
2.2. Sensor Preparation
2.3. Detection of •OH Produced by the Fenton Reaction Using the GSH-Modified Electrode
3. Results and discussion
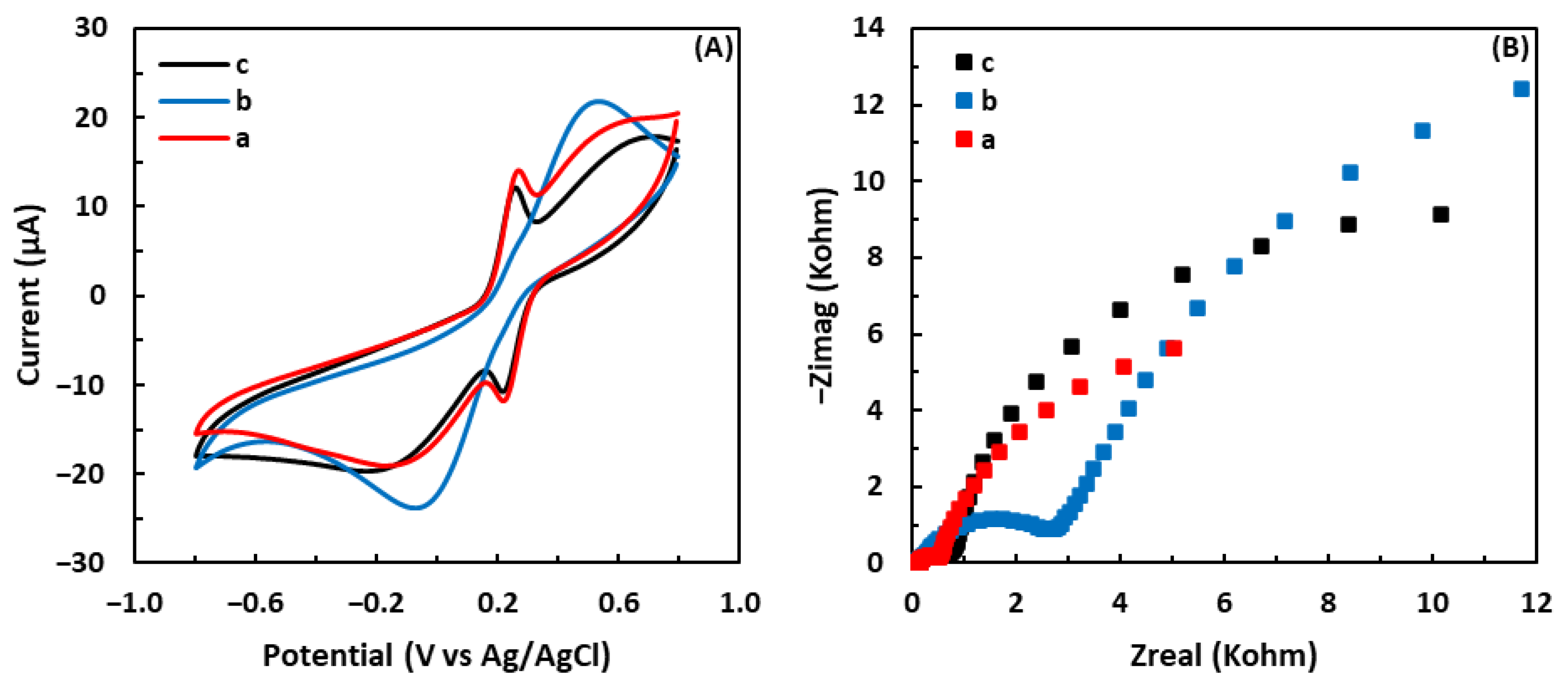
3.1. Electrochemical Characterization of Fabricated Electrodes
3.1.1. Cyclic Voltammetry
3.1.2. Electrochemical Impedance Spectroscopy
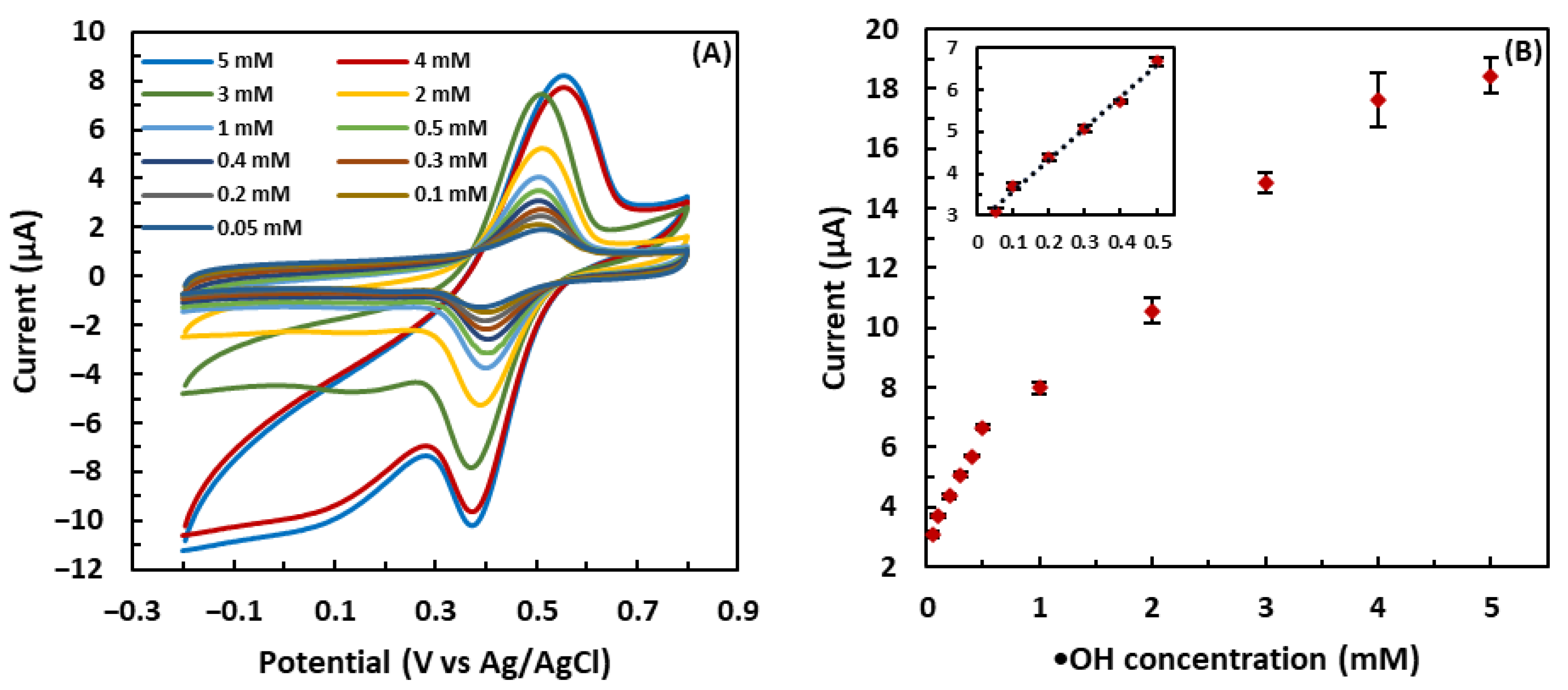
3.2. Detection of •OH in the Fenton Solution by the GSH-Modified Electrode
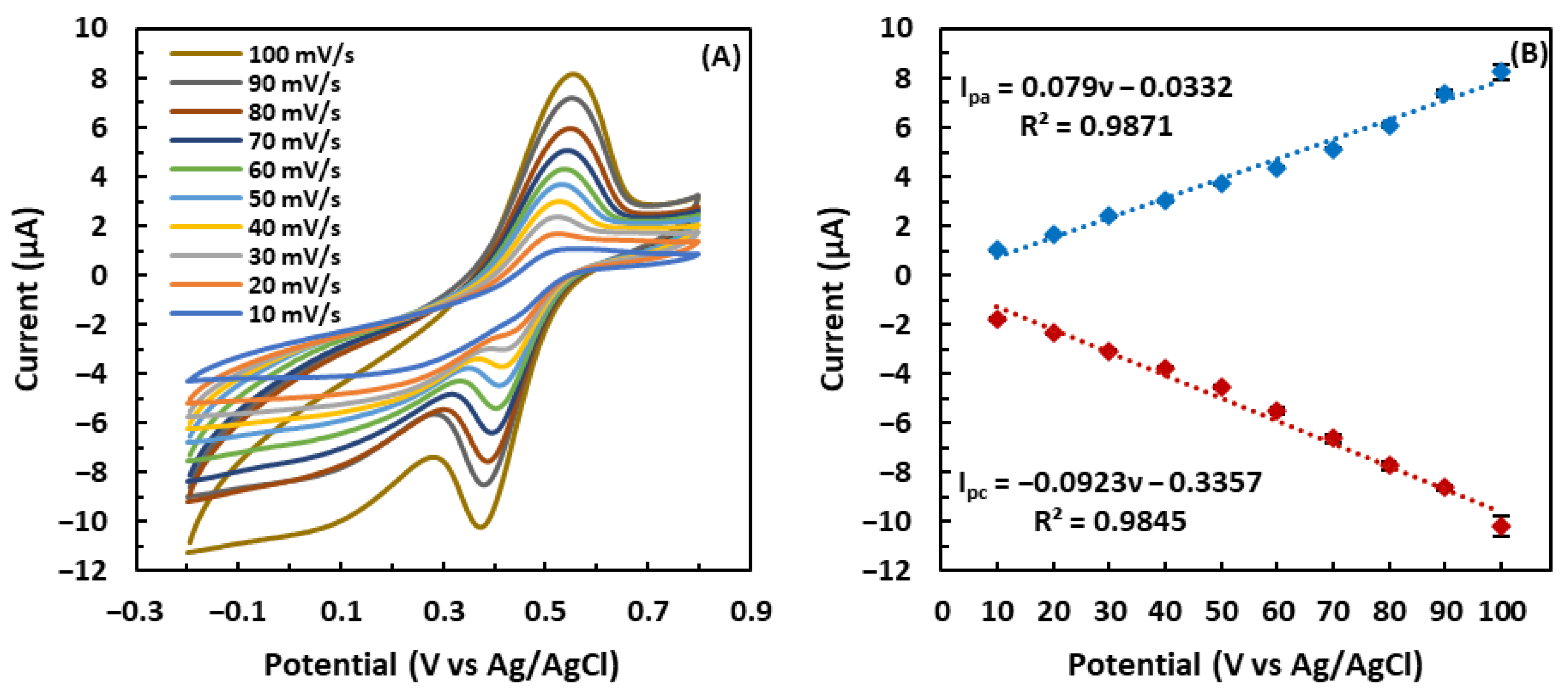
3.3. Effect of the Scan Rate on the GSH-Modified Electrode
3.4. Calibration Curve
3.5. Selectivity and Stability of the GSH-Modified Sensor
3.6. Regeneration of the Sensor
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Sinha, A.; Zhao, H.; Dang, X.; Zhang, Y.; Quan, X. Real Time Detection of Hazardous Hydroxyl Radical Using an Electrochemical Approach. Chemistryselect 2019, 4, 12507–12511. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; de Oliveira, V.A.; da Motta, L.L.; De Bastiani, M.A.; Lopes, F.M.; Müller, C.B.; Gabiatti, B.P.; França, F.S.; Castro, M.A.A.; Klamt, F.; et al. Redox Signaling Across Cell Membranes. Antioxid. Redox Signal. 2009, 11, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signaling transduction. Annu. Rev. Plant Biol. 2004, 55, 373. [Google Scholar] [CrossRef]
- Esposito, F.; Ammendola, R.; Faraonio, R.; Russo, T.; Cimino, F. Redox Control of Signal Transduction, Gene Expression and Cellular Senescence. Neurochem. Res. 2004, 29, 617–628. [Google Scholar] [CrossRef]
- Soh, N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal. Bioanal. Chem. 2006, 386, 532–543. [Google Scholar] [CrossRef]
- Sugino, N. The Role of Oxygen Radical-mediated Signaling Pathways in Endometrial Function. Placenta 2007, 28, S133–S136. [Google Scholar] [CrossRef]
- Clifford, D.P.; Repine, J.E. Hydrogen peroxide mediated killing of bacteria. Mol. Cell. Biochem. 1982, 49, 143–149. [Google Scholar] [CrossRef]
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive Oxygen Species in the Immune System. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef]
- Kajiwara, K.; Ikeda, K.; Kuroi, R.; Hashimoto, R.; Tokumaru, S.; Kojo, S. Hydrogen peroxide and hydroxyl radical involvement in the activation of caspase-3 in chemically induced apoptosis of HL-60 cells. Cell. Mol. Life Sci. 2001, 58, 485–491. [Google Scholar] [CrossRef]
- Carballo, M.; Conde, M.; El Bekay, R.; Martín-Nieto, J.; Camacho, M.J.; Monteseirín, J.; Conde, J.; Bedoya, F.J.; Sobrino, F. Oxidative Stress Triggers STAT3 Tyrosine Phosphorylation and Nuclear Translocation in Human Lymphocytes. J. Biol. Chem. 1999, 274, 17580–17586. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.; Jaruga, P.; Leber, D.; Nyaga, S.G.; Evans, M.K.; Dizdaroglu, M. Lymphoblasts of women with BRCA1 mutations are deficient in cellular repair of 8, 5′-cyclopurine-2′-deoxynucleosides and 8-hydroxy-2′-deoxyguanosine. Biochemistry 2007, 46, 2488–2496. [Google Scholar]
- Seenivasan, R.; Kolodziej, C.; Karunakaran, C.; Burda, C. Nanotechnology for Electroanalytical Biosensors of Reactive Oxygen and Nitrogen Species. Chem. Rec. 2017, 17, 886–901. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547. [Google Scholar] [CrossRef] [PubMed]
- Karakoti, A.; Singh, S.; Dowding, J.M.; Seal, S.; Self, W.T. Redox-active radical scavenging nanomaterials. Chem. Soc. Rev. 2010, 39, 4422–4432. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/ RNS generation. J. Biomed. Sci. 2017, 24, 76. [Google Scholar] [CrossRef]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Li, K.; Muhammad, S.; Ju, M.; Liu, L.; Huang, Y.; Wang, B.; Ding, W.; Shen, B.; et al. Fluorogenic toolbox for facile detecting of hydroxyl radicals: From designing principles to diagnostics applications. TrAC Trends Anal. Chem. 2022, 157, 116734. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.M.; Hasse, U.; Hermes, M.; Scholz, F. Hydroxyl Radicals Attack Metallic Gold. Angew. Chem. 2010, 122, 1079–1081. [Google Scholar] [CrossRef]
- Noël, J.-M.; Médard, J.; Combellas, C.; Kanoufi, F. Prussian Blue Degradation during Hydrogen Peroxide Reduction: A Scanning Electrochemical Microscopy Study on the Role of the Hydroxide Ion and Hydroxyl Radical. Chemelectrochem 2016, 3, 1178–1184. [Google Scholar] [CrossRef]
- Latus, A.; Noël, J.-M.; Volanschi, E.; Lagrost, C.; Hapiot, P. Scanning Electrochemical Microscopy Studies of Glutathione-Modified Surfaces. An Erasable and Sensitive-to-Reactive Oxygen Species Surface. Langmuir 2011, 27, 11206–11211. [Google Scholar] [CrossRef]
- Tachikawa, T.; Majima, T. Single-Molecule Fluorescence Imaging of TiO2 Photocatalytic Reactions. Langmuir 2009, 25, 7791–7802. [Google Scholar] [CrossRef]
- Stoffels, E.; Gonzalvo, Y.A.; Whitmore, T.D.; Seymour, D.L.; Rees, J.A. Mass spectrometric detection of short-living radicals produced by a plasma needle. Plasma Sources Sci. Technol. 2007, 16, 549–556. [Google Scholar] [CrossRef]
- Cao, L.; Wu, Q.; Li, Q.; Shao, S.; Guo, Y. Fluorescence and HPLC Detection of Hydroxyl Radical by a Rhodamine-Nitroxide Probe and its Application in Cell Imaging. J. Fluoresc. 2014, 24, 313–318. [Google Scholar] [CrossRef]
- Li, L.; Zhu, A.; Tian, Y. An electrochemical strategy for fast monitoring of ˙OH released from live cells at an electroactive FcHT-functional surface amplified by Au nanoparticles. Chem. Commun. 2013, 49, 1279–1281. [Google Scholar] [CrossRef]
- Yim, M.B.; Chock, P.B.; Stadtman, E.R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc. Natl. Acad. Sci. USA 1990, 87, 5006–5010. [Google Scholar] [CrossRef]
- Zhu, A.; Liu, Y.; Rui, Q.; Tian, Y. Selective and sensitive determination of hydroxyl radicals generated from living cells through an electrochemical impedance method. Chem. Commun. 2011, 47, 4279–4281. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sui, D.; Zhou, W.; Lu, C. Highly selective chemiluminescence detection of hydroxyl radical via increased π-electron densities of rhodamine B on montmorillonite matrix. Sens. Actuators B: Chem. 2016, 225, 600–606. [Google Scholar] [CrossRef]
- Wang, T.-T.; Huang, Z.-L.; Xu, Q.; Hu, X.-Y. Simple and sensitive determination of hydroxyl radical in atmosphere based on an electrochemically activated glassy carbon electrode. Int. J. Environ. Anal. Chem. 2018, 98, 477–491. [Google Scholar] [CrossRef]
- Ouyang, J.; Li, Z.-Q.; Zhang, J.; Wang, C.; Wang, J.; Xia, X.-H.; Zhou, G.-J. A rapid and sensitive method for hydroxyl radical detection on a microfluidic chip using an N-doped porous carbon nanofiber modified pencil graphite electrode. Analyst 2014, 139, 3416–3422. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiao, X.; Guo, X.; Yao, H.; Pang, H. Synthesis of “Quasi-Ce-MOF” Electrocatalysts for Enhanced Urea Oxidation Reaction Performance. ACS Sustain. Chem. Eng. 2020, 8, 8675–8680. [Google Scholar] [CrossRef]
- Gupta, A.; Rawal, T.B.; Neal, C.J.; Das, S.; Rahman, T.S.; Seal, S. Molybdenum disulfide for ultra-low detection of free radicals: Electrochemical response and molecular modeling. 2d Mater. 2017, 4, 025077. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Tsikas, D.; Rossi, R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit. Rev. Clin. Lab. Sci. 2009, 46, 241–281. [Google Scholar]
- Abbas, M.N.; Saeed, A.A.M.; Ben Ali, M.; Errachid, A.; Zine, N.; Baraket, A.; Singh, B. Biosensor for the oxidative stress biomarker glutathione based on SAM of cobalt phthalocyanine on a thioctic acid modified gold electrode. J. Solid State Electrochem. 2019, 23, 1129–1144. [Google Scholar] [CrossRef]
- Sjöberg, L.; Eriksen, T.E.; Revesz, L. The Reaction of the Hydroxyl Radical with Glutathione in Neutral and Alkaline Aqueous Solution. Radiat. Res. 1982, 89, 255. [Google Scholar] [CrossRef] [PubMed]
- Mezyk, S.P. Rate Constant Determination for the Reaction of Hydroxyl and Glutathione Thiyl Radicals with Glutathione in Aqueous Solution. J. Phys. Chem. 1996, 100, 8861–8866. [Google Scholar] [CrossRef]
- Mi, W.; Tang, S.; Jin, Y.; Shao, N. Au/Ag Bimetallic Nanoclusters Stabilized by Glutathione and Lysozyme for Ratiometric Sensing of H2O2 and Hydroxyl Radicals. ACS Appl. Nano Mater. 2021, 4, 1586–1595. [Google Scholar] [CrossRef]
- Franzoni, F.; Colognato, R.; Galetta, F.; Laurenza, I.; Barsotti, M.C.; Di Stefano, R.; Bocchetti, R.; Regoli, F.; Carpi, A.; Balbarini, A.; et al. An in vitro study on the free radical scavenging capacity of ergothioneine: Comparison with reduced glutathione, uric acid and trolox. Biomed. Pharmacother. 2006, 60, 453–457. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. A Chemically-Bound Glutathione Sensor Bioinspired by the Defense of Organisms against Heavy Metal Contamination: Optimization of the Immobilization Conditions. Chemosensors 2017, 5, 12. [Google Scholar] [CrossRef]
- Duanghathaipornsuk, S.; Alateeq, F.A.O.; Kim, S.S.; Kim, D.-S.; Alba-Rubio, A.C. The effects of size and content of cerium oxide nanoparticles on a composite sensor for hydroxyl radicals detection. Sens. Actuators B Chem. 2020, 321, 128467. [Google Scholar] [CrossRef]
- Ahlberg, E.; Helgee, B.; Parker, V. The reaction of aryl radicals with metallic electrodes. Acta Chem. Scand. B 1980, 34, 181–186. [Google Scholar] [CrossRef]
- Goud, K.Y.; Kumar, V.S.; Hayat, A.; Catanante, G.; Gobi, K.V.; Marty, J.L. Polymer scaffold layers of screen-printed electrodes for homogeneous deposition of silver nanoparticles: Application to the amperometric detection of hydrogen peroxide. Microchim. Acta 2019, 186, 810. [Google Scholar] [CrossRef]
- Veloso, A.J.; Chow, A.M.; Ganesh, H.V.S.; Li, N.; Dhar, D.; Wu, D.C.H.; Mikhaylichenko, S.; Brown, I.R.; Kerman, K. Electrochemical Immunosensors for Effective Evaluation of Amyloid-Beta Modulators on Oligomeric and Fibrillar Aggregation Processes. Anal. Chem. 2014, 86, 4901–4909. [Google Scholar] [CrossRef]
- Xu, D.; Xu, D.; Yu, X.; Liu, Z.; He, W.; Ma, Z. Label-free electrochemical detection for aptamer-based array electrodes. Anal. Chem. 2005, 77, 5107–5113. [Google Scholar] [CrossRef] [PubMed]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.-H.; Lu, H.-T.; Zhang, Q. Electrochemical behavior and voltammetric determination of paracetamol on Nafion/TiO2–graphene modified glassy carbon electrode. Colloids Surf. B Biointerfaces 2011, 85, 289–292. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Guo, F.; Zheng, W.; Zhou, H.; Wang, B.; Zheng, Y.; Wang, Y.; Cheng, Y.; Lou, X.; et al. A novel amperometric hydrogen peroxide biosensor based on immobilized Hb in Pluronic P123-nanographene platelets composite. Colloids Surf. B Biointerfaces 2011, 84, 427–432. [Google Scholar] [CrossRef]
- Niwa, O.; Tabei, H. Voltammetric measurements of reversible and quasi-reversible redox species using carbon film based interdigitated array microelectrodes. Anal. Chem. 1994, 66, 285–289. [Google Scholar] [CrossRef]
- Zhao, N.; He, Y.; Mao, X.; Sun, Y.; Zhang, X.; Li, C.-Z.; Lin, Y.; Liu, G. Electrochemical assay of active prostate-specific antigen (PSA) using ferrocene-functionalized peptide probes. Electrochem. Commun. 2010, 12, 471–474. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Zhang, Y.; Zhang, J.; Jiang, S.; Liang, W.; Zhu, T.; Ye, B.-C. A novel electrochemical sensor for determination of hydroxyl radicals in living cells by coupling nanoporous gold layer with self-assembled 6-(Ferrocenyl) hexanethiol. Anal. Chim. Acta 2020, 1096, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Wu, L.; Yang, Y.; Zhang, H.; Zhu, G.; Zhang, X.; Chen, J. Sensitive electrochemical detection of hydroxyl radical with biobarcode amplification. Anal. Chim. Acta 2012, 756, 1–6. [Google Scholar] [CrossRef]
- Liu, F.; Du, J.; Song, D.; Xu, M.; Sun, G. A sensitive fluorescent sensor for the detection of endogenous hydroxyl radicals in living cells and bacteria and direct imaging with respect to its ecotoxicity in living zebra fish. Chem. Commun. 2016, 52, 4636–4639. [Google Scholar] [CrossRef]
- Korotkova, E.I.; Misini, B.; Dorozhko, E.V.; Bukkel, M.V.; Plotnikov, E.V.; Linert, W. Study of OH● Radicals in Human Serum Blood of Healthy Individuals and Those with Pathological Schizophrenia. Int. J. Mol. Sci. 2011, 12, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Aw, T.Y.; Jones, D.P. Glutathione-dependent projection against oxidative injury. Pharmacol. Ther. 1990, 47, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Gutterer, J.; Hirrlinger, J. Glutathione metabolism in brain: Metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000, 267, 4912–4916. [Google Scholar] [CrossRef] [PubMed]
- Munday, R.; Winterbourn, C.C. Reduced glutathione in combination with superoxide dismutase as an important biological antioxidant defence mechanism. Biochem. Pharmacol. 1989, 38, 4349–4352. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaedamini, H.; Duanghathaipornsuk, S.; Onusko, P.; Binsheheween, A.M.; Kim, D.-S. Reduced Glutathione-Modified Electrode for the Detection of Hydroxyl Free Radicals. Biosensors 2023, 13, 254. https://doi.org/10.3390/bios13020254
Ghaedamini H, Duanghathaipornsuk S, Onusko P, Binsheheween AM, Kim D-S. Reduced Glutathione-Modified Electrode for the Detection of Hydroxyl Free Radicals. Biosensors. 2023; 13(2):254. https://doi.org/10.3390/bios13020254
Chicago/Turabian StyleGhaedamini, Hamidreza, Surachet Duanghathaipornsuk, Patrick Onusko, Abdullah M. Binsheheween, and Dong-Shik Kim. 2023. "Reduced Glutathione-Modified Electrode for the Detection of Hydroxyl Free Radicals" Biosensors 13, no. 2: 254. https://doi.org/10.3390/bios13020254
APA StyleGhaedamini, H., Duanghathaipornsuk, S., Onusko, P., Binsheheween, A. M., & Kim, D.-S. (2023). Reduced Glutathione-Modified Electrode for the Detection of Hydroxyl Free Radicals. Biosensors, 13(2), 254. https://doi.org/10.3390/bios13020254





