Optic Fiber Microsensor Reveals Specific Spatiotemporal Oxygen Uptake Profiles at the Mammalian Ocular Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
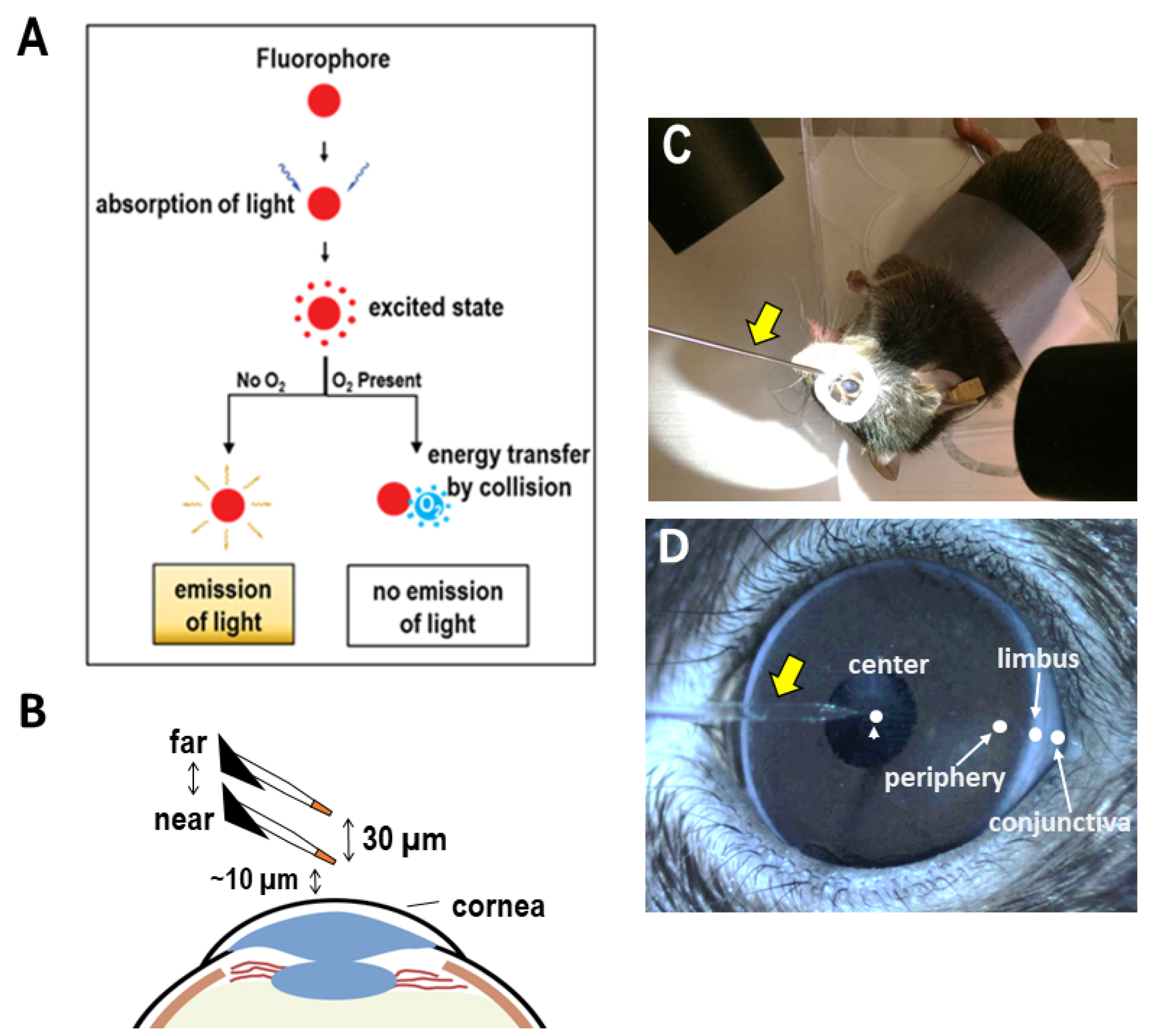
2.2. Scanning Micro-Optrode Technique (SMOT)
2.3. Measurement at Cornea
2.4. Circadian Rhythm Measurements
2.5. Statistical Analysis
3. Results
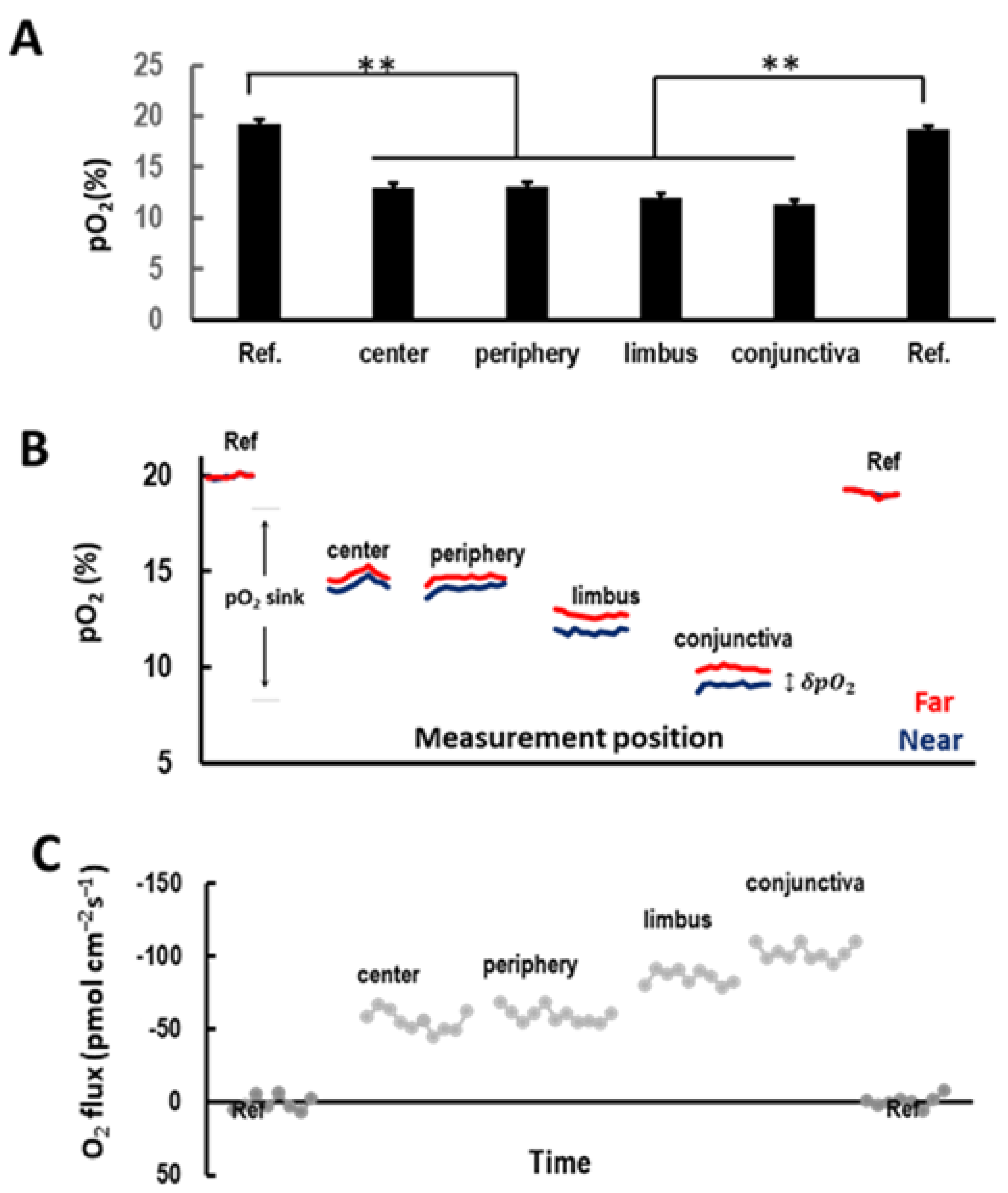
3.1. Ocular Surface Uptakes Oxygen in a Centripetal Gradient In Vivo
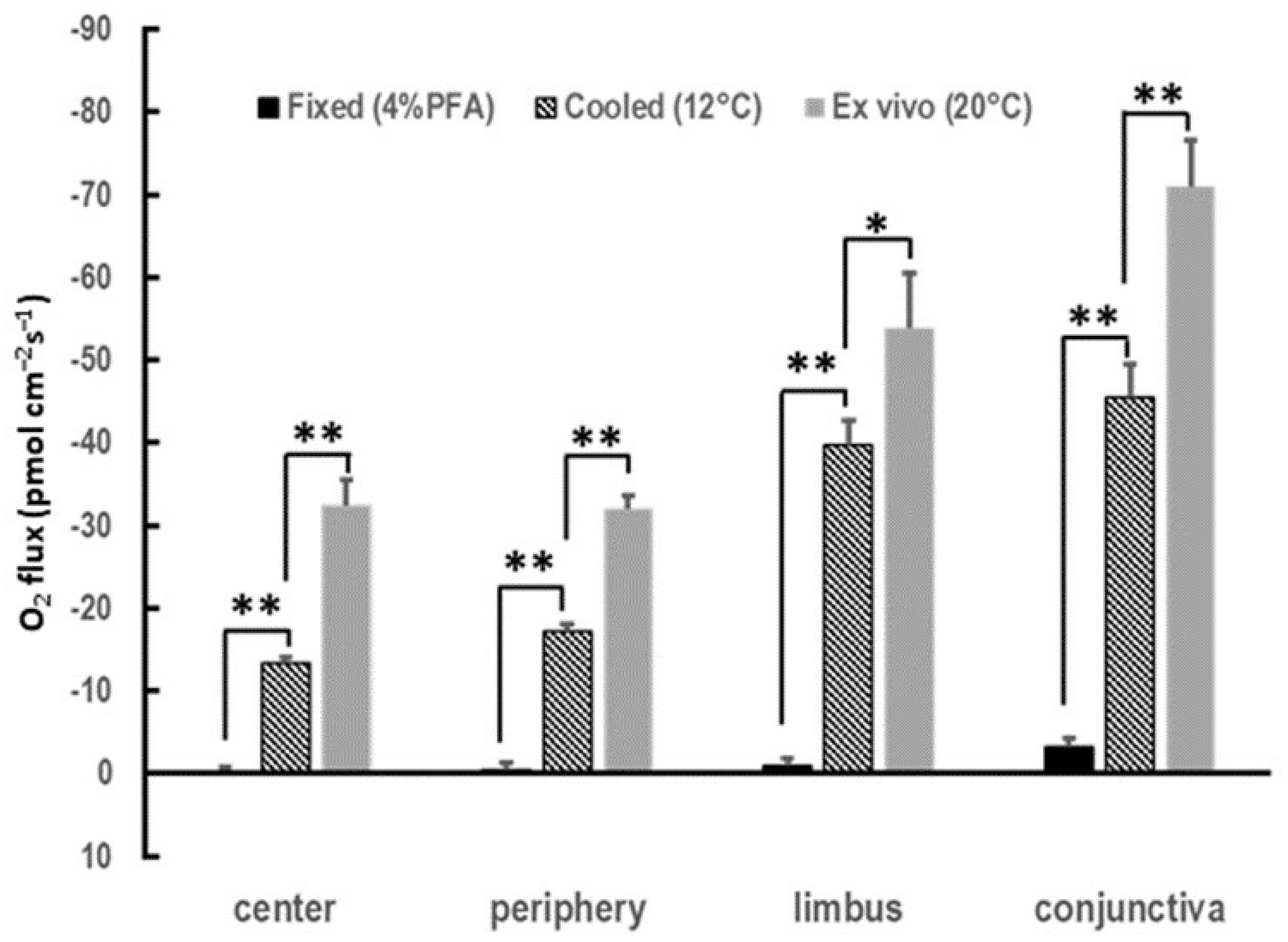
3.2. Cornea of Enucleated Eyes Maintains the Centripetal Gradient
3.3. Spatial COU Profile Is Conserved across Species
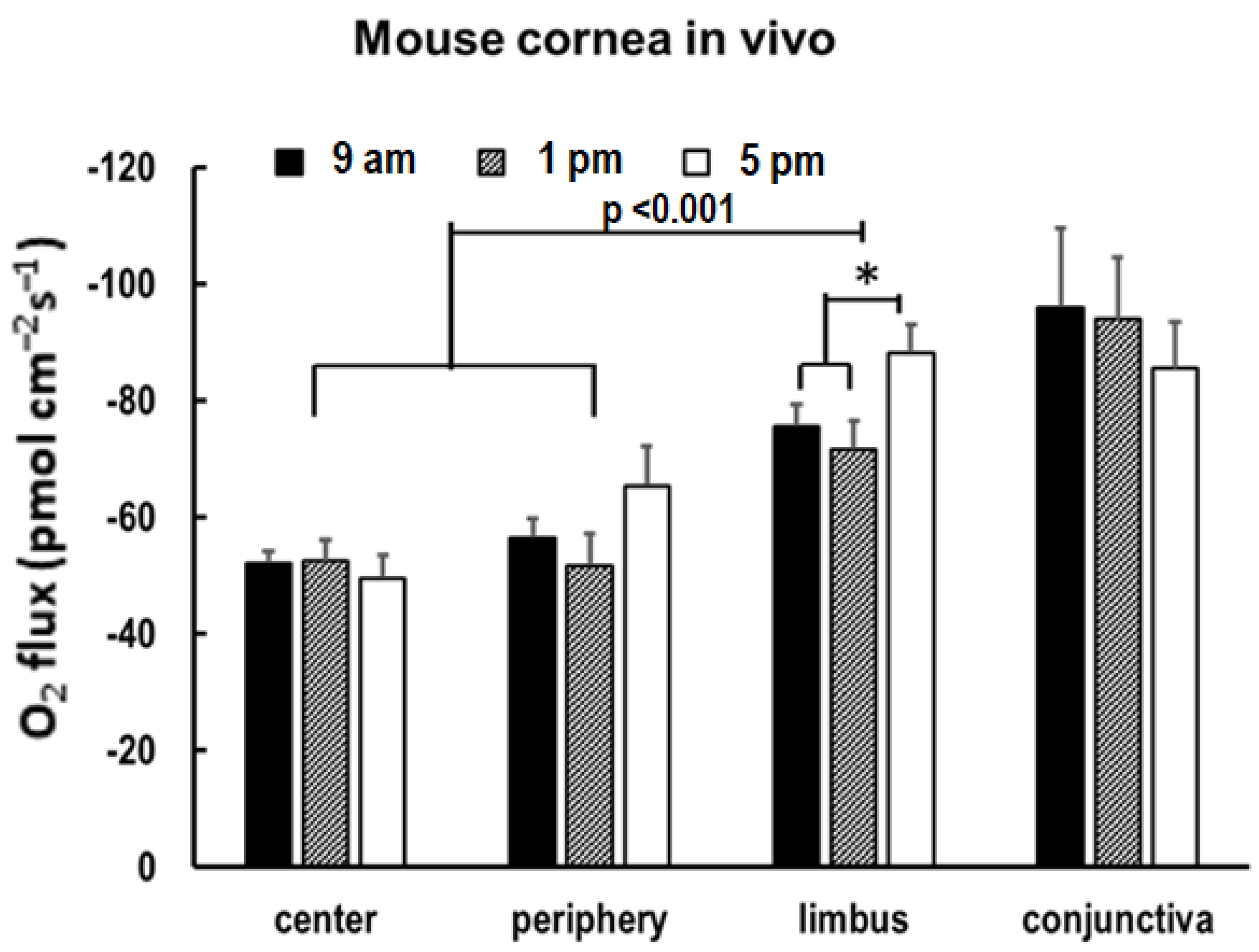
3.4. O2 Uptake Increases in the Limbus in the Evening
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semenza, G.L. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem. J. 2007, 405, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-L.; Liu, H.-Q. Effect of hypoxia on the proliferation of murine cornea limbal epithelial progenitor cells in vitro. Int. J. Ophthalmol. 2011, 4, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.; Raghunathan, V.; Luxardi, G.; Zhu, K.; Zhao, M. Early redox activities modulate Xenopus tail regeneration. Nat. Commun. 2018, 9, 4296. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial pressure of oxygen in the human body: A general review. Am. J. blood Res. 2019, 9, 1–14. [Google Scholar] [PubMed]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef]
- Simon, M.C.; Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 2008, 9, 285–296. [Google Scholar] [CrossRef]
- Mieyal, A.P.; Bonazzi, A.; Jiang, H.; Dunn, M.W.; Schwartzman, M.L. The effect of hypoxia on endogenous corneal epithelial eicosanoids. Investig. Opthalmol. Vis. Sci. 2000, 41, 2170–2176. [Google Scholar]
- Freeman, R.D. Oxygen consumption by the component layers of the cornea. J. Physiol. 1972, 225, 15–32. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, P.; Wang, H.; Xiao, C.; Lin, C.; Liu, J.; Dong, D.; Fu, T.; Yang, Y.; Wang, Z.; et al. Modulation of Circadian Rhythms Affects Corneal Epithelium Renewal and Repair in Mice. Investig. Opthalmol. Vis. Sci. 2017, 58, 1865–1874. [Google Scholar] [CrossRef]
- Pang, K.; Lennikov, A.; Yang, M. Hypoxia adaptation in the cornea: Current animal models and underlying mechanisms. Anim. Model. Exp. Med. 2021, 4, 300–310. [Google Scholar] [CrossRef]
- Takatori, S.C.; De La Jara, P.L.; Holden, B.; Ehrmann, K.; Ho, A.; Radke, C.J. In Vivo Oxygen Uptake into the Human Cornea. Investig. Opthalmol. Vis. Sci. 2012, 53, 6331–6337. [Google Scholar] [CrossRef]
- Fitzgerald, J.P.; Efron, N. Oxygen uptake profile of the human cornea. Clin. Exp. Optom. 1986, 69, 149–152. [Google Scholar] [CrossRef]
- Pal-Ghosh, S.; Tadvalkar, G.; Karpinski, B.A.; Stepp, M.A. Diurnal Control of Sensory Axon Growth and Shedding in the Mouse Cornea. Investig. Opthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef]
- Ferreira, F.; Luxardi, G.; Reid, B.; Ma, L.; Raghunathan, V.; Zhao, M. Real-time physiological measurements of oxygen using a non-invasive self-referencing optical fiber microsensor. Nat. Protoc. 2020, 15, 207–235. [Google Scholar] [CrossRef]
- Song, F.; Xue, Y.; Dong, D.; Liu, J.; Fu, T.; Xiao, C.; Wang, H.; Lin, C.; Liu, P.; Zhong, J.; et al. Insulin Restores an Altered Corneal Epithelium Circadian Rhythm in Mice with Streptozotocin-induced Type 1 Diabetes. Sci. Rep. 2016, 6, 32871. [Google Scholar] [CrossRef]
- Bonanno, A.J.; Stickel, T.; Nguyen, T.; Biehl, T.; Carter, D.; Benjamin, W.J.; Soni, P.S. Estimation of human corneal oxygen consumption by noninvasive measurement of tear oxygen tension while wearing hydrogel lenses. Investig. Opthalmol. Vis. Sci. 2002, 43, 371–376. [Google Scholar]
- Wolfbeis, O.S. Luminescent sensing and imaging of oxygen: Fierce competition to the Clark electrode. Bioessays 2015, 37, 921–928. [Google Scholar] [CrossRef]
- Chatni, M.R.; Li, G.; Porterfield, D.M. Frequency-domain fluorescence lifetime optrode system design and instrumentation without a concurrent reference light-emitting diode. Appl. Opt. 2009, 48, 5528–5536. [Google Scholar] [CrossRef]
- Altshuler, A.; Amitai-Lange, A.; Tarazi, N.; Dey, S.; Strinkovsky, L.; Hadad-Porat, S.; Bhattacharya, S.; Nasser, W.; Imeri, J.; Ben-David, G.; et al. Discrete limbal epithelial stem cell populations mediate corneal homeostasis and wound healing. Cell Stem Cell 2021, 28, 1248–1261.e8. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Taguchi, M.; Burrs, S.L.; Hauser, B.A.; Salim, W.W.A.W.; Claussen, J.C.; McLamore, E.S. Emerging technologies for non-invasive quantification of physiological oxygen transport in plants. Planta 2013, 238, 599–614. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Misrahy, G.; Fox, R.P. Chronically Implanted Polarographic Electrodes. J. Appl. Physiol. 1958, 13, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, W.J.; Hill, R.M. Human cornea: Oxygen uptake immediately following graded deprivation. Graefe’s Arch. Clin. Exp. Ophthalmol. 1985, 223, 47–49. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.W.; Dinslage, S.; Dillon, J.P.; Roberts, J.E.; Brubaker, R.F. Measuring oxygen tension in the anterior chamber of rabbits. Investig. Opthalmol. Vis. Sci. 1998, 39, 1899–1909. [Google Scholar]
- Chatni, M.R.; Porterfield, D.M. Self-referencing optrode technology for non-invasive real-time measurement of biophysical flux and physiological sensing. Analyst 2019, 134, 2224–2232. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Ma, L.; Ferreira, F.; Brown, C.; Reid, B.; Zhao, M. Optic Fiber Microsensor Reveals Specific Spatiotemporal Oxygen Uptake Profiles at the Mammalian Ocular Surface. Biosensors 2023, 13, 245. https://doi.org/10.3390/bios13020245
Sun Q, Ma L, Ferreira F, Brown C, Reid B, Zhao M. Optic Fiber Microsensor Reveals Specific Spatiotemporal Oxygen Uptake Profiles at the Mammalian Ocular Surface. Biosensors. 2023; 13(2):245. https://doi.org/10.3390/bios13020245
Chicago/Turabian StyleSun, Qin, Li Ma, Fernando Ferreira, Chelsea Brown, Brian Reid, and Min Zhao. 2023. "Optic Fiber Microsensor Reveals Specific Spatiotemporal Oxygen Uptake Profiles at the Mammalian Ocular Surface" Biosensors 13, no. 2: 245. https://doi.org/10.3390/bios13020245
APA StyleSun, Q., Ma, L., Ferreira, F., Brown, C., Reid, B., & Zhao, M. (2023). Optic Fiber Microsensor Reveals Specific Spatiotemporal Oxygen Uptake Profiles at the Mammalian Ocular Surface. Biosensors, 13(2), 245. https://doi.org/10.3390/bios13020245





