Reduced Graphene Oxide/Organic Dye Composites for Bioelectroconversion of Saccharides: Application for Detection of Saccharides and α-Amylase Assessments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene Oxide
2.3. Modification of Graphene Oxide with Organic Dyes
2.4. Characterization of Graphene Oxide and His Derivation
2.5. Biosensor Fabrication
2.6. Electrochemical Measurements
2.7. Sulfuric Acid–UV Method
2.8. α-Amylase Activity Determination
3. Results
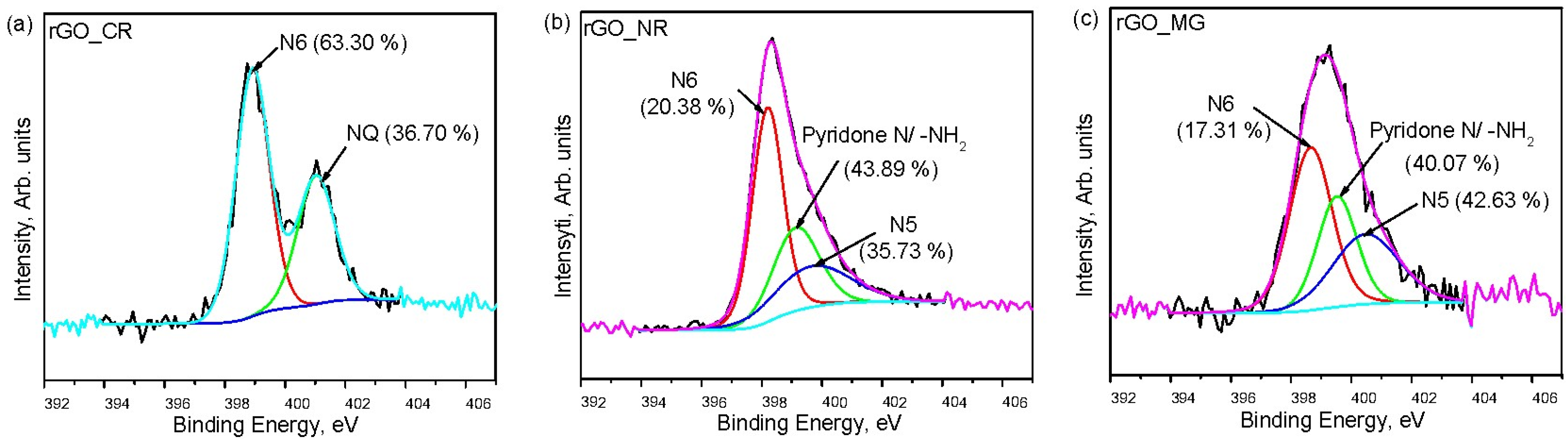
3.1. Characterization of rGO and rGO/Organic Dye Composites
3.2. Bioelectrocatalytic Properties of rGO and rGO/Organic-Dye-Composite-Based Biosensors
3.3. Selectivity of rGO_MG and rGO_NR Based Biosensors
3.4. Stability of rGO_MG and rGO_NR Based Biosensors
3.5. Theoretical Application of Biosensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domínguez, E.; del Río, P.G.; Romaní, A.; Garrote, G.; Domingues, L. Hemicellulosic Bioethanol Production from Fast-Growing Paulownia Biomass. Processes 2021, 9, 173. [Google Scholar] [CrossRef]
- Turner, W.; Greetham, D.; Mos, M.; Squance, M.; Kam, J.; Du, C. Exploring the Bioethanol Production Potential of Miscanthus Cultivars. Appl. Sci. 2021, 11, 9949. [Google Scholar] [CrossRef]
- Roth, J.; Sommerfeld, O.; Birkenfeld, A.L.; Sponholz, C.; Müller, U.A.; von Loeffelholz, C. Blood Sugar Targets in Surgical Intensive Care. Dtsch. Arztebl. Int. 2021, 118, 629. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.V.; Neifar, S.; Merdzo, Z.; Viña-Gonzalez, J.; Fernandez-Arrojo, L.; Ballesteros, A.O.; Fernandez-Lobato, M.; Bejar, S.; Plou, F.J. A Three-Step Process for the Bioconversion of whey Permeate into a Glucose-Free D-Tagatose Syrup. Catalysts 2020, 10, 647. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Hernández-Hernández, O.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Derivatization of Carbohydrates for GC and GC–MS Analyses. J. Chromatogr. B 2011, 879, 1226–1240. [Google Scholar] [CrossRef]
- Bischel, M.D.; Austin, J.H.; Kemeny, M.D.; Hubble, C.M.; Lear, R.K. Separation and Identification of Acid Polysaccharides by Thin-Layer Chromatography. J. Chromatogr. A 1966, 21, 40–45. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.; Zhu, S.; Yin, H.; Wang, M.; Tang, J. Sugar Compositional Determination of Polysaccharides from Dunaliella Salina by Modified RP-HPLC Method of Precolumn Derivatization with 1-Phenyl-3-Methyl-5-Pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Chen, J.; Yang, F.; Guo, H.; Wu, F.; Wang, X. Optimized Hydrolysis and Analysis of Radix asparagi Polysaccharide Monosaccharide Composition by Capillary Zone Electrophoresis. J. Sep. Sci. 2015, 38, 2327–2331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Li, R.; Chang, X.; Jing, R. Development of Near-Infrared Reflectance Spectroscopy Models for Quantitative Determination of Water-Soluble Carbohydrate Content in Wheat Stem and Glume. Anal. Lett. 2011, 44, 2478–2490. [Google Scholar] [CrossRef]
- Şenel, M.; Çevik, E.; Abasıyanık, M.F. Amperometric Hydrogen Peroxide Biosensor Based on Covalent Immobilization of Horseradish Peroxidase on Ferrocene Containing Polymeric Mediator. Sens. Actuators B Chem. 2010, 145, 444–450. [Google Scholar] [CrossRef]
- Gorton, L.; Lindgren, A.; Larsson, T.; Munteanu, F.D.; Ruzgas, T.; Gazaryan, I. Direct Electron Transfer between Heme-Containing Enzymes and Electrodes as Basis for Third Generation Biosensors. Anal. Chim. Acta 1999, 400, 91–108. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Cheng, Y.; Jiang, S.P. Effect of Carbon Nanotubes on Direct Electron Transfer and Electrocatalytic Activity of Immobilized Glucose Oxidase. ACS Omega 2018, 3, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Ludwig, R. Direct Electron Transfer of Enzymes Facilitated by Cytochromes. ChemElectroChem 2019, 6, 958–975. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the Functional Modification of Graphene/Graphene Oxide: A Review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef] [PubMed]
- Razumiene, J.; Barkauskas, J.; Kubilius, V.; Meskys, R.; Laurinavicius, V. Modified Graphitized Carbon Black as Transducing Material for Reagentless HO and Enzyme Sensors. Talanta 2005, 67, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Shinagawa, E.; Adachi, O.; Ameyama, M. Quinoprotein D-Glucose Dehydrogenases InAcinetobacter Calcoaceticus LMD 79. 41: Purification and Characterization of the Membrane-Bound Enzyme Distinct from the Soluble Enzyme. Antonie Leeuwenhoek 1989, 56, 63–72. [Google Scholar] [CrossRef]
- Olsthoorn, A.J.J.; Duine, J.A. On the Mechanism and Specificity of Soluble, Quinoprotein Glucose Dehydrogenase in the Oxidation of Aldose Sugars. Biochemistry 1998, 37, 13854–13861. [Google Scholar] [CrossRef] [PubMed]
- Gružauskaitė, J.; Jasinskaitė, J.; Meškys, R.; Gaidamavičienė, G.; Žalga, A.; Laurynėnas, A.; Tetianec, L.; Dagys, M. Gold-Coated Magnetic Nanocatalyst Containing Wired Oxidoreductases for Mediatorless Catalysis of Carbohydrate Oxidation by Oxygen. Catal. Commun. 2020, 135, 105848. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Ivanauskas, F.; Kaunietis, I.; Laurinavičius, V.; Razumienė, J.; Šimkus, R. Apparent Michaelis Constant of the Enzyme Modified Porous Electrode. J. Math. Chem. 2008, 43, 1516–1526. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A New Method for Rapid Determination of Carbohydrate and Total Carbon Concentrations Using UV Spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Radoń, A.; Włodarczyk, P.; Łukowiec, D. Structure, Temperature and Frequency Dependent Electrical Conductivity of Oxidized and Reduced Electrochemically Exfoliated Graphite. Phys. E Low Dimens. Syst. Nanostruct. 2018, 99, 82–90. [Google Scholar] [CrossRef]
- Puech, P.; Kandara, M.; Paredes, G.; Moulin, L.; Weiss-Hortala, E.; Kundu, A.; Ratel-Ramond, N.; Plewa, J.M.; Pellenq, R.; Monthioux, M. Analyzing the Raman Spectra of Graphenic Carbon Materials from Kerogens to Nanotubes: What Type of Information Can Be Extracted from Defect Bands? C—J. Carbon Res. 2019, 5, 69. [Google Scholar] [CrossRef]
- Gaidukevic, J.; Aukstakojyte, R.; Navickas, T.; Pauliukaite, R.; Barkauskas, J. A Novel Approach to Prepare Highly Oxidized Graphene Oxide: Structural and Electrochemical Investigations. Appl. Surf. Sci. 2021, 567, 150883. [Google Scholar] [CrossRef]
- Drewniak, S.; Muzyka, R.; Stolarczyk, A.; Pustelny, T.; Kotyczka-Morańska, M.; Setkiewicz, M. Studies of Reduced Graphene Oxide and Graphite Oxide in the Aspect of Their Possible Application in Gas Sensors. Sensors 2016, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and Structural Studies of High Quality Graphene Oxide and Reduced Graphene Oxide Prepared by Different Chemical Oxidation Methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Yamada, Y.; Kim, J.; Matsuo, S.; Sato, S. Nitrogen-Containing Graphene Analyzed by X-ray Photoelectron Spectroscopy. Carbon 2014, 70, 59–74. [Google Scholar] [CrossRef]
- Ayiania, M.; Smith, M.; Hensley, A.J.R.; Scudiero, L.; McEwen, J.-S.; Garcia-Perez, M. Deconvoluting the XPS Spectra for Nitrogen-Doped Chars: An Analysis from First Principles. Carbon 2020, 162, 528–544. [Google Scholar] [CrossRef]
- Lin, S.; Ju, S.; Shi, G.; Zhang, J.; He, Y.; Jiang, D. Ultrathin Nitrogen-Doping Graphene Films for Flexible and Stretchable EMI Shielding Materials. J. Mater. Sci. 2019, 54, 7165–7179. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Li, L.; You, T. Direct Electrochemistry of Glucose Oxidase on Novel Free-Standing Nitrogen-Doped Carbon Nanospheres@Carbon Nanofibers Composite Film. Sci. Rep. 2015, 5, 9885. [Google Scholar] [CrossRef] [PubMed]
- Vega-Díaz, S.M.; González, V.J.; Morelos-Gómez, A.; Tristán-López, F.; Labrada-Delgado, G.J.; Rivera-Escoto, B.A.; Sánchez-Salas, R.; Cortés-López, A.J.; Fajardo-Díaz, J.L.; López-Urías, F.; et al. Pyrrolic Nitrogen-Doped Multiwall Carbon Nanotubes Using Ball-Milled Slag-SiC Mixtures as a Catalyst by Aerosol Assisted Chemical Vapor Deposition. Mater. Res. Express 2020, 7, 025602. [Google Scholar] [CrossRef]
- Luo, E.; Xiao, M.; Ge, J.; Liu, C.; Xing, W. Selectively Doping Pyridinic and Pyrrolic Nitrogen into a 3D Porous Carbon Matrix through Template-Induced Edge Engineering: Enhanced Catalytic Activity towards the Oxygen Reduction Reaction. J. Mater. Chem. A 2017, 5, 21709–21714. [Google Scholar] [CrossRef]
- Šakinytė, I.; Butkevičius, M.; Gurevičienė, V.; Stankevičiūtė, J.; Meškys, R.; Razumienė, J. Reagentless D-Tagatose Biosensors Based on the Oriented Immobilization of Fructose Dehydrogenase onto Coated Gold Nanoparticles- or Reduced Graphene Oxide-Modified Surfaces: Application in a Prototype Bioreactor. Biosensors 2021, 11, 466. [Google Scholar] [CrossRef]
- Igarashi, S.; Hirokawa, T.; Sode, K. Engineering PQQ Glucose Dehydrogenase with Improved Substrate Specificity. Biomol. Eng. 2004, 21, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Streďanský, M.; Monošík, R.; Mastihuba, V.; Šturdík, E. Monitoring of PQQ-Dependent Glucose Dehydrogenase Substrate Specificity for Its Potential Use in Biocatalysis and Bioanalysis. Appl. Biochem. Biotechnol. 2013, 171, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tu, Q.; Urban, J.J.; Li, S.; Mi, B. Swelling of Graphene Oxide Membranes in Aqueous Solution: Characterization of Interlayer Spacing and Insight into Water Transport Mechanisms. ACS Nano 2017, 11, 6440–6450. [Google Scholar] [CrossRef]
- Li, G.; Xu, H.; Huang, W.; Wang, Y.; Wu, Y.; Parajuli, R. A Pyrrole Quinoline Quinone Glucose Dehydrogenase Biosensor Based on Screen-Printed Carbon Paste Electrodes Modified by Carbon Nanotubes. Meas. Sci. Technol. 2008, 19, 065203. [Google Scholar] [CrossRef]
- Quintero-Jaime, A.F.; Conzuelo, F.; Cazorla-Amorós, D.; Morallón, E. Pyrroloquinoline Quinone-Dependent Glucose Dehydrogenase Bioelectrodes Based on One-Step Electrochemical Entrapment over Single-Wall Carbon Nanotubes. Talanta 2021, 232, 122386. [Google Scholar] [CrossRef]
- Razumiene, J.; Vilkanauskyte, A.; Gureviciene, V.; Barkauskas, J.; Meskys, R.; Laurinavicius, V. Direct Electron Transfer between PQQ Dependent Glucose Dehydrogenases and Carbon Electrodes: An Approach for Electrochemical Biosensors. Electrochim. Acta 2006, 51, 5150–5156. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butkevicius, M.; Gaidukevic, J.; Gureviciene, V.; Razumiene, J. Reduced Graphene Oxide/Organic Dye Composites for Bioelectroconversion of Saccharides: Application for Detection of Saccharides and α-Amylase Assessments. Biosensors 2023, 13, 1020. https://doi.org/10.3390/bios13121020
Butkevicius M, Gaidukevic J, Gureviciene V, Razumiene J. Reduced Graphene Oxide/Organic Dye Composites for Bioelectroconversion of Saccharides: Application for Detection of Saccharides and α-Amylase Assessments. Biosensors. 2023; 13(12):1020. https://doi.org/10.3390/bios13121020
Chicago/Turabian StyleButkevicius, Marius, Justina Gaidukevic, Vidute Gureviciene, and Julija Razumiene. 2023. "Reduced Graphene Oxide/Organic Dye Composites for Bioelectroconversion of Saccharides: Application for Detection of Saccharides and α-Amylase Assessments" Biosensors 13, no. 12: 1020. https://doi.org/10.3390/bios13121020
APA StyleButkevicius, M., Gaidukevic, J., Gureviciene, V., & Razumiene, J. (2023). Reduced Graphene Oxide/Organic Dye Composites for Bioelectroconversion of Saccharides: Application for Detection of Saccharides and α-Amylase Assessments. Biosensors, 13(12), 1020. https://doi.org/10.3390/bios13121020







