Development of a Direct Non-Puncture Device for Measuring Portal Venous Pressure during Liver Transplantation—A Swine Model
Abstract
1. Introduction
2. Materials and Methods
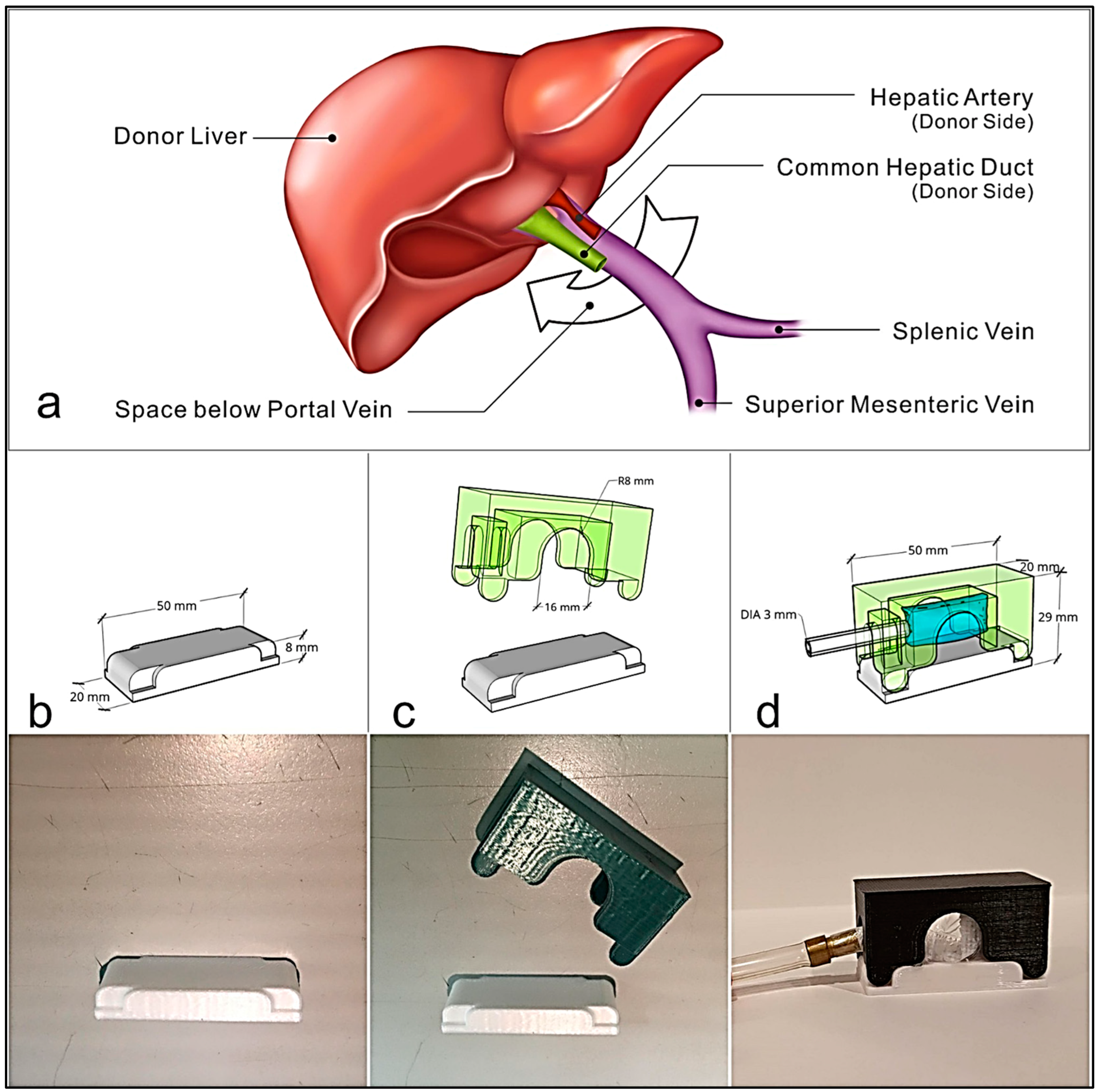
2.1. Device Design
2.2. System and Data Acquisition
2.3. Animal Experiments
2.3.1. Ethical Approval
2.3.2. Preoperative Care
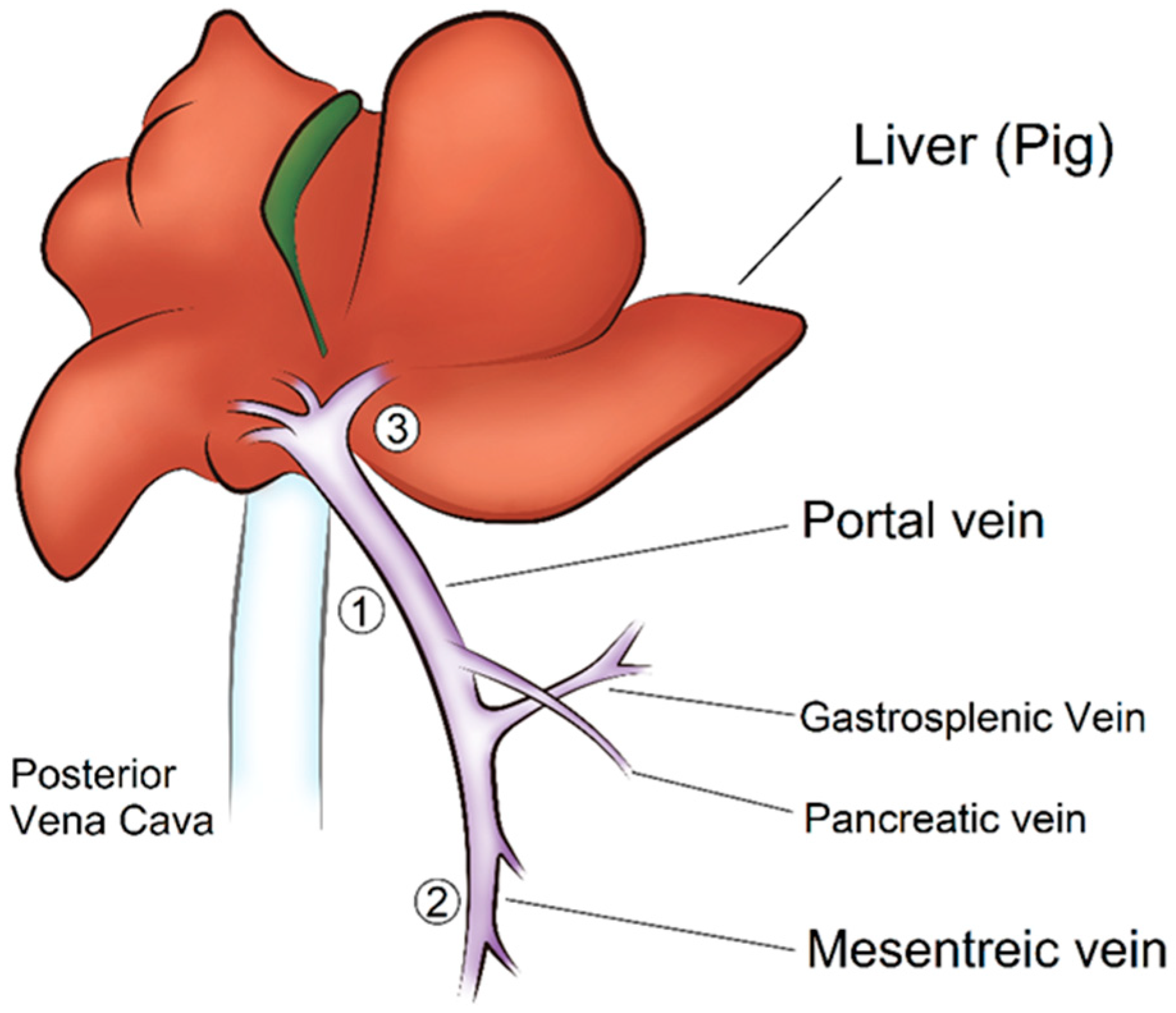
2.3.3. Surgical Procedure
2.4. Data Acquisition
2.5. Statistical Analysis
3. Results
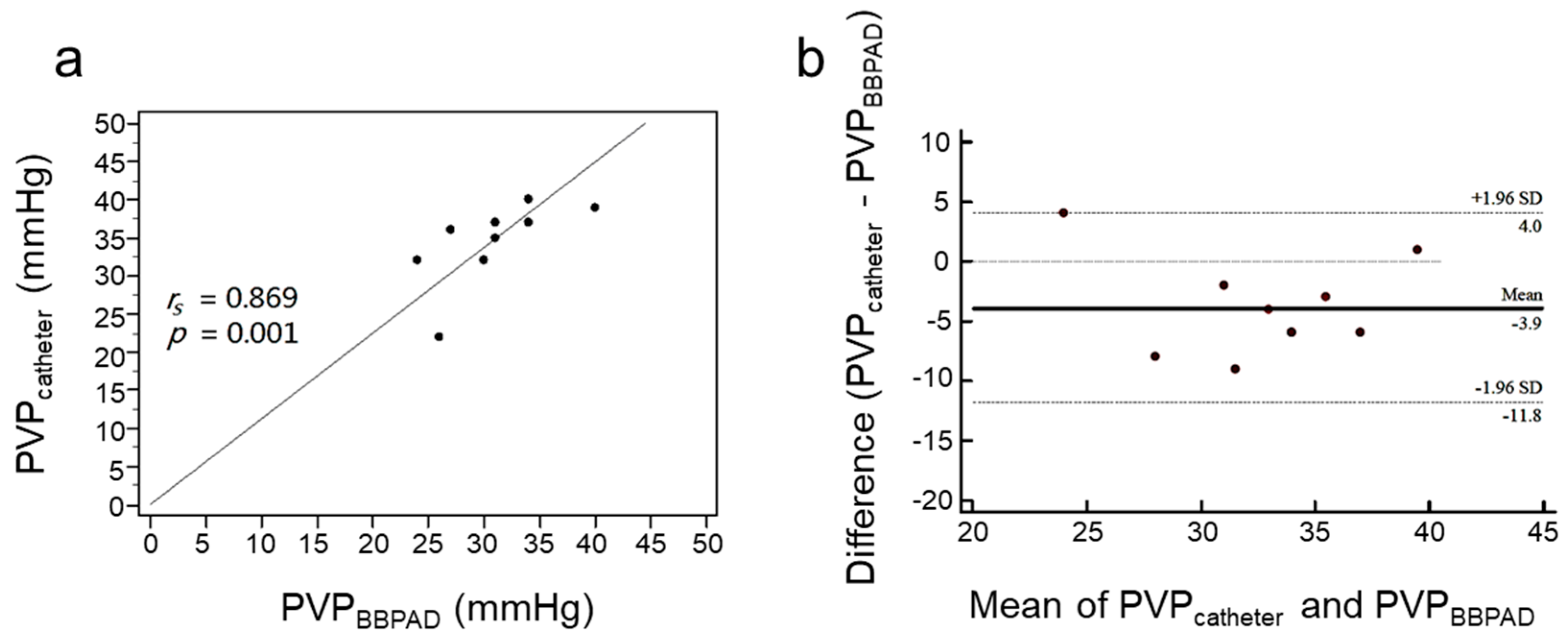
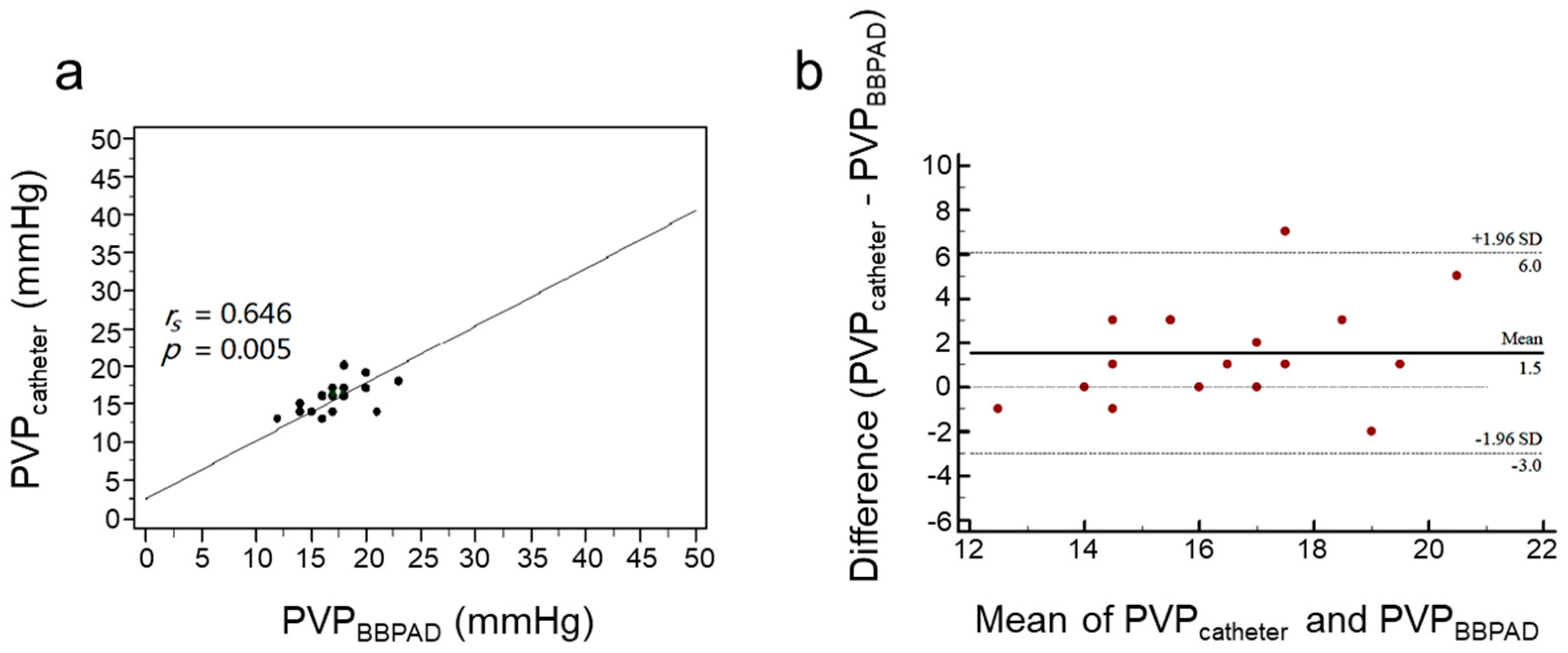
3.1. Assessment of PVP by PVPMD and Catheter
3.2. Repeatability and Reproducibility of the PVPMD-Measured PVP
4. Discussion
Limitation and Future Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar] [CrossRef] [PubMed]
- Ozden, I.; Yavru, H.A.; Durmaz, O.; Orhun, G.; Salmaslioglu, A.; Gulluoglu, M.; Alper, A.; Ibis, C.; Serin, K.R.; Onal, Z.; et al. Complementary Roles of Cadaveric and Living Donor Liver Transplantation in Acute Liver Failure. J. Gastrointest. Surg. 2021, 25, 2516–2523. [Google Scholar] [CrossRef]
- Shoreem, H.; Gad, E.H.; Soliman, H.; Hegazy, O.; Saleh, S.; Zakaria, H.; Ayoub, E.; Kamel, Y.; Abouelella, K.; Ibrahim, T.; et al. Small for size syndrome difficult dilemma: Lessons from 10 years single centre experience in living donor liver transplantation. World J. Hepatol. 2017, 9, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Kinaci, E.; Kayaalp, C. Portosystemic Shunts for “Too Small-for-Size Syndrome” After Liver Transplantation: A Systematic Review. World J. Surg. 2016, 40, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Raut, V.; Alikhanov, R.; Belghiti, J.; Uemoto, S. Review of the surgical approach to prevent small-for-size syndrome in recipients after left lobe adult LDLT. Surg. Today 2014, 44, 1189–1196. [Google Scholar] [CrossRef]
- Buob, S.; Johnston, A.N.; Webster, C.R. Portal hypertension: Pathophysiology, diagnosis, and treatment. J. Vet. Intern. Med. 2011, 25, 169–186. [Google Scholar] [CrossRef]
- Matsushima, H.; Fujiki, M.; Sasaki, K.; Rotroff, D.M.; Sands, M.; Bayona Molano, M.D.P.; Aucejo, F.; Diago Uso, T.; Eghtesad, B.; Miller, C.; et al. Predictive Value of Hepatic Venous Pressure Gradient for Graft Hemodynamics in Living Donor Liver Transplantation. Liver Transpl. 2019, 25, 1034–1042. [Google Scholar] [CrossRef]
- Dajti, E.; Alemanni, L.V.; Marasco, G.; Montagnani, M.; Azzaroli, F. Approaches to the Diagnosis of Portal Hypertension: Non-Invasive or Invasive Tests? Hepat. Med. 2021, 13, 25–36. [Google Scholar] [CrossRef]
- Groszmann, R.J.; Glickman, M.; Blei, A.T.; Storer, E.; Conn, H.O. Wedged and free hepatic venous pressure measured with a balloon catheter. Gastroenterology 1979, 76, 253–258. [Google Scholar] [CrossRef]
- Bochnakova, T. Hepatic Venous Pressure Gradient. Clin. Liver Dis. 2021, 17, 144–148. [Google Scholar] [CrossRef]
- Berzigotti, A.; Seijo, S.; Reverter, E.; Bosch, J. Assessing portal hypertension in liver diseases. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Merkel, C.; Montagnese, S. Hepatic venous pressure gradient measurement in clinical hepatology. Dig. Liver Dis. 2011, 43, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T.B.; Ito, S.; Iwatsuki, S. Measurement of portal pressure and its clinical application. Am. J. Med. 1970, 49, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Viallet, A.; Joly, J.G.; Marleau, D.; Lavoie, P. Comparison of free portal venous pressure and wedged hepatic venous pressure in patients with cirrhosis of the liver. Gastroenterology 1970, 59, 372–375. [Google Scholar] [CrossRef]
- Keiding, S.; Vilstrup, H. Intrahepatic heterogeneity of hepatic venous pressure gradient in human cirrhosis again. Scand. J. Gastroenterol. 2002, 37, 1344. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Cimbala, J.M. Fluid Mechanics: Fundamentals and Applications, 3rd ed.; McGraw Hill: New York, NY, USA, 2014; 1000p. [Google Scholar]
- Hall, J.E.; Hall, M.E. Vascular Distensibility and Functions of the Arterial and Venous Systems. In Guyton and Hall Textbook of Medical Physiology, 14th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020; pp. 183–192. [Google Scholar]
- Weinreb, J.; Kumari, S.; Phillips, G.; Pochaczevsky, R. Portal vein measurements by real-time sonography. AJR Am. J. Roentgenol. 1982, 139, 497–499. [Google Scholar] [CrossRef]
- Lafortune, M.; Marleau, D.; Breton, G.; Viallet, A.; Lavoie, P.; Huet, P.M. Portal venous system measurements in portal hypertension. Radiology 1984, 151, 27–30. [Google Scholar] [CrossRef]
- Fernandez-Bueno, I.; Di Lauro, S.; Alvarez, I.; Lopez, J.C.; Garcia-Gutierrez, M.T.; Fernandez, I.; Larra, E.; Pastor, J.C. Safety and Biocompatibility of a New High-Density Polyethylene-Based Spherical Integrated Porous Orbital Implant: An Experimental Study in Rabbits. J. Ophthalmol. 2015, 2015, 904096. [Google Scholar] [CrossRef]
- Smith, D.G.; Schenk, M.P. A Dissection Guide & Atlas to the Fetal Pig, 3rd ed.; Morton Publishing: Englewood, CO, USA, 2011; 132p. [Google Scholar]
- Marcelino, P.; Germano, N.; Marum, S.; Fernandes, A.P.; Ribeiro, P.; Lopes, M.G. Haemodynamic parameters obtained by transthoracic echocardiography and Swan-Ganz catheter: A comparative study in liver transplant patients. Acta Med. Port. 2006, 19, 197–205. [Google Scholar]
- Wise, E.S.; Hocking, K.M.; Polcz, M.E.; Beilman, G.J.; Brophy, C.M.; Sobey, J.H.; Leisy, P.J.; Kiberenge, R.K.; Alvis, B.D. Hemodynamic Parameters in the Assessment of Fluid Status in a Porcine Hemorrhage and Resuscitation Model. Anesthesiology 2021, 134, 607–616. [Google Scholar] [CrossRef]
- Cherpanath, T.G.; Geerts, B.F.; Lagrand, W.K.; Schultz, M.J.; Groeneveld, A.B. Basic concepts of fluid responsiveness. Neth. Heart J. 2013, 21, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Hauke, J.; Kossowski, T. Comparison of Values of Pearson’s and Spearman’s Correlation Coefficients on The Same Sets of Data. Quaest. Geogr. 2011, 30, 87–93. [Google Scholar] [CrossRef]
- Zayat, R.; Goetzenich, A.; Lee, J.Y.; Kang, H.; Jansen-Park, S.H.; Schmitz-Rode, T.; Musetti, G.; Schnoering, H.; Autschbach, R.; Hatam, N.; et al. Comparison between radial artery tonometry pulse analyzer and pulsed-Doppler echocardiography derived hemodynamic parameters in cardiac surgery patients: A pilot study. PeerJ 2017, 5, e4132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bunce, C. Correlation, agreement, and Bland-Altman analysis: Statistical analysis of method comparison studies. Am. J. Ophthalmol. 2009, 148, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singapore Med. J. 2003, 44, 614–619. [Google Scholar] [PubMed]
- Ferrusquia-Acosta, J.; Bassegoda, O.; Turco, L.; Reverter, E.; Pellone, M.; Bianchini, M.; Perez-Campuzano, V.; Ripoll, E.; Garcia-Criado, A.; Graupera, I.; et al. Agreement between wedged hepatic venous pressure and portal pressure in non-alcoholic steatohepatitis-related cirrhosis. J. Hepatol. 2021, 74, 811–818. [Google Scholar] [CrossRef]
- Leung, J.C.; Loong, T.C.; Pang, J.; Wei, J.L.; Wong, V.W. Invasive and non-invasive assessment of portal hypertension. Hepatol. Int. 2018, 12, 44–55. [Google Scholar] [CrossRef]
- Singal, A.K.; Ahmad, M.; Soloway, R.D. Duplex Doppler ultrasound examination of the portal venous system: An emerging novel technique for the estimation of portal vein pressure. Dig. Dis. Sci. 2010, 55, 1230–1240. [Google Scholar] [CrossRef]
- Yao, H.; Wang, Y. Relationship between hemodynamic parameters and portal venous pressure in cirrhosis patients with portal hypertension. Open Life Sci. 2020, 15, 981–987. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Cimbala, J.M. Bernoulli and Energy Equation. In Fluid Mechanics: Fundamentals and Applications, 3rd ed.; McGraw Hill: New York, NY, USA, 2014; p. 185. [Google Scholar]
- Krause, E. Fluid Mechanics: With Problems and Solutions, and an Aerodynamic Laboratory; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005; 354p. [Google Scholar]
- Feng, A.C.; Chen, T.W.; Fan, H.L.; Yu, J.C.; Hsieh, C.B. The Correlation of Hepatic and Systemic Hemodynamics During Liver Transplantation: Quantification of Hepatic Resistance as an Actual Value. Medicine 2015, 94, e1815. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.D.; Cheng, Y.F.; Chen, T.Y.; Tsang, L.L.; Ou, H.Y.; Yu, C.Y.; Hsu, H.W.; Chen, C.L.; Concejero, A.M.; Huang, T.L. Portal venous pressure in adult living donor liver transplantation. Transplant. Proc. 2014, 46, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Sholkamy, A.; Salman, A.; El-Garem, N.; Hosny, K.; Abdelaziz, O. Portal venous pressure and proper graft function in living donor liver transplants in 69 patients from an Egyptian center. Ann. Saudi Med. 2018, 38, 181–188. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, M.A.; Olde Damink, S.W.; Dejong, C.H.; Lang, H.; Malago, M.; Jalan, R.; Saner, F.H. Liver failure after partial hepatic resection: Definition, pathophysiology, risk factors and treatment. Liver Int. 2008, 28, 767–780. [Google Scholar] [CrossRef]
- Bruix, J.; Castells, A.; Bosch, J.; Feu, F.; Fuster, J.; Garcia-Pagan, J.C.; Visa, J.; Bru, C.; Rodes, J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: Prognostic value of preoperative portal pressure. Gastroenterology 1996, 111, 1018–1022. [Google Scholar] [CrossRef]
- Hidaka, M.; Takatsuki, M.; Soyama, A.; Tanaka, T.; Muraoka, I.; Hara, T.; Kuroki, T.; Kanematsu, T.; Eguchi, S. Intraoperative portal venous pressure and long-term outcome after curative resection for hepatocellular carcinoma. Br. J. Surg. 2012, 99, 1284–1289. [Google Scholar] [CrossRef]
- Biecker, E. Diagnosis and therapy of ascites in liver cirrhosis. World J. Gastroenterol. 2011, 17, 1237–1248. [Google Scholar] [CrossRef]
- Cantre, D.; Schuett, H.; Hildebrandt, A.; Dold, S.; Menger, M.D.; Vollmar, B.; Eipel, C. Nitric oxide reduces organ injury and enhances regeneration of reduced-size livers by increasing hepatic arterial flow. Br. J. Surg. 2008, 95, 785–792. [Google Scholar] [CrossRef]
- Morsiani, E.; Aleotti, A.; Ricci, D. Haemodynamic and ultrastructural observations on the rat liver after two-thirds partial hepatectomy. J. Anat. 1998, 192 Pt 4, 507–515. [Google Scholar] [CrossRef]
- Eipel, C.; Abshagen, K.; Vollmar, B. Regulation of hepatic blood flow: The hepatic arterial buffer response revisited. World J. Gastroenterol. 2010, 16, 6046–6057. [Google Scholar] [CrossRef]
- Bogner, A.; Reissfelder, C.; Striebel, F.; Mehrabi, A.; Ghamarnejad, O.; Rahbari, M.; Weitz, J.; Rahbari, N.N. Intraoperative Increase of Portal Venous Pressure is an Immediate Predictor of Posthepatectomy Liver Failure After Major Hepatectomy: A Prospective Study. Ann. Surg. 2021, 274, e10–e17. [Google Scholar] [CrossRef] [PubMed]



| 1st Experiment | 2nd Experiment | 3rd Experiment | |
|---|---|---|---|
| Weight (kg) | 44.5 | 46.8 | 45.7 |
| Number of measurements (n) | 11 | 10 | 17 |
| Central venous pressure (mmHg), mean ± SD | 4.4 ± 0.7 | 4.7 ± 0.7 | 4.8 ± 0.5 |
| Portal pressure via PVPMD (mmHg), mean ± SD | 35.7 ± 4.4 | 30.8 ± 4.68 | 17.2 ± 2.7 |
| Catheter site | Main trunk | Mesenteric vein | Intrahepatic part |
| Portal pressure via catheter (mmHg), mean ± SD | 31.5 ± 5.3 | 34.7 ± 5.2 | 15.7 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, K.-C.; Huang, T.-S.; Lin, J.-C.; Chiang, H.K. Development of a Direct Non-Puncture Device for Measuring Portal Venous Pressure during Liver Transplantation—A Swine Model. Biosensors 2023, 13, 1007. https://doi.org/10.3390/bios13121007
Ho K-C, Huang T-S, Lin J-C, Chiang HK. Development of a Direct Non-Puncture Device for Measuring Portal Venous Pressure during Liver Transplantation—A Swine Model. Biosensors. 2023; 13(12):1007. https://doi.org/10.3390/bios13121007
Chicago/Turabian StyleHo, Kung-Chen, Tun-Sung Huang, Jiunn-Chang Lin, and Huihua Kenny Chiang. 2023. "Development of a Direct Non-Puncture Device for Measuring Portal Venous Pressure during Liver Transplantation—A Swine Model" Biosensors 13, no. 12: 1007. https://doi.org/10.3390/bios13121007
APA StyleHo, K.-C., Huang, T.-S., Lin, J.-C., & Chiang, H. K. (2023). Development of a Direct Non-Puncture Device for Measuring Portal Venous Pressure during Liver Transplantation—A Swine Model. Biosensors, 13(12), 1007. https://doi.org/10.3390/bios13121007






