Design of a Facile Antifouling Sensor Based on the Synergy between an Antibody and Phase-Transited BSA
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
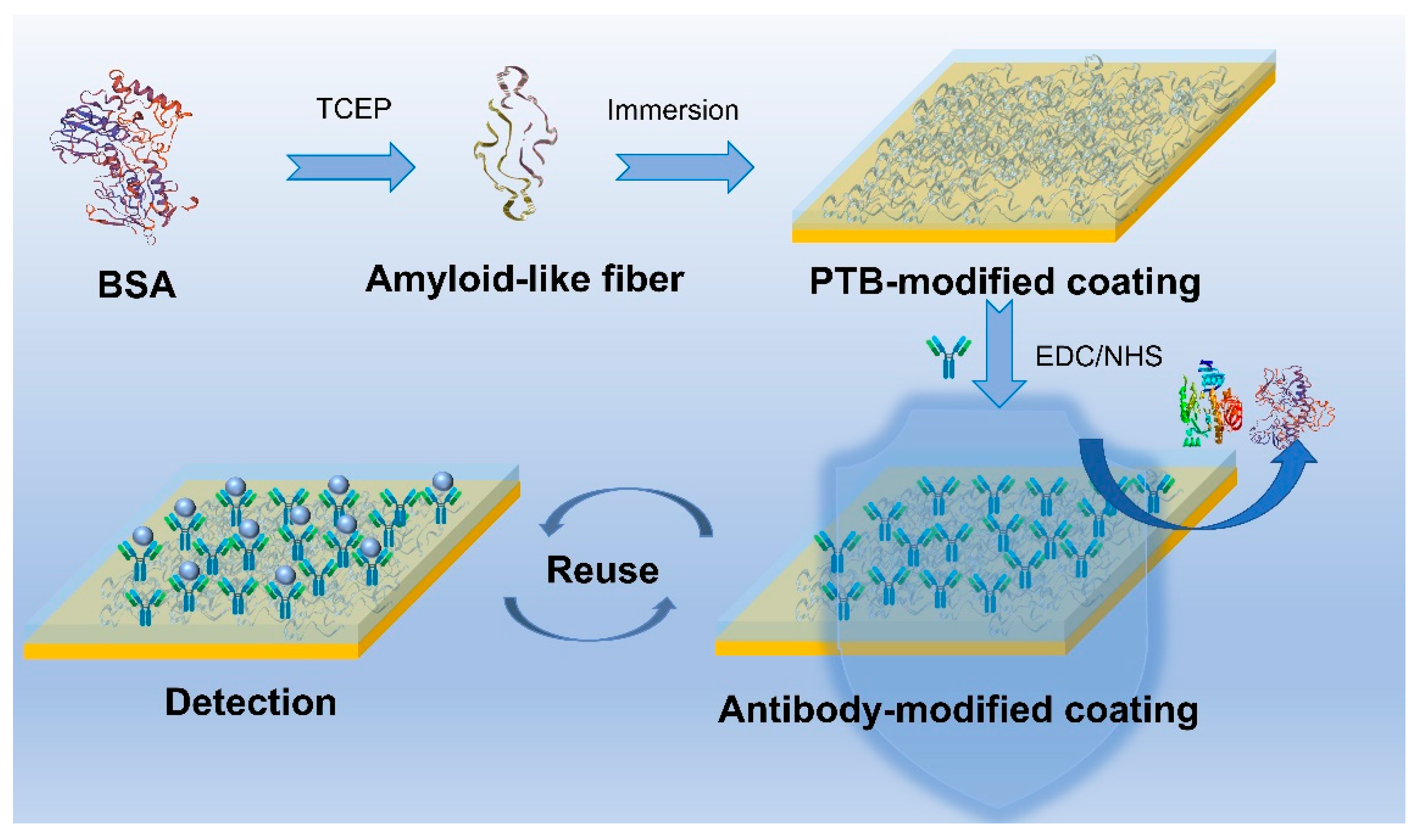
2.2. Modification of Phase-Transited BSA on Au Surfaces
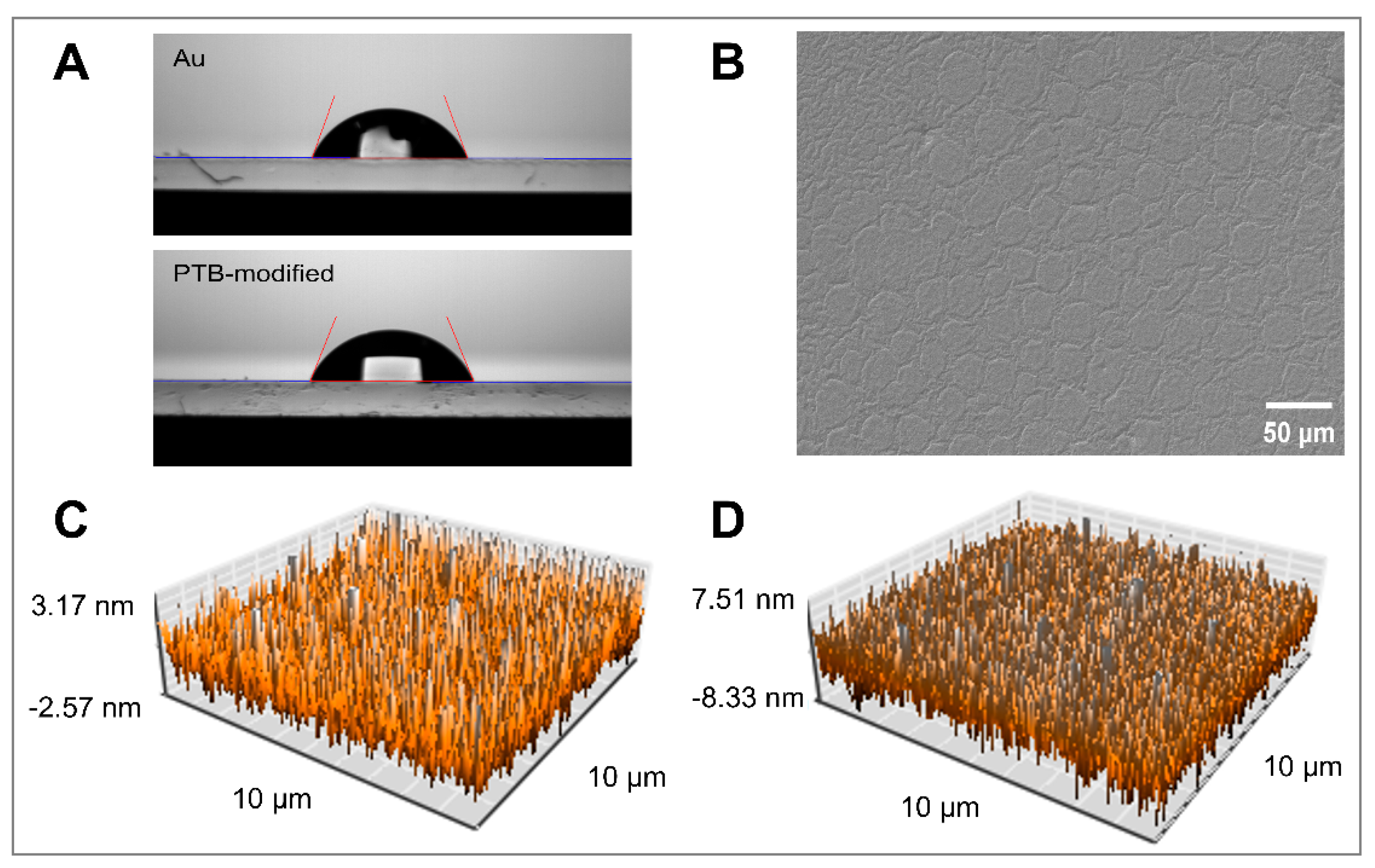
2.3. Morphology and Force Curve Measured by AFM
2.4. Contact Angle Measurements
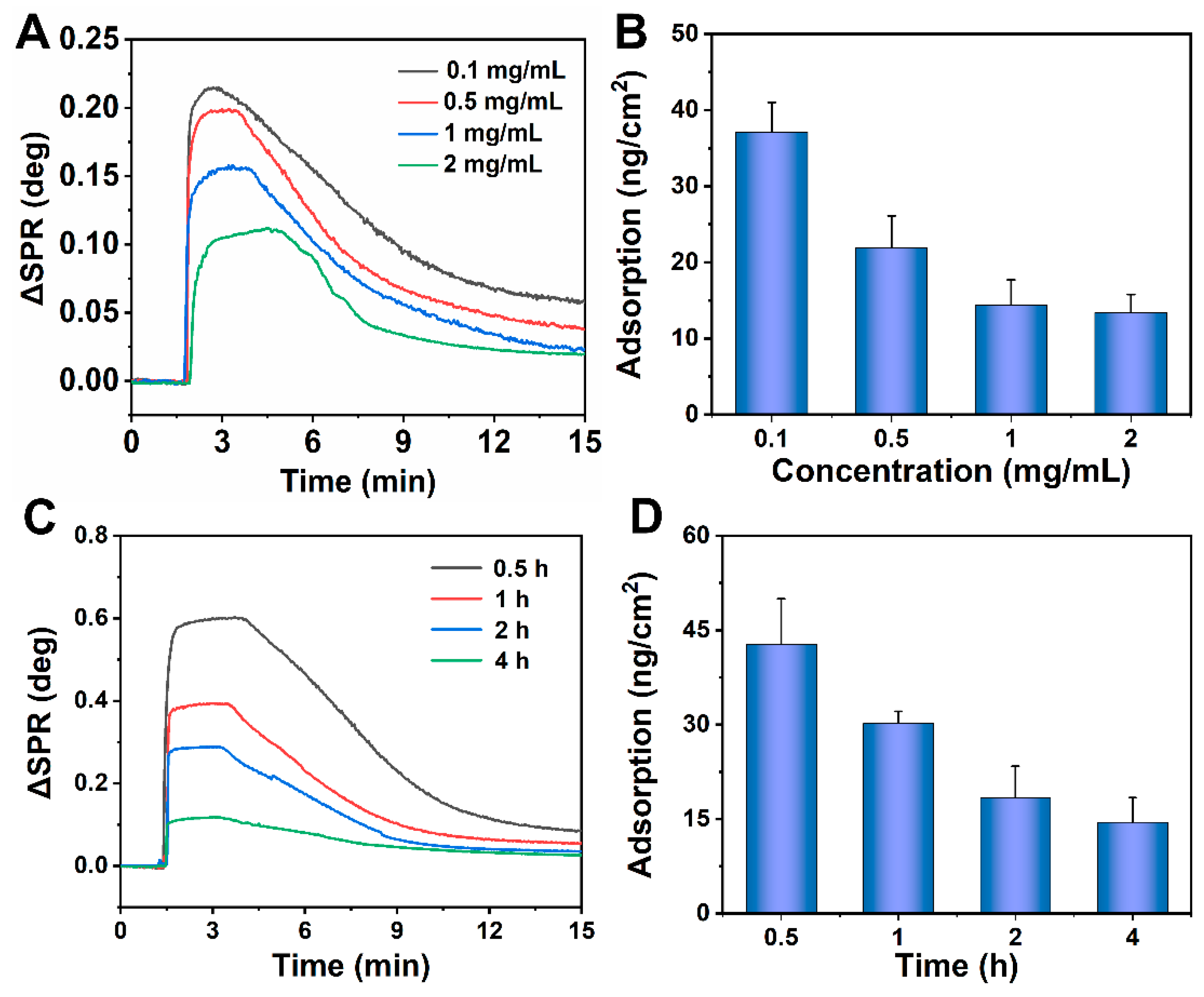
2.5. Measurements of Antifouling Performance on the Phase-Transited BSA Surface
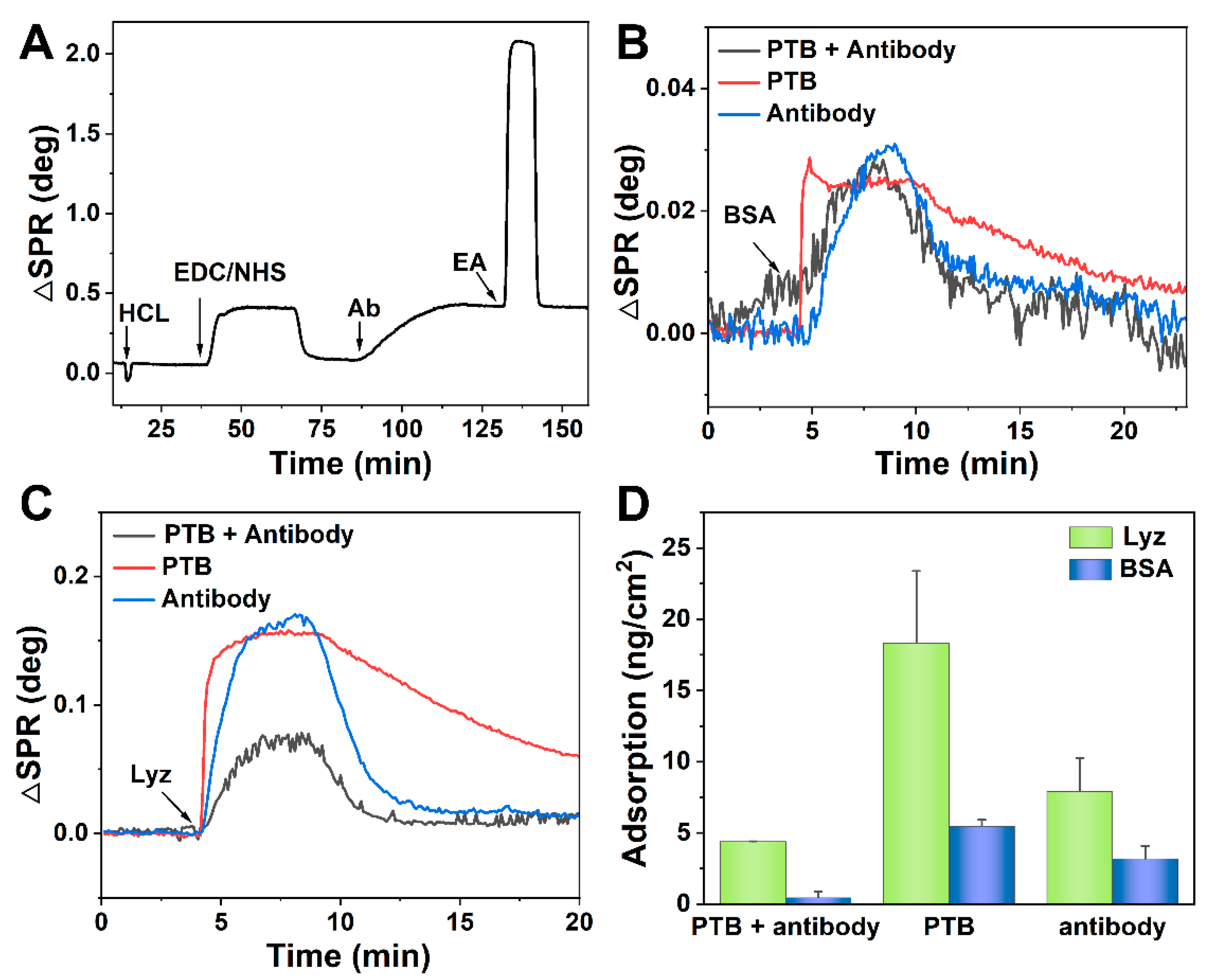
2.6. Fabrication and Evaluation of the Specific SPR Sensor
3. Results and Discussion
3.1. Characterization of PTB-Modified Surfaces
3.2. Nonspecific Adsorption on Modified Surfaces
3.3. Fabrication and Evaluation of Antifouling Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashem, A.; Hossain, M.A.M.; Marlinda, A.R.; Al Mamun, M.; Sagadevan, S.; Shahnavaz, Z.; Simarani, K.; Johan, M.R. Nucleic acid-based electrochemical biosensors for rapid clinical diagnosis: Advances, challenges, and opportunities. Crit. Rev. Clin. Lab. Sci. 2022, 59, 156–177. [Google Scholar] [CrossRef] [PubMed]
- Nnachi, R.C.; Sui, N.; Ke, B.; Luo, Z.; Bhalla, N.; He, D.; Yang, Z. Biosensors for rapid detection of bacterial pathogens in water, food and environment. Environ. Int. 2022, 166, 107357. [Google Scholar] [CrossRef] [PubMed]
- Griesche, C.; Baeumner, A.J. Biosensors to support sustainable agriculture and food safety. TrAC-Trends Anal. Chem. 2020, 128, 115906. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Xia, Y.; Su, R.; Huang, R.; Ding, L.; Wang, L.; Qi, W.; He, Z. Design of elution strategy for simultaneous detection of chloramphenicol and gentamicin in complex samples using surface plasmon resonance. Biosens. Bioelectron. 2017, 92, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Wang, Q.; Zou, L.; Zheng, Y.; Liu, X.; Yang, X.; Wang, K. Low-Fouling Surface Plasmon Resonance Sensor for Highly Sensitive Detection of MicroRNA in a Complex Matrix Based on the DNA Tetrahedron. Anal. Chem. 2018, 90, 12584–12591. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Shi, S.; Huang, R.; Su, R.; Qi, W.; He, Z. Design and mechanisms of antifouling materials for surface plasmon resonance sensors. Acta Biomater. 2016, 40, 100–118. [Google Scholar] [CrossRef]
- Jarvas, G.; Guttman, A.; Miekus, N.; Baczek, T.; Jeong, S.; Chung, D.S.; Patoprsty, V.; Masar, M.; Hutta, M.; Datinska, V.; et al. Practical sample pretreatment techniques coupled with capillary electrophoresis for real samples in complex matrices. TrAC-Trends Anal. Chem. 2020, 122, 115702. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J.J. Antifouling Strategies for Selective In Vitro and In Vivo Sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef]
- Lin, P.-H.; Li, B.-R. Antifouling strategies in advanced electrochemical sensors and biosensors. Analyst 2020, 145, 1110–1120. [Google Scholar] [CrossRef]
- Su, R.; Xia, Y.; Li, C.; Ye, H.; Duan, Y.; Huang, R. Sensing Interfaces: Antifouling Materials for Sensors; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Desai, N.P.; Hubbell, J.A. Solution technique to incorporate polyethylene oxide and other water-soluble polymers into surfaces of polymeric biomaterials. Biomaterials 1991, 12, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, M.; Zhao, Z.; Li, S.; Cheng, Z.; Zhu, J. Controlled hierarchical architecture in poly [oligo (ethylene glycol) methacrylate-b-glycidyl methacrylate] brushes for enhanced label-free biosensing. Appl. Surf. Sci. 2018, 450, 236–243. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, M.; Zhou, W.; Zhang, B.; Cheng, Z.; Huang, J.; Zhang, M.; Wang, Z.; Wang, R.; Chen, Z.; et al. Kinetic and high-throughput profiling of epigenetic interactions by 3D-carbene chip-based surface plasmon resonance imaging technology. Proc. Natl. Acad. Sci. USA 2017, 114, 7245–7254. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Johnson, R.W.; Desai, T.A. Evaluation of the stability of nonfouling ultrathin poly(ethylene glycol) films for silicon-based microdevices. Langmuir 2004, 20, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Jiang, S. Integrated Antimicrobial and Nonfouling Zwitterionic Polymers. Angew. Chem.-Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jiang, S. Super-hydrophilic zwitterionic poly(carboxybetaine) and amphiphilic non-ionic poly(ethylene glycol) for stealth nanoparticles. Nano Today 2012, 7, 404–413. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, J.; Li, X.; Wu, J.; Chen, S.; Chen, Q.; Wang, Q.; Gong, X.; Li, L.; Zheng, J. Probing structure-antifouling activity relationships of polyacrylamides and polyacrylates. Biomaterials 2013, 34, 4714–4724. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Y.; Miao, S.; Yang, Q.; Hu, X.; Han, Q.; Xue, L.; Yang, P. Amyloid-like protein aggregates combining antifouling with antibacterial activity. Biomater. Sci. 2020, 8, 6903–6911. [Google Scholar] [CrossRef]
- Ma, G.J.; Ferhan, A.R.; Jackman, J.A.; Cho, N.-J. Conformational flexibility of fatty acid-free bovine serum albumin proteins enables superior antifouling coatings. Commun. Mater. 2020, 1, 45. [Google Scholar] [CrossRef]
- del Rio, J.S.; Henry, O.Y.F.; Jolly, P.; Ingber, D.E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 2019, 14, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, K.; Tian, J.; Gao, A.; Tian, L.; Su, H.; Miao, S.; Tao, F.; Ren, H.; Yang, Q.; et al. Synthesis of robust underwater glues from common proteins via unfolding-aggregating strategy. Nat. Commun. 2023, 14, 5145. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tian, J.; Li, C.; Su, H.; Qin, R.; Wang, Y.; Cao, X.; Yang, P. Amyloid-Like Protein Aggregates: A New Class of Bioinspired Materials Merging an Interfacial Anchor with Antifouling. Adv. Mater. 2020, 32, 2000128. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, R.; Yang, X.; Ambrosi, A.; Luo, X. Ultralow fouling electrochemical detection of uric acid directly in serum based on phase-transited bovine serum albumin and conducting polymer. Chin. Chem. Lett. 2023, 34, 108314. [Google Scholar] [CrossRef]
- de los Santos Pereira, A.; Riedel, T.; Brynda, E.; Rodriguez-Emmenegger, C. Hierarchical antifouling brushes for biosensing applications. Sens. Actuators B-Chem. 2014, 202, 1313–1321. [Google Scholar] [CrossRef]
- Vaisocherova, H.; Sevcu, V.; Adam, P.; Spackova, B.; Hegnerova, K.; Pereira, A.d.l.S.; Rodriguez-Emmenegger, C.; Riedel, T.; Houska, M.; Brynda, E.; et al. Functionalized ultra-low fouling carboxy- and hydroxy-functional surface platforms: Functionalization capacity, biorecognition capability and resistance to fouling from undiluted biological media. Biosens. Bioelectron. 2014, 51, 150–157. [Google Scholar] [CrossRef]
- Vaisocherova, H.; Yang, W.; Zhang, Z.; Cao, Z.; Cheng, G.; Piliarik, M.; Homola, J.; Jiang, S. Ultralow fouling and functionalizable surface chemistry based on a zwitterionic polymer enabling sensitive and specific protein detection in undiluted blood plasma. Anal. Chem. 2008, 80, 7894–7901. [Google Scholar] [CrossRef]
- Hedayati, M.; Marruecos, D.F.; Krapf, D.; Kaar, J.L.; Kipper, M.J. Protein adsorption measurements on low fouling and ultralow fouling surfaces: A critical comparison of surface characterization techniques. Acta Biomater. 2020, 102, 169–180. [Google Scholar] [CrossRef]
- Xia, Y.; Adibnia, V.; Shan, C.; Huang, R.; Qi, W.; He, Z.; Xie, G.; Olszewski, M.; De Crescenzo, G.; Matyjaszewski, K.; et al. Synergy between Zwitterionic Polymers and Hyaluronic Acid Enhances Antifouling Performance. Langmuir 2019, 35, 15535–15542. [Google Scholar] [CrossRef]
- Xing, C.-M.; Meng, F.-N.; Quan, M.; Ding, K.; Dang, Y.; Gong, Y.-K. Quantitative fabrication, performance optimization and comparison of PEG and zwitterionic polymer antifouling coatings. Acta Biomater. 2017, 59, 129–138. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Dong, X.; Li, J.; Liu, J.; Ruan, Y.; Xia, Y. Design of a Facile Antifouling Sensor Based on the Synergy between an Antibody and Phase-Transited BSA. Biosensors 2023, 13, 1004. https://doi.org/10.3390/bios13121004
Wang S, Dong X, Li J, Liu J, Ruan Y, Xia Y. Design of a Facile Antifouling Sensor Based on the Synergy between an Antibody and Phase-Transited BSA. Biosensors. 2023; 13(12):1004. https://doi.org/10.3390/bios13121004
Chicago/Turabian StyleWang, Siqi, Xinru Dong, Jialu Li, Jialei Liu, Yifei Ruan, and Yinqiang Xia. 2023. "Design of a Facile Antifouling Sensor Based on the Synergy between an Antibody and Phase-Transited BSA" Biosensors 13, no. 12: 1004. https://doi.org/10.3390/bios13121004
APA StyleWang, S., Dong, X., Li, J., Liu, J., Ruan, Y., & Xia, Y. (2023). Design of a Facile Antifouling Sensor Based on the Synergy between an Antibody and Phase-Transited BSA. Biosensors, 13(12), 1004. https://doi.org/10.3390/bios13121004



