Detection of Reverse Transcriptase LAMP-Amplified Nucleic Acid from Oropharyngeal Viral Swab Samples Using Biotinylated DNA Probes through a Lateral Flow Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. SARS-CoV-2 Swab Sample
2.2. Reverse Transcriptase LAMP
2.3. RT-LAMP Programme
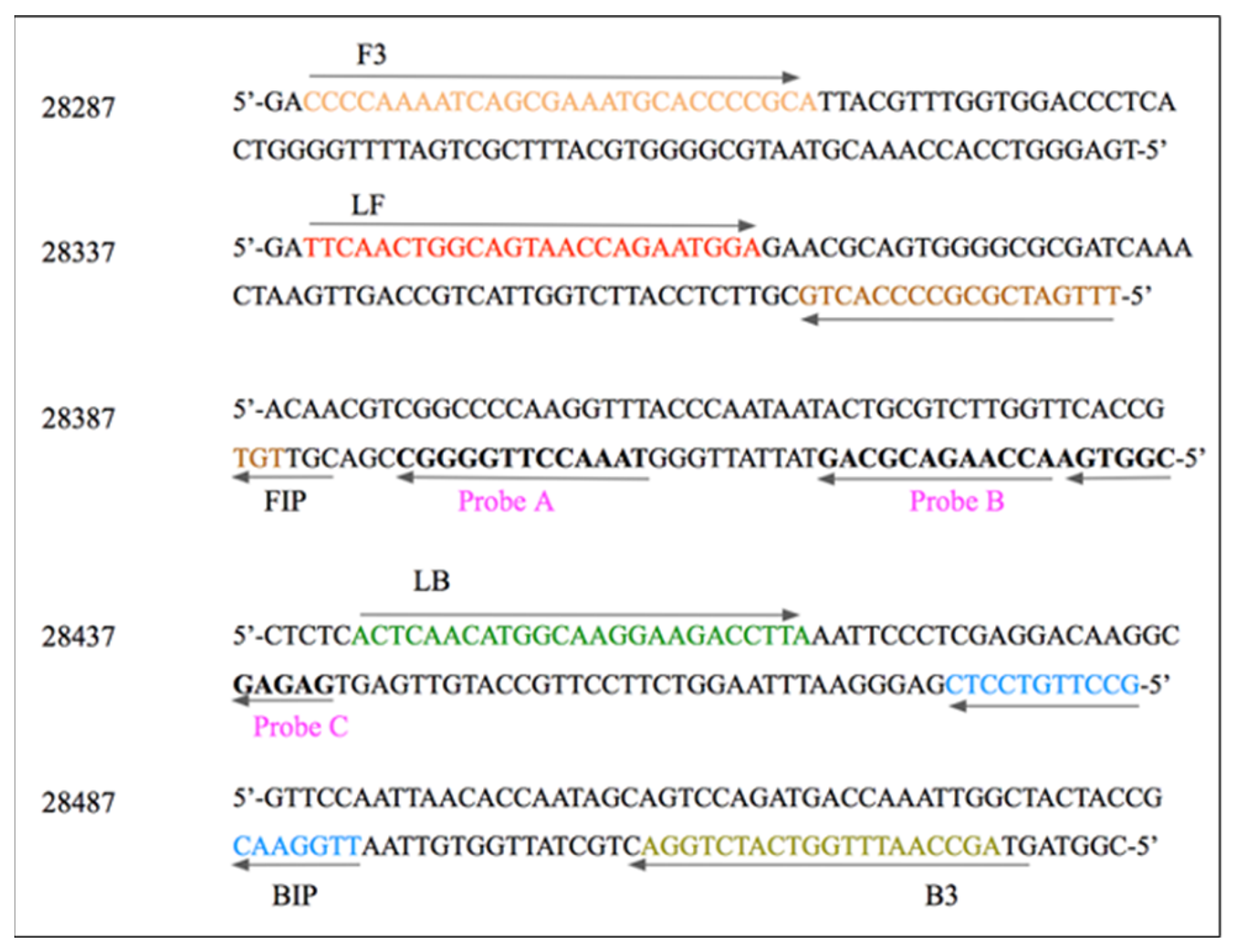
2.4. N-Gene-Specific Probe Design
2.5. Biotin-Labelled Probe Hybridization with the RT-LAMP Amplicon
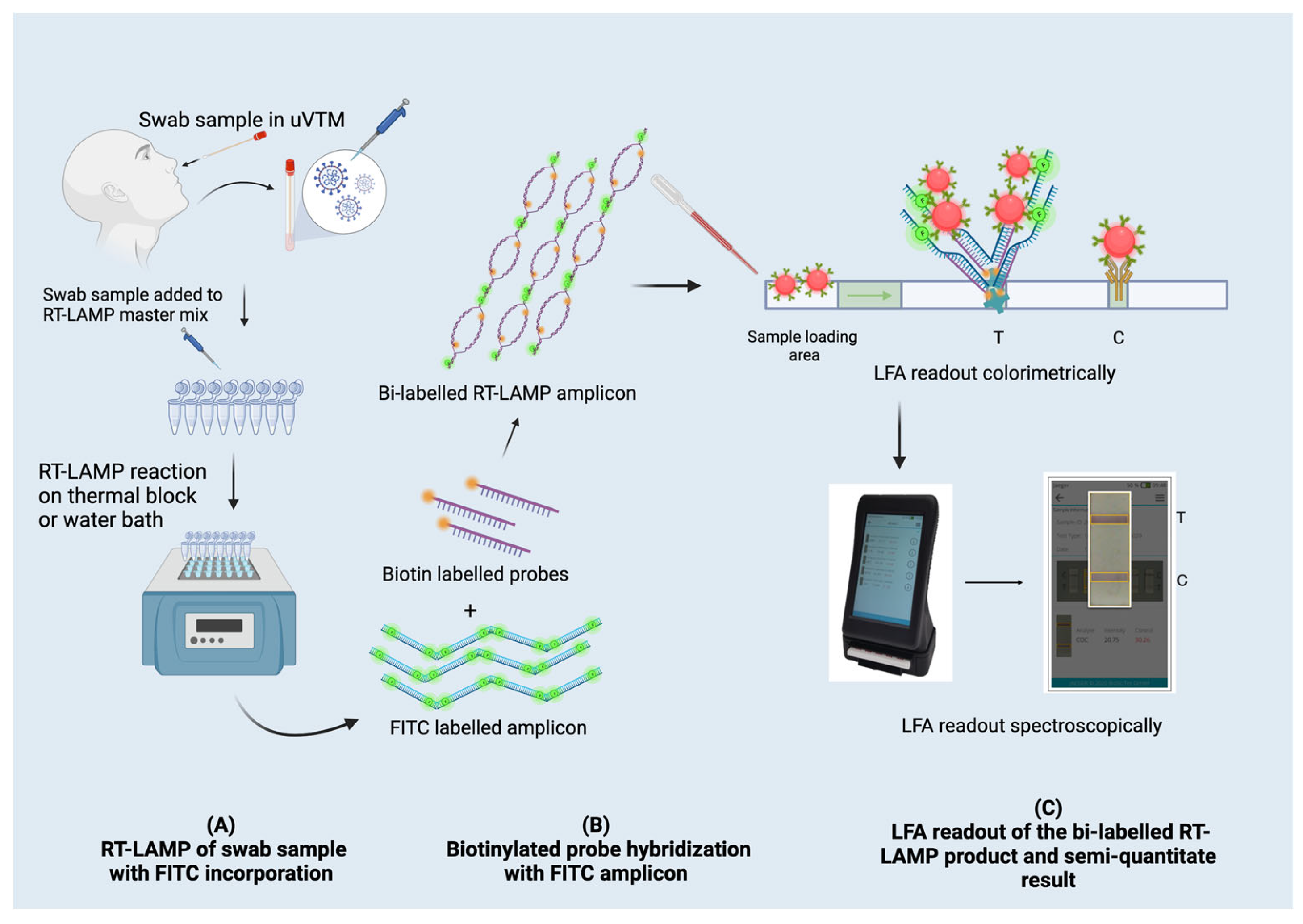
2.6. Lateral Flow Assay
2.7. Agarose Gel Electrophoresis
2.8. Smartphone-Based In Vitro Diagnostic Device-Based LFA Readout
2.9. Analytical Efficacy Calculations
3. Results and Discussion
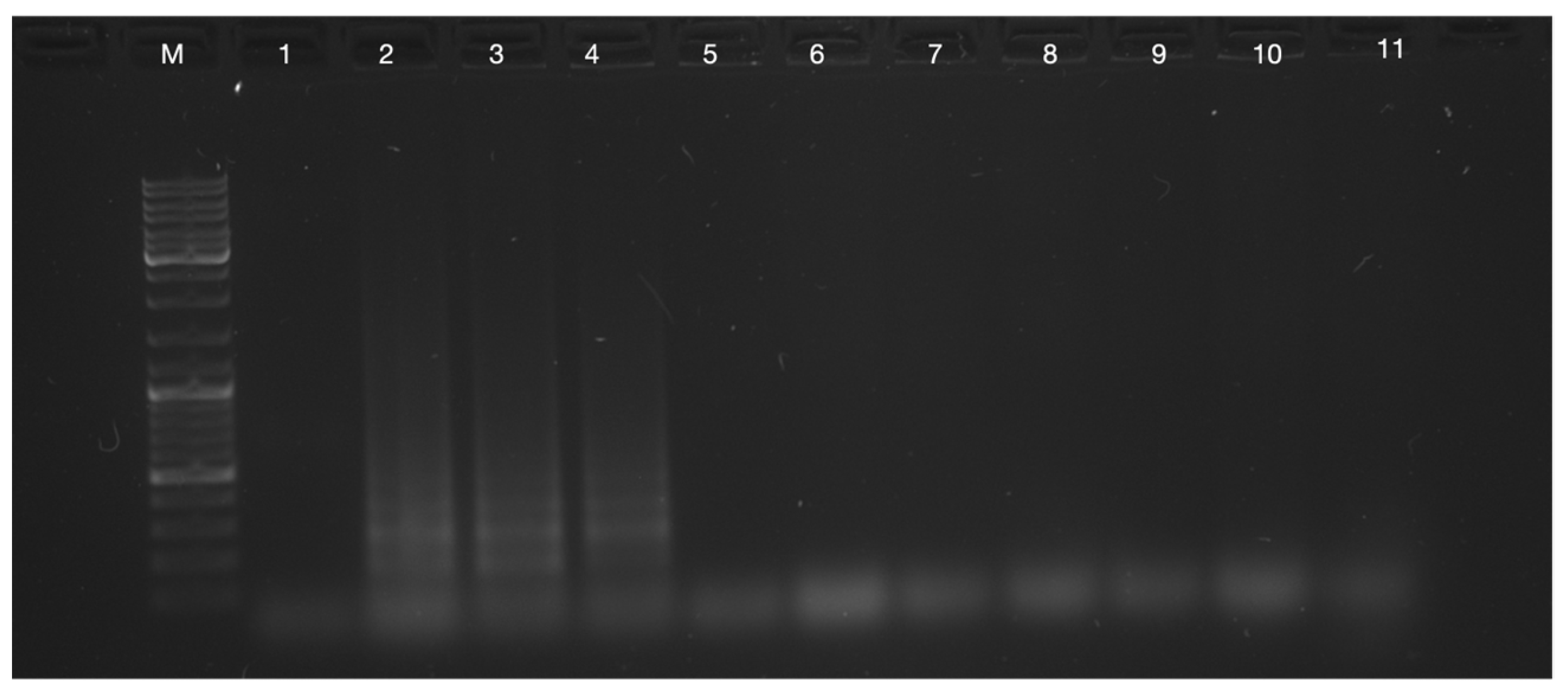
3.1. RT-LAMP for SARS-CoV-2 Positive and Negative Throat Swab Samples as Template Optimisation
Optimisation of Processing Times for Swab Sample Amplification with RT-LAMP
3.2. Bi-Labelled RT-LAMP-Amplified Product for an LFA Readout
3.2.1. Hybridization of Biotin-Labelled Probes with FITC-Labelled RT-LAMP Products
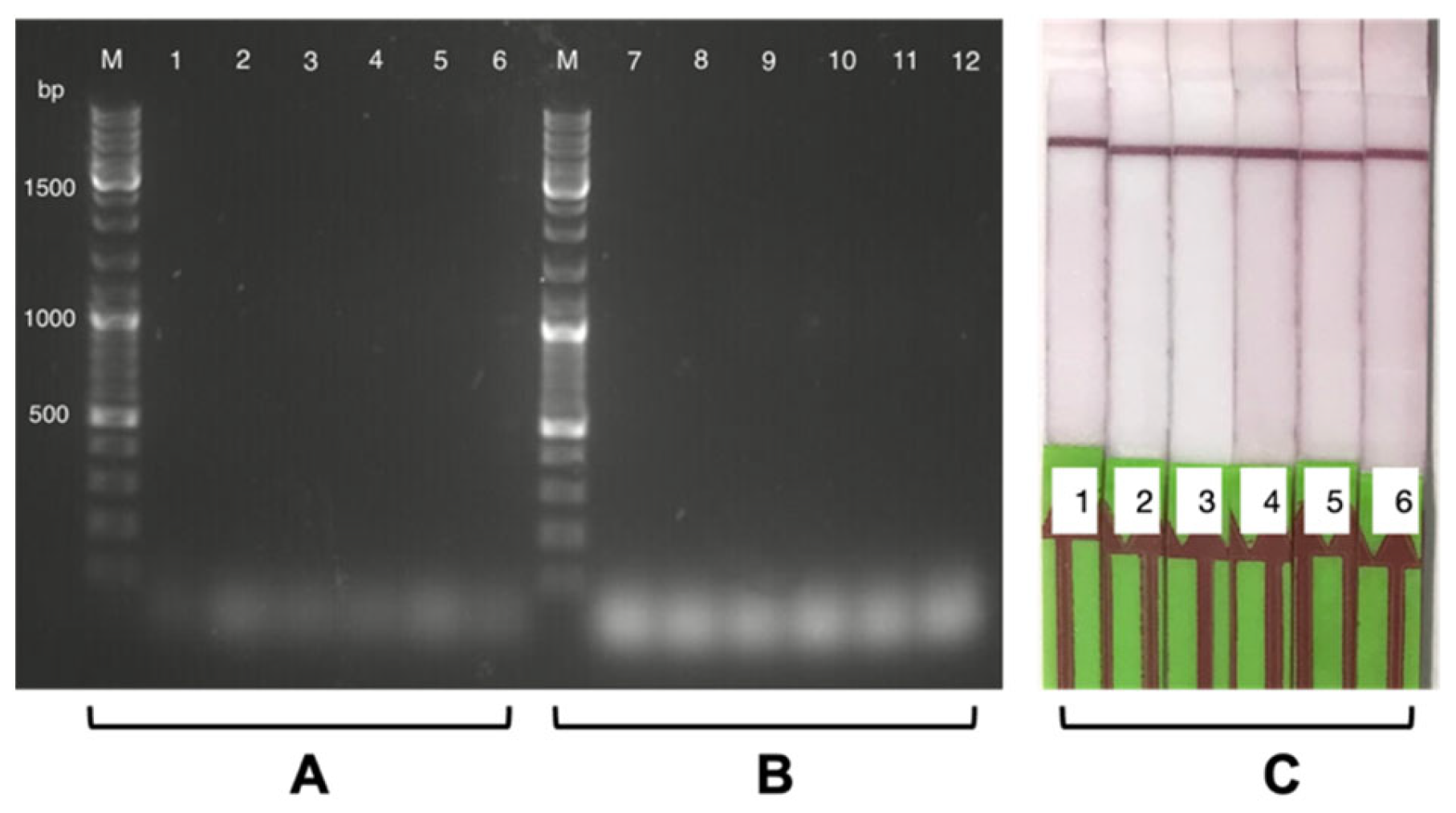
3.2.2. Individual Specificity Test for Each Probe
3.2.3. Positive and Negative Swab Sample RT-LAMP-LFA Readouts
3.3. Analytical Efficacy
3.4. Digitalization of the LFA Test Band Intensity Readout Using a Smartphone-Based IVD Device
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primers | Sequences (5′ -> 3′) | Length (nt) |
|---|---|---|
| F3 | CCCAAAATCAGCGAAATGCA | 20 |
| B3 | AGCCAATTTGGTCATCTGGA | 20 |
| FIP | TGTTTTGATCGCGCCCCACTGATTACGTTTGGTGGACCCTC | 41 |
| BIP | TGCGTCTTGGTTCACCGCTCATTGGAACGCCTTGTCCTC | 39 |
| FITC-LF | FITC—TCCATTCTGGTTACTGCCAGTTGAA | 25 |
| LB | ACTCAACATGGCAAGGAAGACCTTA | 25 |
| Fwd_PCR | GACCCCAAAATCAGCGAAAT | 20 |
| Rev_PCR | TGTAGCACGATTGCAGCATTG | 20 |
| Reagent | Stock Concentration | End Concentration | Company |
|---|---|---|---|
| 10X Isothermal buffer | 10 X | 1 X | New England BioLabs Inc. |
| MgSO4 | 100 mM | 6 mM | |
| ATP | 10 mM | 0.4 mM | |
| dNTP mix | 40 mM | 5 mM | |
| F3 Primer | 10 µM | 0.6 µM | Biomers.net GmbH |
| B3 Primer | 10 µM | 1 µM | |
| FIP Primer | 10 µM | 0.6 µM | |
| BIP Primer | 10 µM | 0.6 µM | |
| FITC-LF Primer | 20 µM | 1 µM | |
| LB Primer | 10 µM | 0.6 µM | |
| Tte UvrD Helicase | 20 µg/ml | 0.8 µg/ml | New England BioLabs Inc. |
| WarmStart® RTx Reverse Transcriptase | 15 U/µL | 0.6 U/µL | |
| Bst 3.0 DNA polymerase | 8 U/µL | 0.32 U/µL | |
| Nuclease free PCR grade water | — | — | Roth Industries Inc. |
| Swab Sample | CT 18 to CT 32 | In.vent Diagnostics GmbH | |
| Lid Temperature 72 °C | ||
|---|---|---|
| Step | Temperature (°C) | Duration (min) |
| Annealing | 65 | 20 |
| Inactivation | 83 | 2 |
| Cooling | 4 | 2 |
| Probe | Sequences (5′ → 3′) | Tm (°C) | Length (nt) | GC % |
|---|---|---|---|---|
| A | Biotin-TAAACCTTGGGGC | 40 | 13 | 53.8 |
| B | Biotin-ACCAAGACGCAG | 38 | 12 | 58.3 |
| C | Biotin-GAGAGCGGTGA | 36 | 11 | 63.6 |
| Step | Temperature (°C) | Duration (min) | |
|---|---|---|---|
| 1. Denaturation of amplified products | 52 | 2 | |
| 2. Biotin probe (0.25 μM) | Probe A | 46 | 2 |
| Probe B | 39 | 2 | |
| Probe C | 39 | 2 | |
| All 3 probes | 46 | 2 | |
| 3. Cooling | RT | 5 | |
References
- Gupta, R.; Sagar, P.; Priyadarshi, N.; Kaul, S.; Sandhir, R.; Rishi, V.; Singhal, N.K. Nanotechnology-Based Approaches for the Detection of SARS-CoV-2. Front. Nanotechnol. 2020, 2, 589832. [Google Scholar] [CrossRef]
- Mina, M.J.; Parker, R.; Larremore, D.B. Rethinking COVID-19 Test Sensitivity—A Strategy for Containment. N. Engl. J. Med. 2020, 383, e120. [Google Scholar] [CrossRef] [PubMed]
- Alwan, N.A. Surveillance Is Underestimating the Burden of the COVID-19 Pandemic. Lancet 2020, 396, e24. [Google Scholar] [CrossRef]
- Mouliou, D.S. The Deceptive COVID-19: Lessons from Common Molecular Diagnostics and a Novel Plan for the Prevention of the Next Pandemic. Diseases 2023, 11, 20. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-nCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Bielicki, J.A.; Duval, X.; Gobat, N.; Goossens, H.; Koopmans, M.; Tacconelli, E.; van der Werf, S. Monitoring Approaches for Health-Care Workers during the COVID-19 Pandemic. Lancet Infect. Dis. 2020, 20, e261–e267. [Google Scholar] [CrossRef]
- Notomi, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Kashir, J.; Yaqinuddin, A. Loop Mediated Isothermal Amplification (LAMP) Assays as a Rapid Diagnostic for COVID-19. Medical Hypotheses 2020, 141, 109786. [Google Scholar] [CrossRef] [PubMed]
- Menting, S.; Erhart, A.; Schallig, H.D.F.H. Laboratory Evaluation of a SARS-CoV-2 RT-LAMP Test. Trop. Med. Infect. Dis. 2023, 8, 320. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Nucleic Acid Lateral Flow Assay with Recombinase Polymerase Amplification: Solutions for Highly Sensitive Detection of RNA Virus. Talanta 2020, 210, 120616. [Google Scholar] [CrossRef] [PubMed]
- Warmt, C.; Fenzel, C.K.; Henkel, J.; Bier, F.F. Using Cy5-dUTP Labelling of RPA-Amplicons with Downstream Microarray Analysis for the Detection of Antibiotic Resistance Genes. Sci. Rep. 2021, 11, 20137. [Google Scholar] [CrossRef] [PubMed]
- Zasada, A.A.; Mosiej, E.; Prygiel, M.; Polak, M.; Wdowiak, K.; Formińska, K.; Ziółkowski, R.; Żukowski, K.; Marchlewicz, K.; Nowiński, A.; et al. Detection of SARS-CoV-2 Using Reverse Transcription Helicase Dependent Amplification and Reverse Transcription Loop-Mediated Amplification Combined with Lateral Flow Assay. Biomedicines 2022, 10, 2329. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, M.; Yang, J.Y.C.; Zou, R.S.; Ma, W.; Lee, C.-Y.; Mohapatra, S.; Kang, J.; Liao, T.-W.; Myong, S.; Ha, T. Engineered Helicase Replaces Thermocycler in DNA Amplification While Retaining Desired PCR Characteristics. Nat. Commun. 2022, 13, 6312. [Google Scholar] [CrossRef]
- Lucia, C.; Federico, P.-B.; Alejandra, G.C. An Ultrasensitive, Rapid, and Portable Coronavirus SARS-CoV-2 Sequence Detection Method Based on CRISPR-Cas12. Mol. Biol. 2020, 26, 971127. [Google Scholar] [CrossRef]
- van Dongen, J.E.; Berendsen, J.T.W.; Steenbergen, R.D.M.; Wolthuis, R.M.F.; Eijkel, J.C.T.; Segerink, L.I. Point-of-Care CRISPR/Cas Nucleic Acid Detection: Recent Advances, Challenges and Opportunities. Biosens. Bioelectron. 2020, 166, 112445. [Google Scholar] [CrossRef]
- Ortiz-Cartagena, C.; Fernández-García, L.; Blasco, L.; Pacios, O.; Bleriot, I.; López, M.; Cantón, R.; Tomás, M. Reverse Transcription-Loop-Mediated Isothermal Amplification-CRISPR-Cas13a Technology as a Promising Diagnostic Tool for SARS-CoV-2. Microbiol. Spectr. 2022, 10, e02398-22. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Moehling, T.J.; Meagher, R.J. Advances in RT-LAMP for COVID-19 Testing and Diagnosis. Expert Rev. Mol. Diagn. 2023, 23, 9–28. [Google Scholar] [CrossRef]
- Lai, M.Y.; Bukhari, F.D.M.; Zulkefli, N.Z.; Ismail, I.; Mustapa, N.I.; Soh, T.S.T.; Hassan, A.H.; Peariasamy, K.M.; Lee, Y.L.; Suppiah, J.; et al. Clinical Testing on SARS-CoV-2 Swab Samples Using Reverse-Transcription Loop-Mediated Isothermal Amplification (RT-LAMP). BMC Infect. Dis. 2022, 22, 697. [Google Scholar] [CrossRef]
- Warmt, C.; Yaslanmaz, C.; Henkel, J. Investigation and Validation of Labelling Loop Mediated Isothermal Amplification (LAMP) Products with Different Nucleotide Modifications for Various Downstream Analysis. Sci. Rep. 2022, 12, 7137. [Google Scholar] [CrossRef]
- Zhang, Y.; Chai, Y.; Hu, Z.; Xu, Z.; Li, M.; Chen, X.; Yang, C.; Liu, J. Recent Progress on Rapid Lateral Flow Assay-Based Early Diagnosis of COVID-19. Front. Bioeng. Biotechnol. 2022, 10, 866368. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis. ACS Nano 2021, 15, 3593–3611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zheng, T.; Wang, H.; Chen, W.; Huang, X.; Liang, J.; Qiu, L.; Han, D.; Tan, W. Rapid One-Pot Detection of SARS-CoV-2 Based on a Lateral Flow Assay in Clinical Samples. Anal. Chem. 2021, 93, 3325–3330. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Yip, C.C.-Y.; To, K.K.-W.; Tang, T.H.-C.; Wong, S.C.-Y.; Leung, K.-H.; Fung, A.Y.-F.; Ng, A.C.-K.; Zou, Z.; Tsoi, H.-W.; et al. Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. J. Clin. Microbiol. 2020, 58, e00310-20. [Google Scholar] [CrossRef]
- Guan, B.; Frank, K.M.; Maldonado, J.O.; Beach, M.; Pelayo, E.; Warner, B.M.; Hufnagel, R.B. Sensitive Extraction-Free SARS-CoV-2 RNA Virus Detection Using a Chelating Resin. iScience 2021, 24, 102960. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Neumann, M.; Krause, E.; Rinner, T.; Muzeniek, T.; Grossegesse, M.; Hille, G.; Schwarz, F.; Puyskens, A.; Förster, S.; et al. Resource-Efficient Internally Controlled in-House Real-Time PCR Detection of SARS-CoV-2. Virol. J. 2021, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, V.K.; Bakthavathsalam, P.; Bergquist, P.L.; Sunna, A. Smartphone Detection of Antibiotic Resistance Using Convective PCR and a Lateral Flow Assay. Sens. Actuators B Chem. 2019, 298, 126849. [Google Scholar] [CrossRef]
- Agarwal, S.; Warmt, C.; Henkel, J.; Schrick, L.; Nitsche, A.; Bier, F.F. Lateral Flow–Based Nucleic Acid Detection of SARS-CoV-2 Using Enzymatic Incorporation of Biotin-Labeled dUTP for POCT Use. Anal. Bioanal. Chem. 2022, 414, 3177–3186. [Google Scholar] [CrossRef]
- Wang, T.; Lien, C.; Liu, S.; Selveraj, P. Effective Heat Inactivation of SARS-CoV-2. medRxiv 2020. [Google Scholar] [CrossRef]
- Milenia HybriDetect—A Universal Lateral Flow Development Platform. Milenia Biotech. 2021. Available online: https://www.milenia-biotec.com/en/universal-lateral-flow-development-tool (accessed on 15 December 2020).
- Zou, J.; Zhi, S.; Chen, M.; Su, X.; Kang, L.; Li, C.; Su, X.; Zhang, S.; Ge, S.; Li, W. Heat Inactivation Decreases the Qualitative Real-Time RT-PCR Detection Rates of Clinical Samples with High Cycle Threshold Values in COVID-19. Diagn. Microbiol. Infect. Dis. 2020, 98, 115109. [Google Scholar] [CrossRef] [PubMed]
- Subsoontorn, P.; Lohitnavy, M.; Kongkaew, C. The Diagnostic Accuracy of Isothermal Nucleic Acid Point-of-Care Tests for Human Coronaviruses: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 22349. [Google Scholar] [CrossRef]
- Jhou, Y.-R.; Wang, C.-H.; Tsai, H.-P.; Shan, Y.-S.; Lee, G.-B. An Integrated Microfluidic Platform Featuring Real-Time Reverse Transcription Loop-Mediated Isothermal Amplification for Detection of COVID-19. Sens. Actuators B Chem. 2022, 358, 131447. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Li, B.-R. Passively Driven Microfluidic Device with Simple Operation in the Development of Nanolitre Droplet Assay in Nucleic Acid Detection. Sci. Rep. 2021, 11, 21019. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Fu, J.-J.; Zhou, Y.; Chen, G.; Fang, C.; Lu, Z.S.; Yu, L. On-Chip RT-LAMP and Colorimetric Detection of the Prostate Cancer 3 Biomarker with an Integrated Thermal and Imaging Box. Talanta 2020, 208, 120407. [Google Scholar] [CrossRef] [PubMed]




| Organism | Template | Sample Size | Amplification Technique | Processing Time | Readout | Efficacy | References |
|---|---|---|---|---|---|---|---|
| SARS-CoV-2 E-gene | Extracted RNA | 55 positive 55 negative | RT-LAMP | 30 min (63 °C) | Fluorometric detection | 97.2% sensitivity 100% specificity | [9] |
| SARS-CoV-2 | Extracted RNA | 25 positive 25 negative | RT-HDA | 30 to 90 min (42 °C) | LFA | 100% sensitivity 100% specificity | [12] |
| SARS-CoV-2 E- and N-gene | Extracted RNA | 25 positive 25 negative | RT-LAMP | 60 min (65 °C) | LFA | 100% sensitivity 83.3% specificity | [12] |
| SARS-CoV-2 N-gene | Extracted RNA | 150 positive 110 negative | RT-LAMP | 1 h (65 °C) | pH based colorimetric | 92.7% sensitivity 93.6% specificity | [18] |
| SARS-CoV-2 N and ORF1ab gene | Lysed RNA | 12 synthetic positive samples, 8 positive swab samples, 4 negative swab samples from healthy individuals, 6 negative samples (Mycoplasma infection) | RT-LAMP | 30 min (60–65 °C) | LFA | 100% sensitivity 100% specificity | [22] |
| SARS-CoV-2 N-gene | Extracted RNA and cDNA | 82 positive and non-template control | RT-LAMP | 15 min (65 °C) | LFA | 95.4% sensitivity 96.5% specificity | [27] |
| SARS-CoV-2 N-gene | Swab sample in uVTM | 54 positive and 51 negative SARS-CoV-2 throat swab samples | RT-LAMP | 20 min (65 °C) | LFA | 98.11% sensitivity 96.15% specificity | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, S.; Hamidizadeh, M.; Bier, F.F. Detection of Reverse Transcriptase LAMP-Amplified Nucleic Acid from Oropharyngeal Viral Swab Samples Using Biotinylated DNA Probes through a Lateral Flow Assay. Biosensors 2023, 13, 988. https://doi.org/10.3390/bios13110988
Agarwal S, Hamidizadeh M, Bier FF. Detection of Reverse Transcriptase LAMP-Amplified Nucleic Acid from Oropharyngeal Viral Swab Samples Using Biotinylated DNA Probes through a Lateral Flow Assay. Biosensors. 2023; 13(11):988. https://doi.org/10.3390/bios13110988
Chicago/Turabian StyleAgarwal, Saloni, Mojdeh Hamidizadeh, and Frank F. Bier. 2023. "Detection of Reverse Transcriptase LAMP-Amplified Nucleic Acid from Oropharyngeal Viral Swab Samples Using Biotinylated DNA Probes through a Lateral Flow Assay" Biosensors 13, no. 11: 988. https://doi.org/10.3390/bios13110988
APA StyleAgarwal, S., Hamidizadeh, M., & Bier, F. F. (2023). Detection of Reverse Transcriptase LAMP-Amplified Nucleic Acid from Oropharyngeal Viral Swab Samples Using Biotinylated DNA Probes through a Lateral Flow Assay. Biosensors, 13(11), 988. https://doi.org/10.3390/bios13110988





