The Development of Reagentless Amperometric Glucose Biosensor Based on Gold Nanostructures, Prussian Blue and Glucose Oxidase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. The Preparation, Modification and Characterization of the Graphite Rod Electrode
2.3. Immobilization of GOx on a PB/AuNS/GR Electrode
2.4. Electrochemical Measurements
3. Results
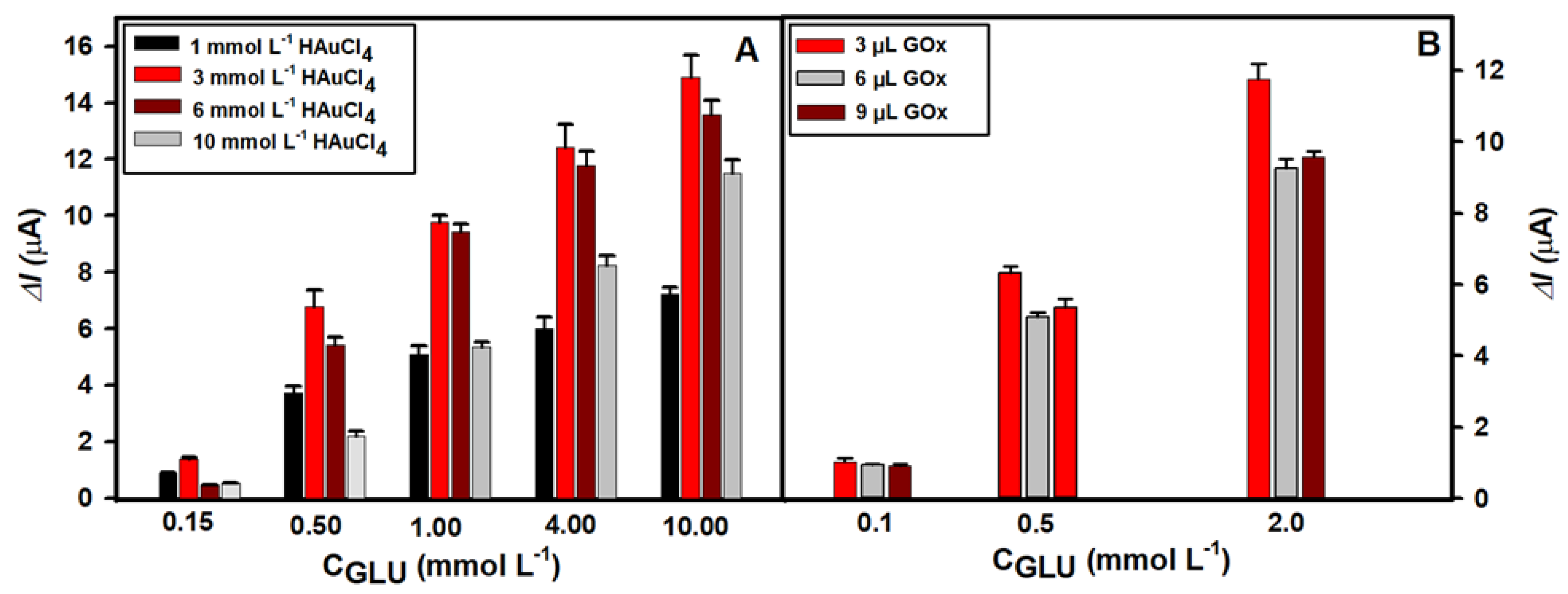
3.1. Selection of the Optimal Glucose Biosensor Performance Conditions
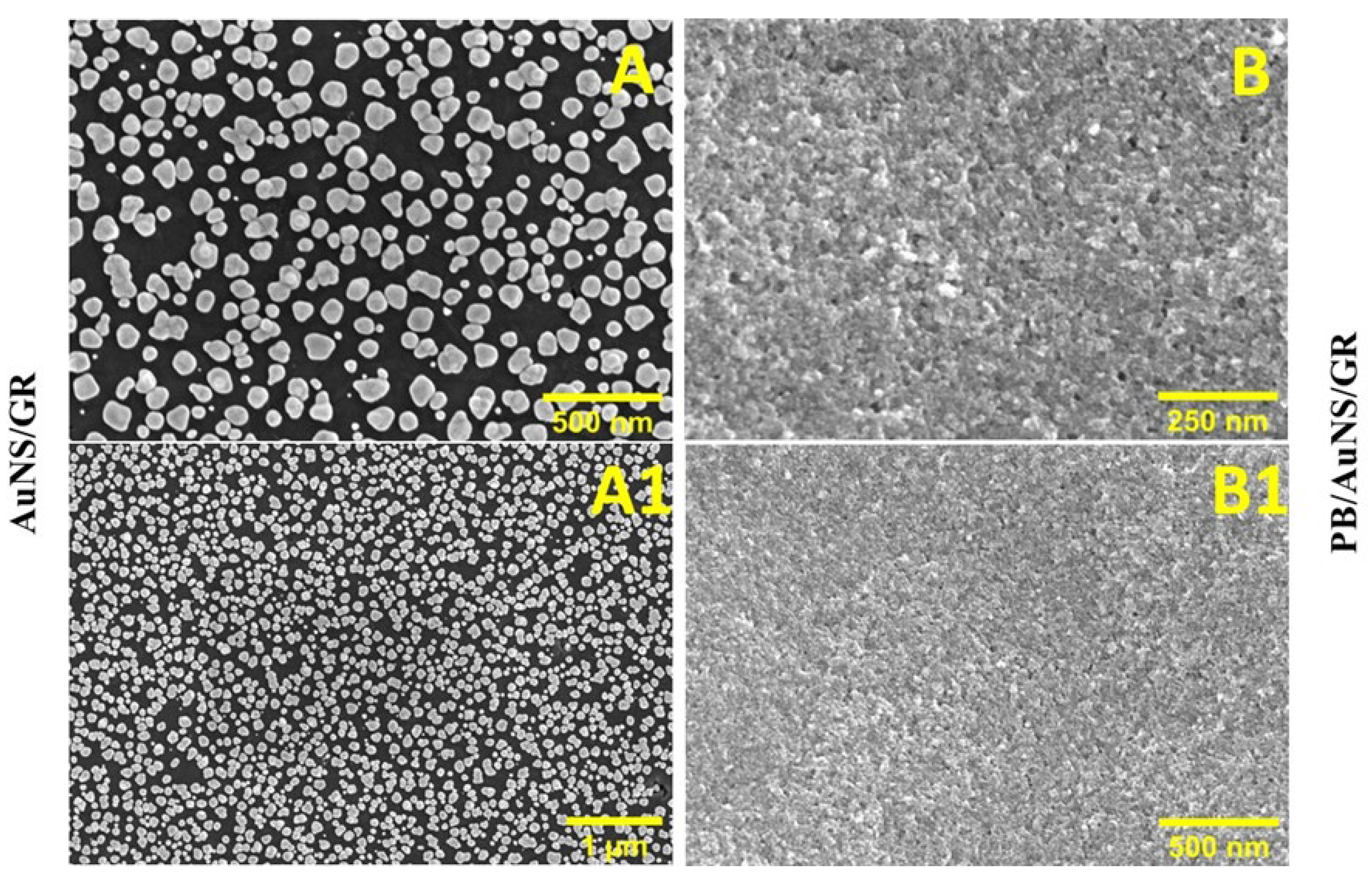
3.2. Characterization of AuNS and PB/AuNS Nanocomposite Electrochemically Deposited on GR Electrode
3.3. Characterization of the Developed Glucose Biosensor Based on Nf-GOx/PB/AuNS/GR Electrode
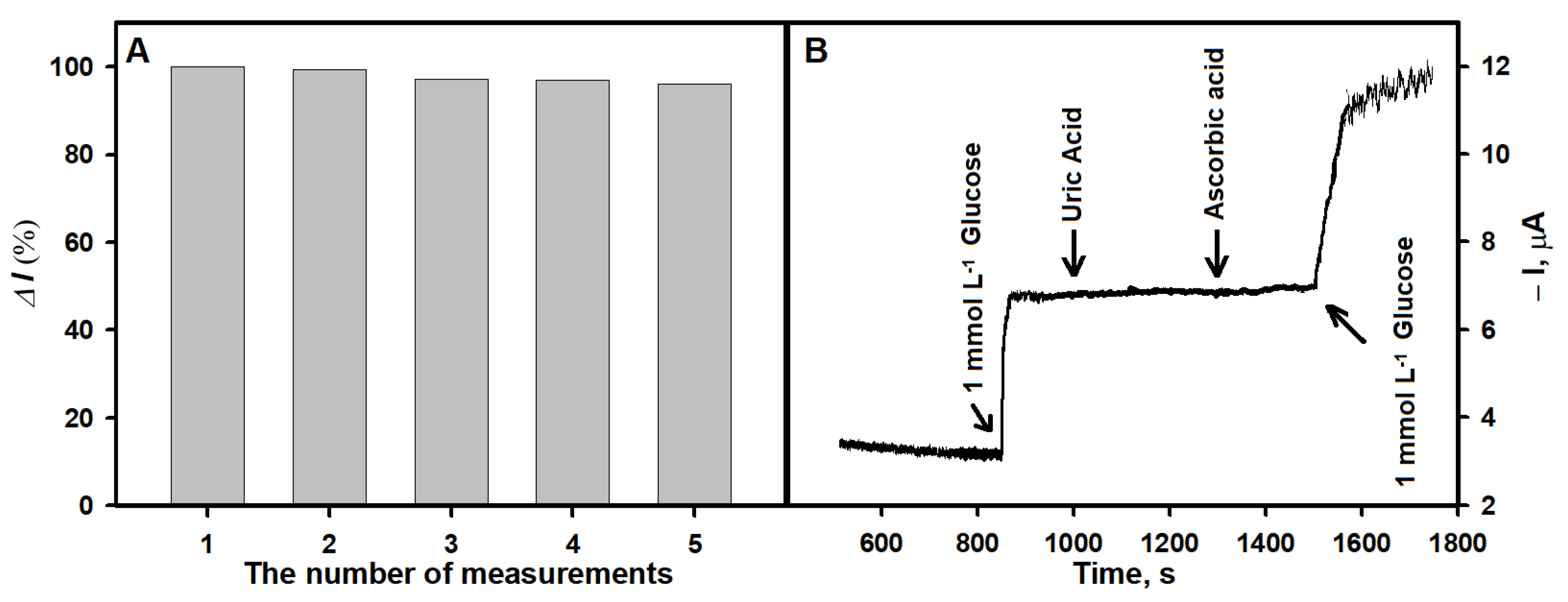
3.4. Study of Analytical Signal Repeatability and Influence of Interfering Substances on the Analytical Signal
3.5. Determination of H2O2 Using Biosensor Based on PB/AuNS/GR Electrode
3.6. Application of Developed Biosensor for Glucose Determination in Human Serum Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakalauskiene, L.; Popov, A.; Kausaite-Minkstimiene, A.; Ramanavicius, A.; Ramanaviciene, A. The Impact of Glucose Oxidase Immobilization on Dendritic Gold Nanostructures on the Performance of Glucose Biosensors. Biosensors 2022, 12, 320. [Google Scholar] [CrossRef]
- Jayakumar, K.; Bennett, R.; Leech, D. Electrochemical Glucose Biosensor Based on an Osmium Redox Polymer and Glucose Oxidase Grafted to Carbon Nanotubes: A Design-of-Experiments Optimisation of Current Density and Stability. Electrochim. Acta 2021, 371, 137845. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanaviciene, A. The Development and Evaluation of Reagentless Glucose Biosensors Using Dendritic Gold Nanostructures as a Promising Sensing Platform. Biosensors 2023, 13, 727. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Xiang, Y.; Li, W.; Zhong, X.; Che, X.; Li, J. Glucose Biosensor Based on the Highly Efficient Immobilization of Glucose Oxidase on Prussian Blue-Gold Nanocomposite Films. J. Mol. Catal. B Enzym. 2011, 69, 1–7. [Google Scholar] [CrossRef]
- Yadav, H.M.; Lee, J.-J. One-Pot Synthesis of Copper Nanoparticles on Glass: Applications for Non-Enzymatic Glucose Detection and Catalytic Reduction of 4-Nitrophenol. J. Solid State Electrochem. 2019, 23, 503–512. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Ramanaviciene, A.; German, N.; Kausaite-Minkstimiene, A.; Ramanavicius, A. Glucose Biosensor Based on Dendritic Gold Nanostructures Electrodeposited on Graphite Electrode by Different Electrochemical Methods. Chemosensors 2021, 9, 188. [Google Scholar] [CrossRef]
- Clark, L.C.J.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef]
- Guilbault, G.G.; Lubrano, G.J. An Enzyme Electrode for the Amperometric Determination of Glucose. Anal. Chim. Acta 1973, 64, 439–455. [Google Scholar] [CrossRef]
- der Wissenschaften, B.K.A. Miscellanea Berolinensia Ad Incrementum Scientiarum ex Scriptis Societati Regiae Scientiarum Exhibitis Edita; Natural History Museum Library: London, UK, 2009; Volume 1, p. 1710. [Google Scholar]
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. A High-Sensitive Glucose Amperometric Biosensor Based on Prussian Blue Modified Electrodes. Anal. Lett. 1994, 27, 2861–2869. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, J.-J.; Shi, C.-G.; Chen, H.-Y. Multilayer Membranes via Layer-by-Layer Deposition of Organic Polymer Protected Prussian Blue Nanoparticles and Glucose Oxidase for Glucose Biosensing. Langmuir 2005, 21, 9630–9634. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.M.B.F.; Fátima Barroso, M.; Morais, S.; Araújo, M.; Freire, C.; de Lima-Neto, P.; Correia, A.N.; Oliveira, M.B.P.P.; Delerue-Matos, C. Laccase-Prussian Blue Film-Graphene Doped Carbon Paste Modified Electrode for Carbamate Pesticides Quantification. Biosens. Bioelectron. 2013, 47, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.-Y.; Yu, C.-M.; Yen, M.-J.; Chen, L.-C. Glucose Sensing Electrodes Based on a Poly(3,4-Ethylenedioxythiophene)/Prussian Blue Bilayer and Multi-Walled Carbon Nanotubes. Biosens. Bioelectron. 2009, 24, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Miao, K.; Ma, P.; Ma, X.; Bi, R.; Chen, F. A Feasible Electrochemical Biosensor for Determination of Glucose Based on Prussian Blue—Enzyme Aggregates Cascade Catalytic System. Bioelectrochemistry 2021, 141, 107838. [Google Scholar] [CrossRef]
- Cinti, S.; Cusenza, R.; Moscone, D.; Arduini, F. Paper-Based Synthesis of Prussian Blue Nanoparticles for the Development of Whole Blood Glucose Electrochemical Biosensor. Talanta 2018, 187, 59–64. [Google Scholar] [CrossRef]
- Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Communication—Accessing Stability of Oxidase-Based Biosensors via Stabilizing the Advanced H2O2 Transducer. J. Electrochem. Soc. 2017, 164, B3056. [Google Scholar] [CrossRef]
- Nikolaev, K.; Ermakov, S.; Ermolenko, Y.; Averyaskina, E.; Offenhäusser, A.; Mourzina, Y. A Novel Bioelectrochemical Interface Based on in Situ Synthesis of Gold Nanostructures on Electrode Surfaces and Surface Activation by Meerwein’s Salt. A Bioelectrochemical Sensor for Glucose Determination. Bioelectrochemistry 2015, 105, 34–43. [Google Scholar] [CrossRef]
- Seker, E.; Reed, M.L.; Begley, M.R. Nanoporous Gold: Fabrication, Characterization, and Applications. Materials 2009, 2, 2188–2215. [Google Scholar] [CrossRef]
- Li, C.; Ya, Y.; Zhan, G. Electrochemical Investigation of Tryptophan at Gold Nanoparticles Modified Electrode in the Presence of Sodium Dodecylbenzene Sulfonate. Colloids Surf. B Biointerfaces 2010, 76, 340–345. [Google Scholar] [CrossRef]
- German, N.; Ramanavicius, A.; Ramanaviciene, A. Electrochemical Deposition of Gold Nanoparticles on Graphite Rod for Glucose Biosensing. Sens. Actuators B Chem. 2014, 203, 25–34. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of Pani–Pedot Film Deposited on Transparent Electrode. Polymers 2020, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- Esmail Tehrani, S.; Quang Nguyen, L.; Garelli, G.; Jensen, B.M.; Ruzgas, T.; Emnéus, J.; Sylvest Keller, S. Hydrogen Peroxide Detection Using Prussian Blue-Modified 3D Pyrolytic Carbon Microelectrodes. Electroanalysis 2021, 33, 2516–2528. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Moscone, D.; Palleschi, G.; Killard, A.J. Development of a Hydrogen Peroxide Sensor Based on Screen-Printed Electrodes Modified with Inkjet-Printed Prussian Blue Nanoparticles. Sensors 2014, 14, 14222–14234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, G.; Wu, D.; Li, X.; Hu, N.; Chen, J.; Chen, G.; Wu, Y. Recent Progress in the Design Fabrication of Metal-Organic Frameworks-Based Nanozymes and Their Applications to Sensing and Cancer Therapy. Biosens. Bioelectron. 2019, 137, 178–198. [Google Scholar] [CrossRef]
- Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Catalytically Synthesized Prussian Blue Nanoparticles Defeating Natural Enzyme Peroxidase. J. Am. Chem. Soc. 2018, 140, 11302–11307. [Google Scholar] [CrossRef]
- Tran, M.; Mundt, C.; Lan, T.; Padalkar, S. Electrodeposition of Gold Nanostructures Having Controlled Morphology. J. Nanosci. Nanotechnol. 2018, 18, 3492–3498. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, B.; Yu, F.; Yuan, Q.; Gu, M.; Ji, J.; Zhang, Y.; Li, Y. 3D Nitrogen-Doped Graphite Foam@Prussian Blue: An Electrochemical Sensing Platform for Highly Sensitive Determination of H2O2 and Glucose. Microchim. Acta 2018, 185, 86. [Google Scholar] [CrossRef]
- Hu, T.; Wang, D.; Xu, J.; Chen, K.; Li, X.; Yi, H.; Ni, Z. Glucose Sensing on Screen-Printed Electrochemical Electrodes Based on Porous Graphene Aerogel @prussian Blue. Biomed. Microdevices 2022, 24, 14. [Google Scholar] [CrossRef]
- Thakur, B.; Guo, X.; Chang, J.; Kron, M.; Chen, J. Porous Carbon and Prussian Blue Composite: A Highly Sensitive Electrochemical Platform for Glucose Biosensing. Sens. Bio-Sens. Res. 2017, 14, 47–53. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, L.; Yin, L.; Guo, L. Electrochemical Glucose Biosensor with Improved Performance Based on the Use of Glucose Oxidase and Prussian Blue Incorporated into a Thin Film of Self-Polymerized Dopamine. Sens. Actuators B Chem. 2015, 210, 513–518. [Google Scholar] [CrossRef]
- Chu, Z.; Shi, L.; Zhang, Y.; Jin, W.; Warren, S.; Ward, D.; Dempsey, E. Single Layer Prussian Blue Grid as a Versatile Enzyme Trap for Low-Potential Biosensors. J. Mater. Chem. 2012, 22, 14874–14879. [Google Scholar] [CrossRef]
- Garjonyte, R.; Malinauskas, A. Amperometric Glucose Biosensors Based on Prussian Blue– and Polyaniline–Glucose Oxidase Modified Electrodes. Biosens. Bioelectron. 2000, 15, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Niamsi, W.; Larpant, N.; Kalambate, P.K.; Primpray, V.; Karuwan, C.; Rodthongkum, N.; Laiwattanapaisal, W. Paper-Based Screen-Printed Ionic-Liquid/Graphene Electrode Integrated with Prussian Blue/MXene Nanocomposites Enabled Electrochemical Detection for Glucose Sensing. Biosensors 2022, 12, 852. [Google Scholar] [CrossRef] [PubMed]




| Modified Working Electrode | Potential/Solution | LOD | LR | Ref. |
|---|---|---|---|---|
| Nf-GOx/Pt-NCs/PB-Au/GC | −0.15 V/0.1 mol L−1 KNO3 (pH 6.0) | 0.001 mmol L−1 | 0.003–1.1 mmol L−1 | [4] |
| CTS/GOx/GA@PB/carbon SPE | −0.35 V/0.01 mol L−1 PBS | 0.15 mmol L−1 | 0.5–6.0 mmol·L−1 | [30] |
| GOx@PB/coral-likeAuNS/CC | −0.05 V/0.1 mol L−1 PBS with 0.1 mol L−1 KCl (pH 6.0) | 0.05 mmol L−1 | 0.15–6.65 mmol·L−1 | [16] |
| GOx-PCPB/Pt | −0.05 V/0.1 mol L−1 PBS with 0.1 mol L−1 KCl (pH 6.5) | 0.03 mmol L−1 | 0.03–0.4 mmol·L−1 | [31] |
| GOx/PB/NGF | −0.05 V/0.1 mol L−1 PBS with 0.1 M KCl (pH 6.0) | 0.1 mmol L−1 | 0.2–20.0 mmol L−1 | [29] |
| GOx–PDA/PB/GC | −0.0 V/0.05 mol L−1 PBS with 0.1 mol L−1 KCl (pH 7.4). | 0.0462 mmol L−1 | 0.2–3.4 mmol L−1 | [32] |
| Nf/GOx/PB/AuNS/GR | −0.05 V/0.05 mol L−1 PBS with 0.1 mol L−1 KCl (pH 5.8). | 0.0088 mmol L−1 | 0.025–1.0 mmol L−1 | This work |
| Concentration in the Serum, mmol L−1 * | Added Concentration, mmol L−1 | Detected Concentration, mmol L−1 | Recovery, % | RSD, % (n = 3) |
|---|---|---|---|---|
| 0.49 | 0.5 | 1.01 | 102 | 0.84 |
| 0.49 | 1.0 | 1.51 | 101 | 0.74 |
| 0.49 | 1.5 | 2.02 | 101 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakalauskiene, L.; Brasiunas, B.; Popov, A.; Kausaite-Minkstimiene, A.; Ramanaviciene, A. The Development of Reagentless Amperometric Glucose Biosensor Based on Gold Nanostructures, Prussian Blue and Glucose Oxidase. Biosensors 2023, 13, 942. https://doi.org/10.3390/bios13100942
Sakalauskiene L, Brasiunas B, Popov A, Kausaite-Minkstimiene A, Ramanaviciene A. The Development of Reagentless Amperometric Glucose Biosensor Based on Gold Nanostructures, Prussian Blue and Glucose Oxidase. Biosensors. 2023; 13(10):942. https://doi.org/10.3390/bios13100942
Chicago/Turabian StyleSakalauskiene, Laura, Benediktas Brasiunas, Anton Popov, Asta Kausaite-Minkstimiene, and Almira Ramanaviciene. 2023. "The Development of Reagentless Amperometric Glucose Biosensor Based on Gold Nanostructures, Prussian Blue and Glucose Oxidase" Biosensors 13, no. 10: 942. https://doi.org/10.3390/bios13100942
APA StyleSakalauskiene, L., Brasiunas, B., Popov, A., Kausaite-Minkstimiene, A., & Ramanaviciene, A. (2023). The Development of Reagentless Amperometric Glucose Biosensor Based on Gold Nanostructures, Prussian Blue and Glucose Oxidase. Biosensors, 13(10), 942. https://doi.org/10.3390/bios13100942








