Evaluation of the Information Content for Determining the Vascular Tone Type of the Lower Extremities in Varicose Veins: A Case Study
Abstract
1. Introduction
1.1. Pulse Wave Velocity
1.2. Problem Statement
2. Materials and Methods
2.1. Subject
2.2. Equipment
- 30 precordial electrical impedance channels.
- One transthoracic bioimpedance channel: This channel is specifically designed for measuring bioimpedance through the chest, enabling the assessment of the cardiovascular system and other thoracic tissues.
- One ECG channel.
- A pulse impedance measurement range of −2 to +2 Ohms.
- A channel sampling rate of 500 Hz. This high sampling rate ensures that the measurements are taken quickly and accurately, improving the overall precision of the system.
- The bioimpedance measurement method is tetrapolar.
- The probe current amplitude is 1 mA. A low probe current amplitude minimizes the potential for discomfort and ensures the safety of the measurement process.
- The bandwidth of the bioimpedance channel is 0.01–117 Hz. This wide bandwidth allows for the measurement of a wide range of frequencies, improving the versatility of the system.
- The probing frequency is 100 kHz.
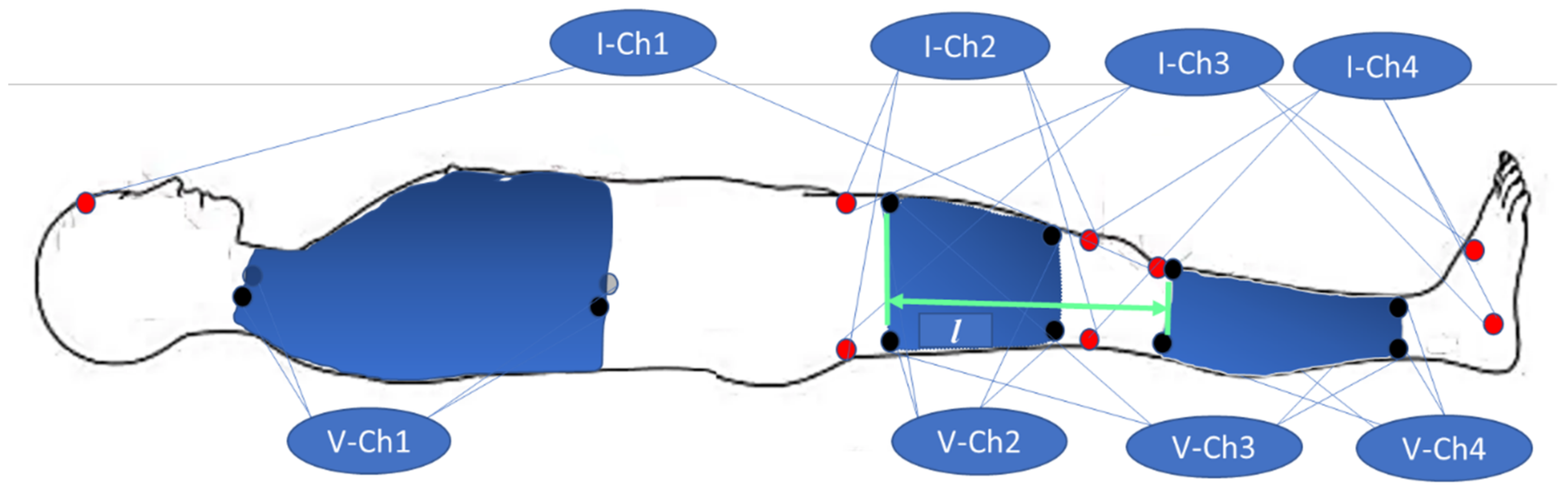
2.3. Electrode Configurations
- Ch1—transthoracic channel;
- Ch2—right thigh (RLU);
- Ch3—right leg (RL);
- Ch4—lower part of the right leg (RLD).
2.4. Experimental Procedure
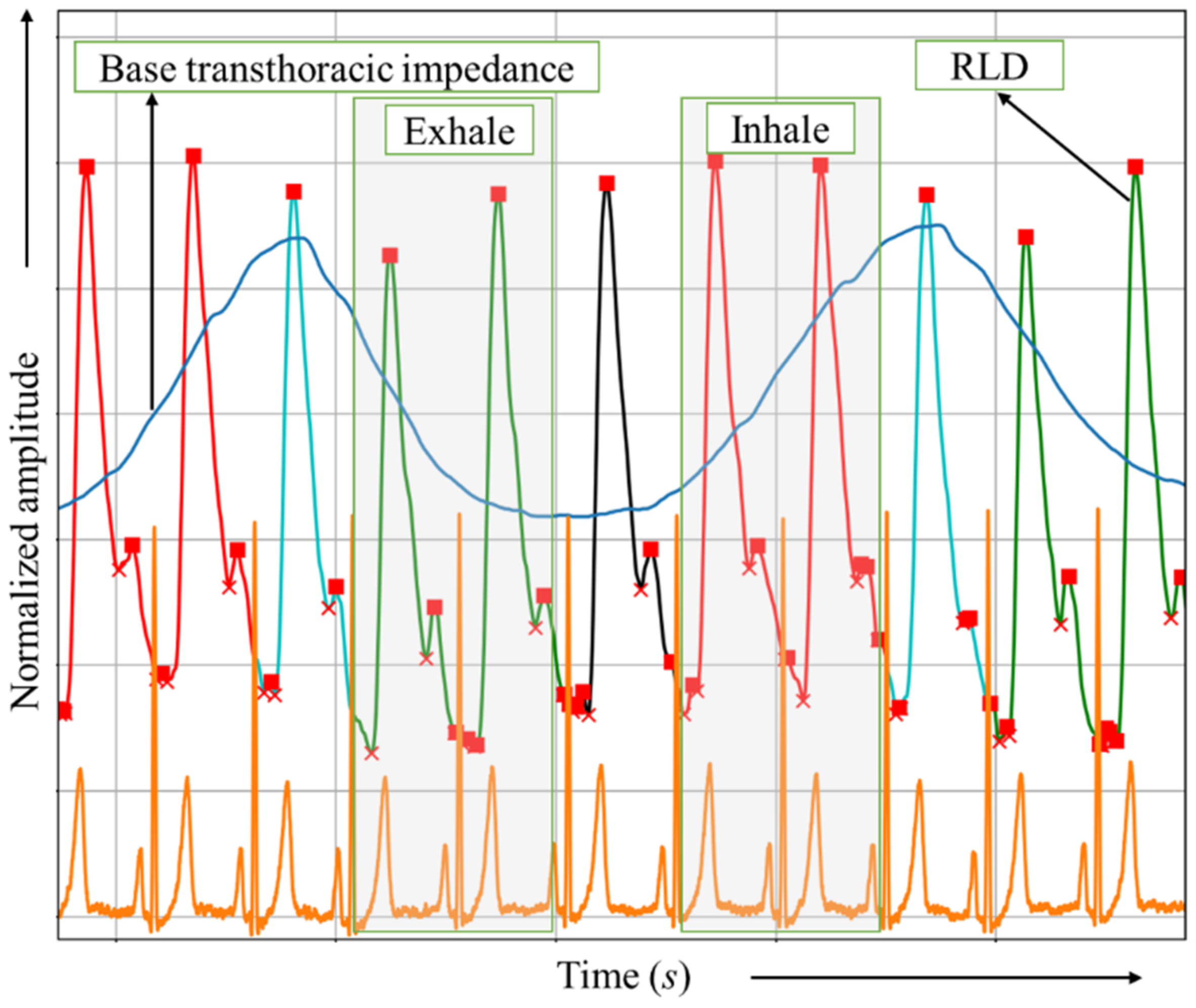
2.5. Signal Processing and Breathing Phase Detection
2.6. Vascular Tone and Hemodynamic Evaluation
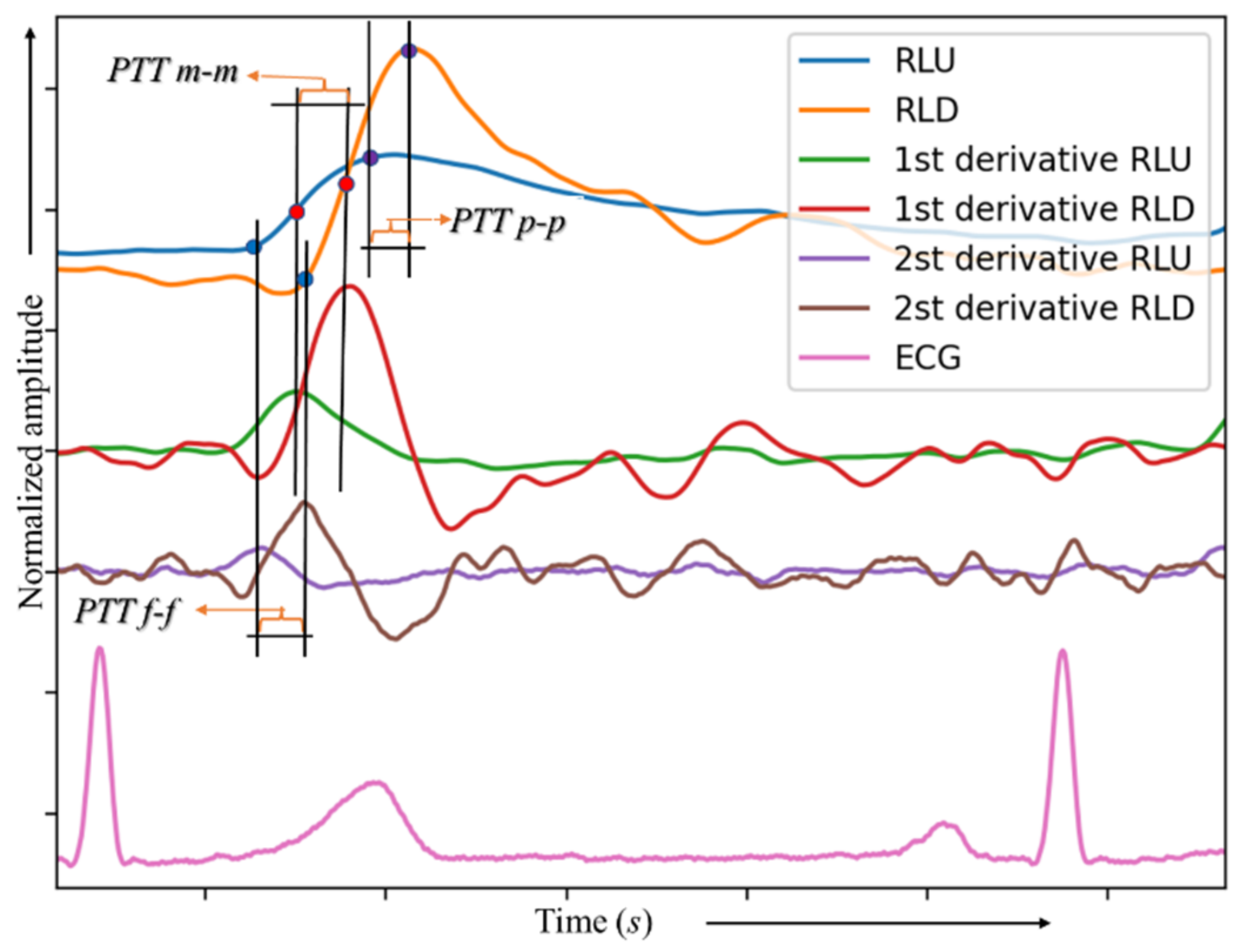
2.7. Local Pulse Wave Velocity Measurement
3. Results
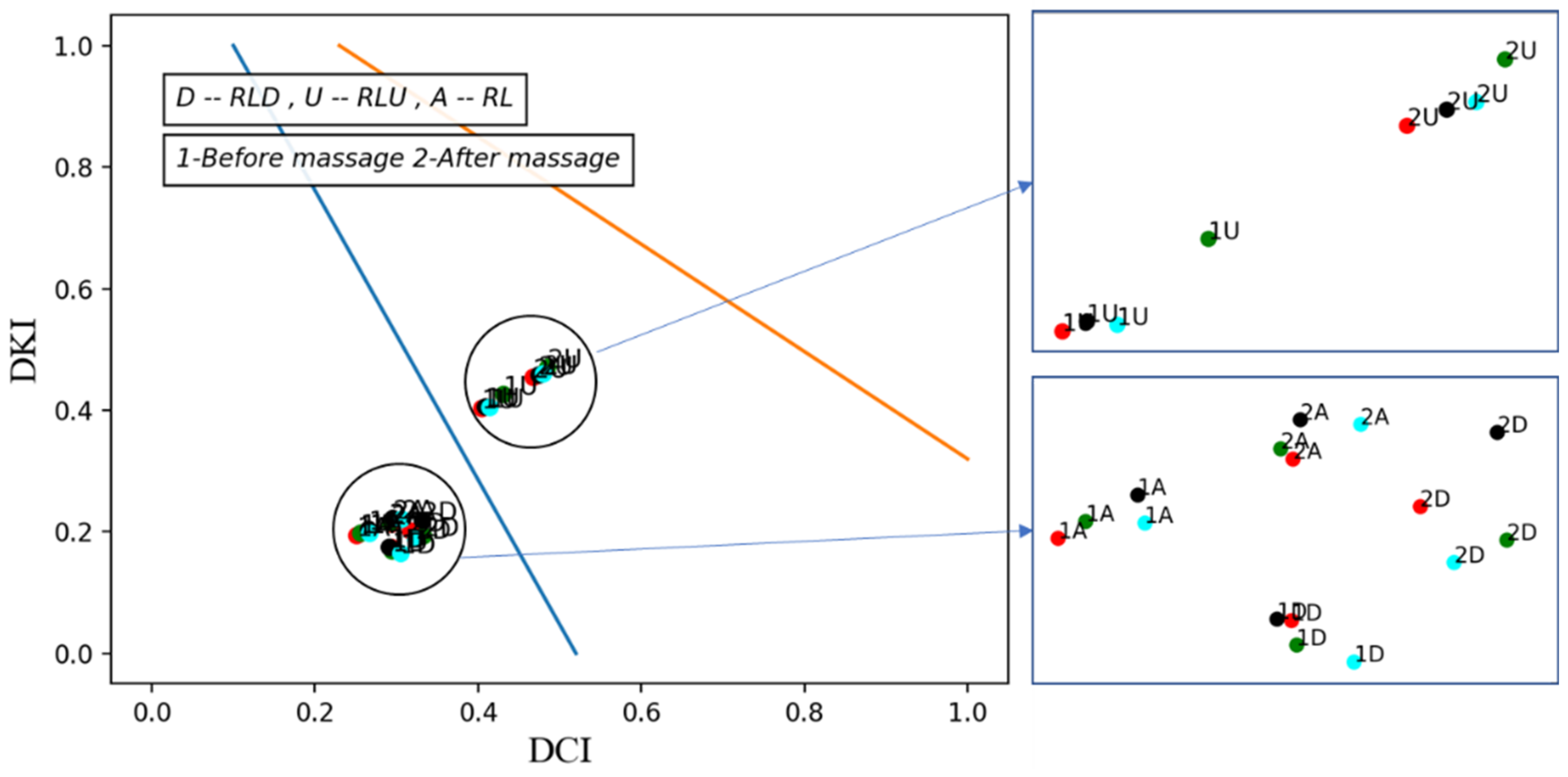
3.1. Vascular Tone for Multi Cycles
3.2. Local-Time Vascular Tone Changes
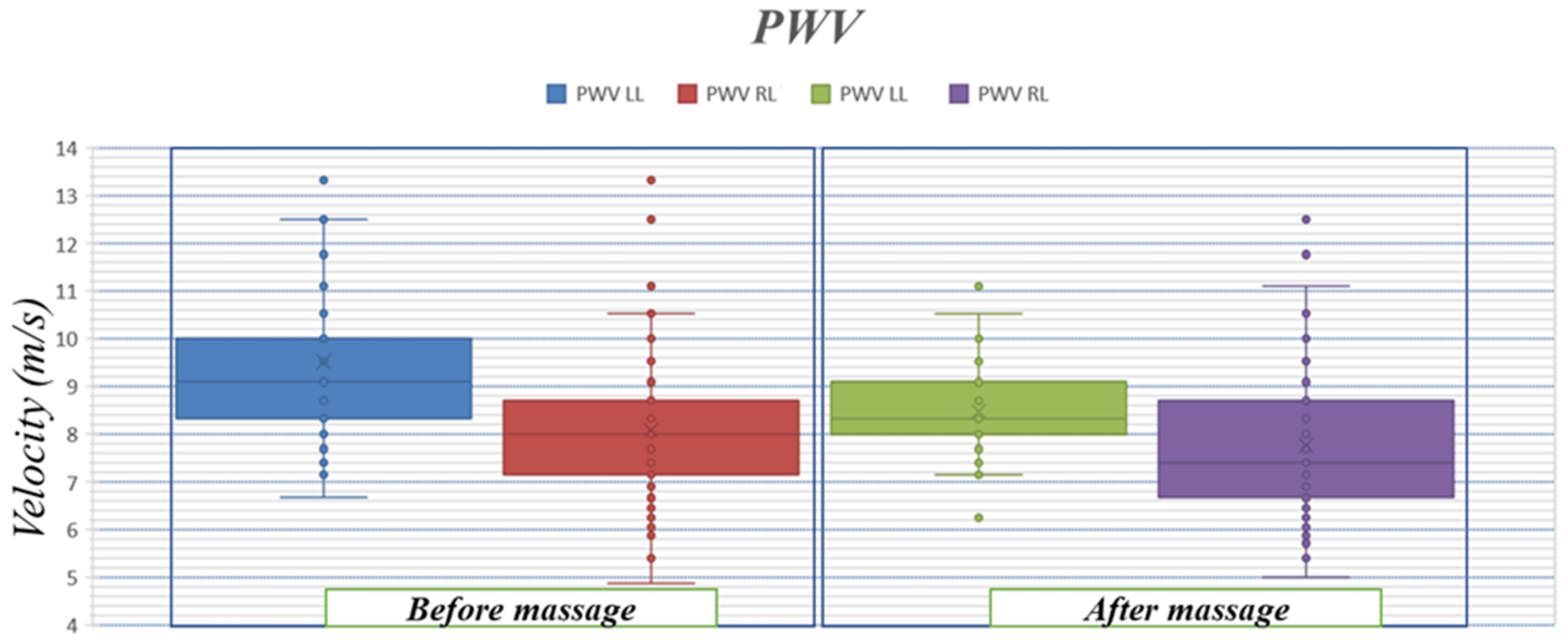
3.3. Pulse Wave Velocity
4. Discussions
4.1. Vascular Tone Changes
4.2. Pulse Wave Velocity
4.3. Limitations
4.4. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef] [PubMed]
- Flores, N.; Reyna, M.A.; Avitia, R.L.; Cardenas-Haro, J.A.; Garcia-Gonzalez, C. Non-Invasive Systems and Methods Patents Review Based on Electrocardiogram for Diagnosis of Cardiovascular Diseases. Algorithms 2022, 15, 82. [Google Scholar] [CrossRef]
- Tamura, T. Blood Flow Measurement; Elsevier: Amsterdam, The Netherlands, 2014; Volume 5, ISBN 9780444536327. [Google Scholar]
- Heijboer, H.; Buller, H.R.; Lensing, A.; Turpie, A.; Colly, L.P.; ten Cate, J.W. A Comparison of Real-Time Compression Ultrasonography with Impedance Plethysmography for the Diagnosis of Deep-Vein Thrombosis in Symptomatic Outpatients. N. Engl. J. Med. 1993, 329, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Nyboer, J. Electrical Impedance Plethysmography; a Physical and Physiologic Approach to Peripheral Vascular Study. Circulation 1950, 2, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Min, M. Fundamentals, recent advances, and future challenges in bioimpedance devices for healthcare applications. J. Sens. 2019, 2019, 9210258. [Google Scholar] [CrossRef]
- Irzmańska, E.; Padula, G.; Irzmański, R. Impedance Plethysmography as a Tool for Assessing Exertion-Related Blood Flow Changes in the Lower Limbs in Healthy Subjects. Measurement 2014, 47, 110–115. [Google Scholar] [CrossRef]
- Haapala, M.; Lyytikäinen, L.-P.; Peltokangas, M.; Koivistoinen, T.; Hutri-Kähönen, N.; Laurila, M.-M.; Mäntysalo, M.; Raitakari, O.T.; Kähönen, M.; Lehtimäki, T.; et al. Impedance Plethysmography-Based Method in the Assessment of Subclinical Atherosclerosis. Atherosclerosis 2021, 319, 101–107. [Google Scholar] [CrossRef]
- Cojocaru, A.-L.; Dănilă, D.M. Study Concerning the Efficiency of the Reflex Massage in the Treatment of Varicose Veins. Procedia Soc. Behav. Sci. 2014, 117, 559–565. [Google Scholar] [CrossRef]
- Rubik, B. Manual Healing Methods. In A Report to the National Institutes of Health on Alternative Medical Systems and Practices in the United States; Expanding Medical Horizons: Washington, DC, USA, 1995. [Google Scholar]
- Duprez, D.A.; Cohn, J.N. The Arterial System in Human Hypertension. In Comprehensive Hypertension; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 135–143. ISBN 9780323039611. [Google Scholar]
- Haynes, W.G.; Ferro, C.J.; O’Kane, K.P.J.; Somerville, D.; Lomax, C.C.; Webb, D.J. Systemic Endothelin Receptor Blockade Decreases Peripheral Vascular Resistance and Blood Pressure in Humans. Circulation 1996, 93, 1860–1870. [Google Scholar] [CrossRef]
- Antle, D.M.; Cormier, L.; Findlay, M.; Miller, L.L.; Côté, J.N. Lower Limb Blood Flow and Mean Arterial Pressure during Standing and Seated Work: Implications for Workplace Posture Recommendations. Prev. Med. Rep. 2018, 10, 117–122. [Google Scholar] [CrossRef]
- Mattace-Raso, F.U.S.; Hofman, A.; Verwoert, G.C.; Wittemana, J.C.M.; Wilkinson, I.; Cockcroft, J.; McEniery, C.; Yasmina; Laurent, S.; Boutouyrie, P.; et al. Determinants of Pulse Wave Velocity in Healthy People and in the Presence of Cardiovascular Risk Factors: ‘Establishing Normal and Reference Values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef]
- Kusche, R.; Klimach, P.; Ryschka, M. A Multichannel Real-Time Bioimpedance Measurement Device for Pulse Wave Analysis. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yoo, H.-J. Simultaneous Electrical Bio-Impedance Plethysmography at Different Body Parts: Continuous and Non-Invasive Monitoring of Pulse Wave Velocity. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.H.; Jafari, R.; Chung, W.-Y. Noninvasive Cuffless Blood Pressure Estimation Using Pulse Transit Time and Impedance Plethysmography. IEEE Trans. Biomed. Eng. 2019, 66, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.; Yu, G.; Niu, F.; He, P. Design and preliminary evaluation of a portable device for the measurement of bioimpedance spectroscopy. Physiol. Meas. 2006, 27, 1293. [Google Scholar] [CrossRef]
- Kusche, R.; Ryschka, M. Combining bioimpedance and EMG measurements for reliable muscle contraction detection. IEEE Sens. J. 2019, 19, 11687–11696. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Composition of the ESPEN Working Group. Bioelectrical impedance analysis--part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef]
- Hammoud, A.; Tikhomirov, A.N.; Shaheen, Z. Automatic Bio-Impedance Signal Analysis: Smoothing Processes Efficacy Evaluation in Determining the Vascular Tone Type. In Proceedings of the 2021 Ural Symposium on Biomedical Engineering, Radioelectronics and Information Technology, USBEREIT 2021, Yekaterinburg, Russia, 13–14 May 2021; pp. 113–116. [Google Scholar]
- Van Gent, P.; Farah, H.; van Nes, N.; van Arem, B. HeartPy: A Novel Heart Rate Algorithm for the Analysis of Noisy Signals. Transp. Res. Part F Traffic Psychol. Behav. 2019, 66, 368–378. [Google Scholar] [CrossRef]
- Piuzzi, E.; Pisa, S.; Pittella, E.; Podesta, L.; Sangiovanni, S. Low-Cost and Portable Impedance Plethysmography System for the Simultaneous Detection of Respiratory and Heart Activities. IEEE Sens. J. 2019, 19, 2735–2746. [Google Scholar] [CrossRef]
- Hammoud, A.; Myasishcheva, G.; Shaheen, Z.; Tikhomirov, A.; Briko, A.; Shchukin, S. Extraction of Respiratory Patterns Using Thoracic Bio-Impedance Channels. In Proceedings of the 2022 Ural-Siberian Conference on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT), Yekaterinburg, Russian, 19–21 September 2022; pp. 74–77. [Google Scholar] [CrossRef]
- Finkelstein, S.M.; Cohn, J.N. First- and Third-Order Models for Determining Arterial Compliance. J. Hypertens. 1992, 10, S11–S14. [Google Scholar] [CrossRef]
- Luzhnov, P.V. Development of a Computer System for Biosynchronized Electromagnetic Impact; Bauman Moscow State Technical University: Moscow, Russia, 2005. [Google Scholar]
- Hammoud, A.; Tikhomirov, A.; Myasishcheva, G.; Shaheen, Z.; Volkov, A.; Briko, A.; Shchukin, S. Multi-Channel Bioimpedance System for Detecting Vascular Tone in Human Limbs: An Approach. Sensors 2021, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Chirinos, J.A. Arterial stiffness: Basic concepts and measurement techniques. J. Cardiovasc. Transl. Res. 2012, 5, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Barvik, D.; Cerny, M.; Penhaker, M.; Noury, N. Noninvasive Continuous Blood Pressure Estimation from Pulse Transit Time: A Review of the Calibration Models. IEEE Rev. Biomed. Eng. 2022, 15, 138–151. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Rocha, C.; Ferreira, H.T.; Silva, H.N. Lower Limb Massage in Humans Increases Local Perfusion and Impacts Systemic Hemodynamics. J. Appl. Physiol. 2020, 128, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, P.A.; Somers, V.K. Cardiovascular Physiology: Autonomic Control in Health and in Sleep Disorders; Elsevier Inc.: Amsterdam, The Netherlands, 2010; ISBN 9781416066453. [Google Scholar]
- Yokoyama, H.; Shoji, T.; Kimoto, E.; Shinohara, K.; Tanaka, S.; Koyama, H.; Emoto, M.; Nishizawa, Y. Pulse Wave Velocity in Lower-Limb Arteries among Diabetic Patients with Peripheral Arterial Disease. J. Atheroscler. Thromb. 2003, 10, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Wiegerinck, A.I.P.; Thomsen, A.; Hisdal, J.; Kalvøy, H.; Tronstad, C. Electrical Impedance Plethysmography versus Tonometry to Measure the Pulse Wave Velocity in Peripheral Arteries in Young Healthy Volunteers: A Pilot Study. J. Electr. Bioimpedance 2021, 12, 169–177. [Google Scholar] [CrossRef]





| Record Duration (min) | Average Heart Rate (PPM) | Heart Cycle Count | Respiratory Rate (Cycle/min) | Inhalation Cycle Count | Exhalation Cycle Count | |
|---|---|---|---|---|---|---|
| 1—Before massage | 3.04 | 61.8 | 188 | 9.5 | 55 | 66 |
| 2—After massage | 2.07 | 61.35 | 127 | 8.9 | 37 | 49 |
| Before Massage | After Massage | |||||||
|---|---|---|---|---|---|---|---|---|
| Segment | HC (B) | HT (B) | NT(B) | HT/HC (B) % | HC (M) | HT (M) | NT(M) | HT/HC (M) % |
| LLU | 188 | 43 | 145 | 22.8% | 127 | 3 | 124 | 2.4% |
| RLU | 188 | 91 | 97 | 48.4 | 127 | 14 | 113 | 11% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammoud, A.; Tikhomirov, A.; Briko, A.; Volkov, A.; Karapetyan, A.; Shchukin, S. Evaluation of the Information Content for Determining the Vascular Tone Type of the Lower Extremities in Varicose Veins: A Case Study. Biosensors 2023, 13, 96. https://doi.org/10.3390/bios13010096
Hammoud A, Tikhomirov A, Briko A, Volkov A, Karapetyan A, Shchukin S. Evaluation of the Information Content for Determining the Vascular Tone Type of the Lower Extremities in Varicose Veins: A Case Study. Biosensors. 2023; 13(1):96. https://doi.org/10.3390/bios13010096
Chicago/Turabian StyleHammoud, Ahmad, Alexey Tikhomirov, Andrey Briko, Alexander Volkov, Aida Karapetyan, and Sergey Shchukin. 2023. "Evaluation of the Information Content for Determining the Vascular Tone Type of the Lower Extremities in Varicose Veins: A Case Study" Biosensors 13, no. 1: 96. https://doi.org/10.3390/bios13010096
APA StyleHammoud, A., Tikhomirov, A., Briko, A., Volkov, A., Karapetyan, A., & Shchukin, S. (2023). Evaluation of the Information Content for Determining the Vascular Tone Type of the Lower Extremities in Varicose Veins: A Case Study. Biosensors, 13(1), 96. https://doi.org/10.3390/bios13010096






