Colorimetric Paper Sensor for Food Spoilage Based on Biogenic Amine Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Instrumentation
2.2. Experimental Design and Method Optimization in Liquid Format
2.3. Method Optimization on Paper
2.4. Reflectance Signal Acquisition and Data Analysis
2.5. Design of Colorimetric BA Sensing Paper
2.6. Real Sample Analysis
2.7. Stability Studies
3. Results and Discussion
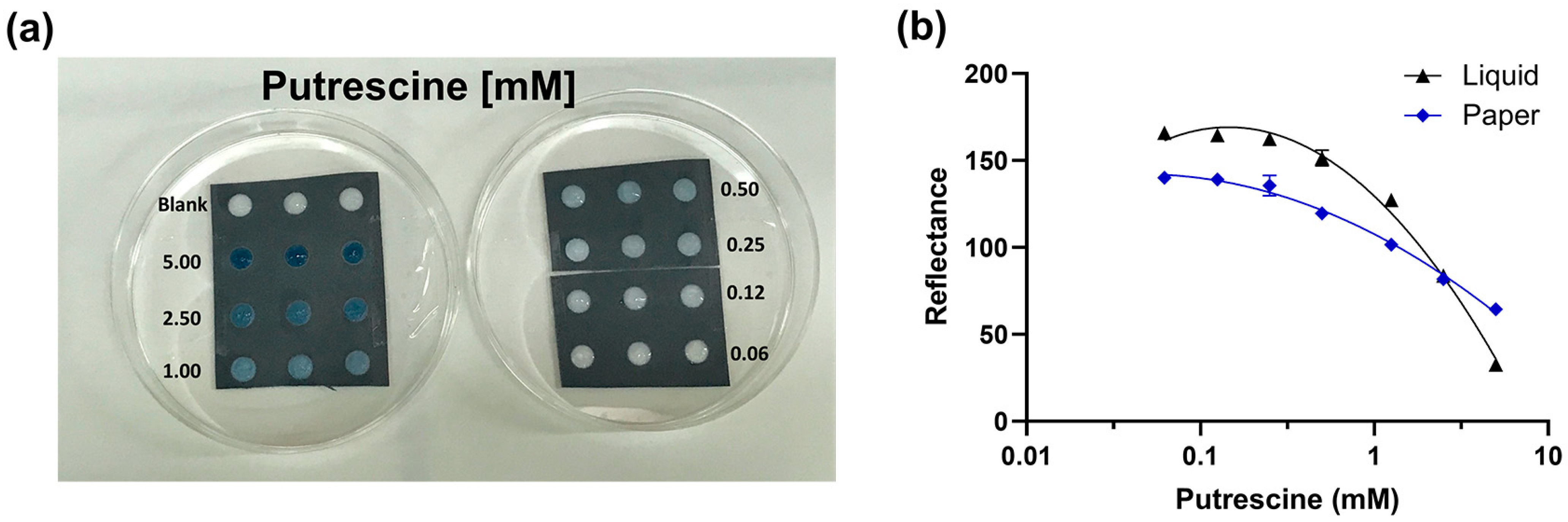
3.1. Method Optimization in Liquid and Paper Format
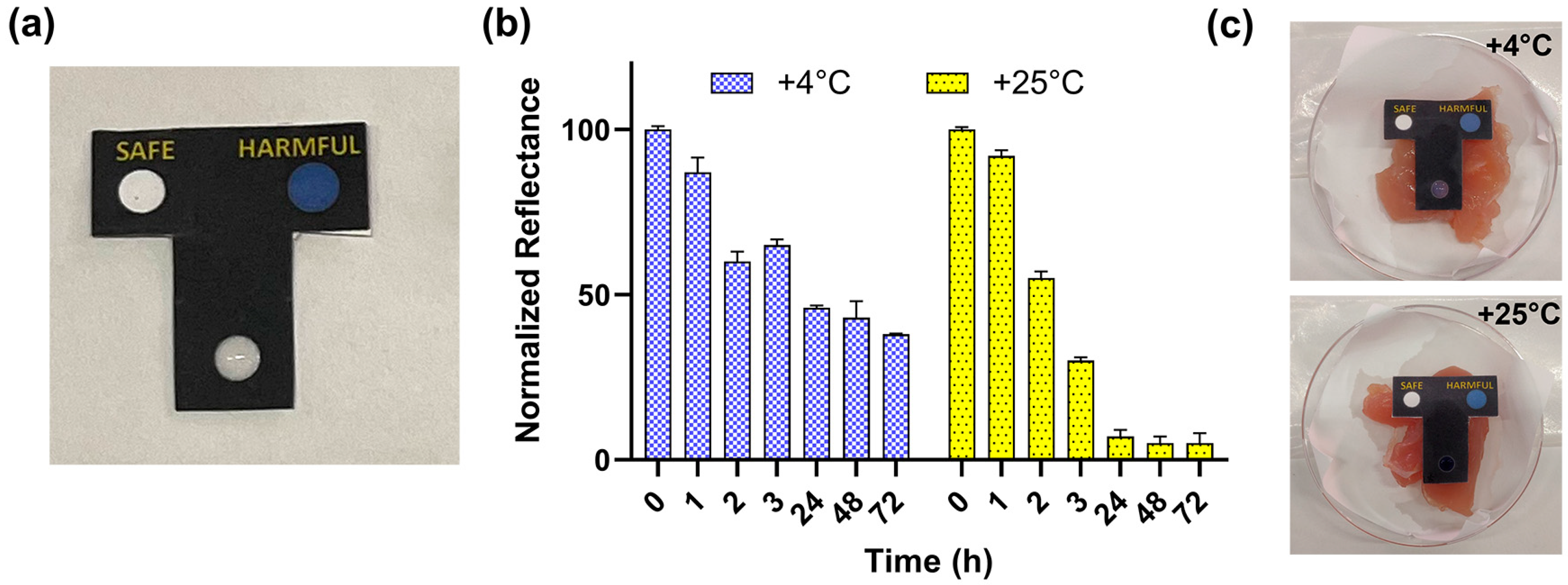
3.2. Design and Fabrication of BA Paper Sensor
3.3. Detection of BAs in Real Chicken Meat Samples
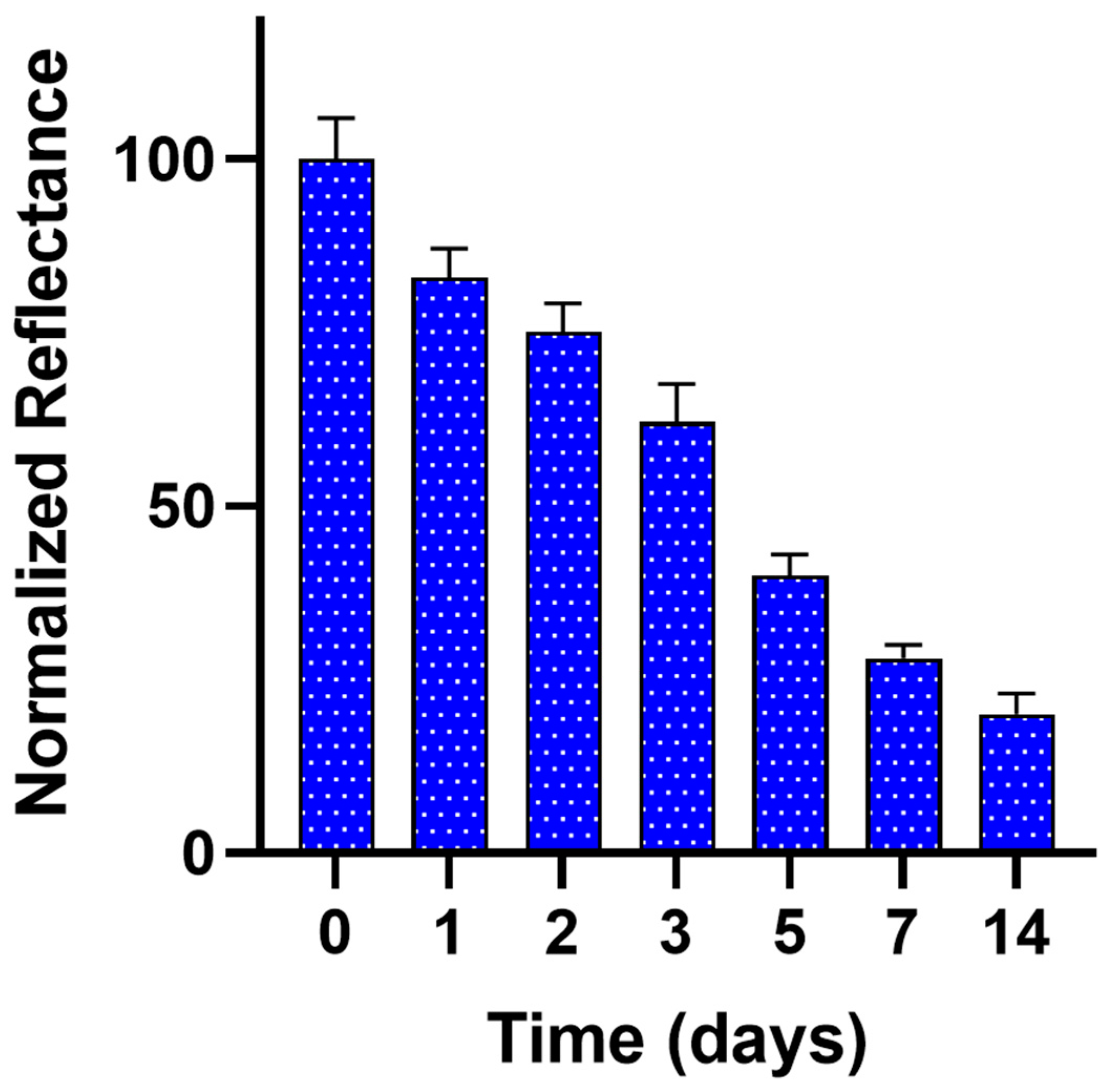
3.4. Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (accessed on 10 January 2022).
- Calabretta, M.M.; Álvarez-Diduk, R.; Michelini, E.; Roda, A.; Merkoçi, A. Nano-Lantern on Paper for Smartphone-Based ATP Detection. Biosens. Bioelectron. 2020, 150, 111902. [Google Scholar] [CrossRef] [PubMed]
- Seddaoui, N.; Attaallah, R.; Amine, A. Development of an Optical Immunoassay Based on Peroxidase-Mimicking Prussian Blue Nanoparticles and a Label-Free Electrochemical Immunosensor for Accurate and Sensitive Quantification of Milk Species Adulteration. Microchim. Acta 2022, 189, 209. [Google Scholar] [CrossRef] [PubMed]
- Michelini, E.; Cevenini, L.; Mezzanotte, L.; Simoni, P.; Baraldini, M.; de Laude, L.; Roda, A. One-Step Triplex-Polymerase Chain Reaction Assay for the Authentication of Yellowfin (Thunnus Albacares), Bigeye (Thunnus Obesus), and Skipjack (Katsuwonus Pelamis) Tuna DNA from Fresh, Frozen, and Canned Tuna Samples. J. Agric. Food Chem. 2007, 55, 7638–7647. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez-Cejuela, H.; Gregucci, D.; Calabretta, M.M.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M.; Michelini, E. Novel Nanozeolitic Imidazolate Framework (ZIF-8)–Luciferase Biocomposite for Nanosensing Applications. Anal. Chem. 2023; in press. [Google Scholar] [CrossRef]
- Lopreside, A.; Montali, L.; Wang, B.; Tassoni, A.; Ferri, M.; Calabretta, M.M.; Michelini, E. Orthogonal Paper Biosensor for Mercury(II) Combining Bioluminescence and Colorimetric Smartphone Detection. Biosens. Bioelectron. 2021, 194, 113569. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]
- Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods. EFSA J. 2011, 9, 2393. [CrossRef]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef]
- Schirone, M.; Esposito, L.; D’Onofrio, F.; Visciano, P.; Martuscelli, M.; Mastrocola, D.; Paparella, A. Biogenic Amines in Meat and Meat Products: A Review of the Science and Future Perspectives. Foods 2022, 11, 788. [Google Scholar] [CrossRef]
- Makhamrueang, N.; Sirilun, S.; Sirithunyalug, J.; Chaiyana, W.; Wangcharoen, W.; Peerajan, S.; Chaiyasut, C. Effect of Pretreatment Processes on Biogenic Amines Content and Some Bioactive Compounds in Hericium Erinaceus Extract. Foods 2021, 10, 996. [Google Scholar] [CrossRef]
- Bedia Erim, F. Recent Analytical Approaches to the Analysis of Biogenic Amines in Food Samples. TrAC Trends Anal. Chem. 2013, 52, 239–247. [Google Scholar] [CrossRef]
- Pandith, A.; Dasagrandhi, C.; Kim, H.-R.; Kim, H.-S. Selective Discrimination of Putrescine and Cadaverine Based on a Fe3+-Morpholinoanthracene Ensemble in Solution and Solid State and Logic Gate Aided Biological Applications in Mixed Aqueous Medium. Sens. Actuators B Chem. 2018, 254, 842–854. [Google Scholar] [CrossRef]
- Xu, X.; Lian, X.; Hao, J.; Zhang, C.; Yan, B. A Double-Stimuli-Responsive Fluorescent Center for Monitoring of Food Spoilage Based on Dye Covalently Modified EuMOFs: From Sensory Hydrogels to Logic Devices. Adv. Mater. 2017, 29, 1702298. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Petty, A.R.; Sazama, G.T.; Swager, T.M. Single-Walled Carbon Nanotube/Metalloporphyrin Composites for the Chemiresistive Detection of Amines and Meat Spoilage. Angew. Chem. Int. Ed. 2015, 54, 6554–6557. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Singh, G.; Kaur, N.; Singh, N. Pattern-Based Colorimetric Sensor Array to Monitor Food Spoilage Using Automated High-Throughput Analysis. Biosens. Bioelectron. 2022, 196, 113687. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, C.S.; Park, S.J.; Kim, J.; Seo, S.E.; An, J.E.; Ha, S.; Bae, J.; Phyo, S.; Lee, J.; et al. In-Situ Food Spoilage Monitoring Using a Wireless Chemical Receptor-Conjugated Graphene Electronic Nose. Biosens. Bioelectron. 2022, 200, 113908. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature Update of Analytical Methods for Biogenic Amines Determination in Food and Beverages. TrAC Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef]
- Viri, V.; Cornaglia, M.; Atakan, H.B.; Lehnert, T.; Gijs, M.A.M. An in Vivo Microfluidic Study of Bacterial Transit in C. Elegans Nematodes. Lab. Chip. 2020, 20, 2696–2708. [Google Scholar] [CrossRef]
- Janči, T.; Valinger, D.; Gajdoš Kljusurić, J.; Mikac, L.; Vidaček, S.; Ivanda, M. Determination of Histamine in Fish by Surface Enhanced Raman Spectroscopy Using Silver Colloid SERS Substrates. Food Chem. 2017, 224, 48–54. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Lee, S.; Lee, J.; Ha, J.-H.; Kang, S.H. Ultrasensitive Biogenic Amine Sensor Using an Enhanced Multiple Nanoarray Chip Based on Competitive Reactions in an Evanescent Field. Sens. Actuators B Chem. 2021, 345, 130354. [Google Scholar] [CrossRef]
- Abo Dena, A.S.; Khalid, S.A.; Ghanem, A.F.; Shehata, A.I.; El-Sherbiny, I.M. User-Friendly Lab-on-Paper Optical Sensor for the Rapid Detection of Bacterial Spoilage in Packaged Meat Products. RSC Adv. 2021, 11, 35165–35173. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Capone, F.; Zanoni, C.; Alberti, G.; Quadrelli, P.; Biesuz, R. Colorimetric Sensor Array for Monitoring, Modelling and Comparing Spoilage Processes of Different Meat and Fish Foods. Foods 2020, 9, 684. [Google Scholar] [CrossRef] [PubMed]
- Wells, N.; Yusufu, D.; Mills, A. Colourimetric Plastic Film Indicator for the Detection of the Volatile Basic Nitrogen Compounds Associated with Fish Spoilage. Talanta 2019, 194, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gurr, P.A.; Qiao, G.G. Irreversible Spoilage Sensors for Protein-Based Food. ACS Sens. 2020, 5, 2903–2908. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Shukla, V.; Lalawmpuii, H.; Kumarand, S. Indicator Sensors for Monitoring Meat Quality: A Review. J. Pharmacogn. Phytochem. 2018, 7, 809–812. [Google Scholar]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent Gelatin/Oxidized Chitin Nanocrystals Nanocomposite Films Containing Black Rice Bran Anthocyanins for Fish Freshness Monitorings. Int. J. Biol. Macromol. 2020, 155, 1296–1306. [Google Scholar] [CrossRef]
- Talukder, S.; Mendiratta, S.K.; Kumar, R.R.; Agrawal, R.K.; Soni, A.; Luke, A.; Chand, S. Jamun Fruit (Syzgium Cumini) Skin Extract Based Indicator for Monitoring Chicken Patties Quality during Storage. J. Food Sci. Technol. 2020, 57, 537–548. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lim, J.-M.; Bhoo, S.-H.; Paik, Y.-S.; Hahn, T.-R. Colorimetric Determination of Amino Acids Using Genipin from Gardenia Jasminoides. Anal. Chim. Acta. 2003, 480, 267–274. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Cok, M.; Asaro, F.; Scognamiglio, F.; Marsich, E.; Lopez, F.; Donati, I.; Sacco, P. On the Mechanism of Genipin Binding to Primary Amines in Lactose-Modified Chitosan at Neutral pH. Int. J. Mol. Sci. 2020, 21, 6831. [Google Scholar] [CrossRef]
- Available online: Https://Www.Biocrick.Com/Genipin-BCN5932.Html (accessed on 10 January 2022).
- Montali, L.; Calabretta, M.M.; Lopreside, A.; D’Elia, M.; Guardigli, M.; Michelini, E. Multienzyme chemiluminescent foldable biosensor for on-site detection of acetylcholinesterase inhibitors. Biosens. Bioelectron. 2020, 162, 112232. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Alvarez-Diduk, R.; Michelini, E.; Merkoçi, A. ATP Sensing Paper with Smartphone Bioluminescence-Based Detection. Methods Mol. Biol. 2022, 2525, 297–307. [Google Scholar] [CrossRef]
- Gu, Z.; Zhao, M.; Sheng, Y.; Bentolila, L.A.; Tang, Y. Detection of Mercury Ion by Infrared Fluorescent Protein and Its Hydrogel-Based Paper Assay. Anal. Chem. 2011, 83, 2324–2329. [Google Scholar] [CrossRef]
- Selim, A.S.; Perry, J.M.; Nasr, M.A.; Pimprikar, J.M.; Shih, S.C.C. A Synthetic Biosensor for Detecting Putrescine in Beef Samples. ACS Appl. Bio. Mater. 2022, 5, 5487–5496. [Google Scholar] [CrossRef] [PubMed]
- Neri-Numa, I.A.; Pessoa, M.G.; Paulino, B.N.; Pastore, G.M. Genipin: A natural blue pigment for food and health purposes. Trends Food Sci. Technol. 2017, 67, 271–279. [Google Scholar] [CrossRef]
- Torres-Sánchez, R.; Martínez-Zafra, M.T.; Castillejo, N.; Guillamón-Frutos, A.; Artés-Hernández, F. Real-Time Monitoring System for Shelf Life Estimation of Fruit and Vegetables. Sensors 2020, 20, 1860. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, J.; Liu, Z.; Wang, X.; Zhou, H. Effect of Storage Temperature and Time on Biogenic Amines in Canned Seafood. Foods 2022, 11, 2743. [Google Scholar] [CrossRef]
- Danchuk, A.I.; Komova, N.S.; Mobarez, S.N.; Doronin, S.Y.; Burmistrova, N.A.; Markin, A.V.; Duerkop, A. Optical sensors for determination of biogenic amines in food. Anal. Bioanal. Chem. 2020, 412, 4023–4036. [Google Scholar] [CrossRef]
- Bueno, L.; Meloni, G.N.; Reddy, S.M.; Paixão, T.R.L.C. Use of plastic-based analytical device, smartphone and chemometric tools to discriminate amines. RSC Adv. 2015, 5, 20148–20154. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabretta, M.M.; Gregucci, D.; Desiderio, R.; Michelini, E. Colorimetric Paper Sensor for Food Spoilage Based on Biogenic Amine Monitoring. Biosensors 2023, 13, 126. https://doi.org/10.3390/bios13010126
Calabretta MM, Gregucci D, Desiderio R, Michelini E. Colorimetric Paper Sensor for Food Spoilage Based on Biogenic Amine Monitoring. Biosensors. 2023; 13(1):126. https://doi.org/10.3390/bios13010126
Chicago/Turabian StyleCalabretta, Maria Maddalena, Denise Gregucci, Riccardo Desiderio, and Elisa Michelini. 2023. "Colorimetric Paper Sensor for Food Spoilage Based on Biogenic Amine Monitoring" Biosensors 13, no. 1: 126. https://doi.org/10.3390/bios13010126
APA StyleCalabretta, M. M., Gregucci, D., Desiderio, R., & Michelini, E. (2023). Colorimetric Paper Sensor for Food Spoilage Based on Biogenic Amine Monitoring. Biosensors, 13(1), 126. https://doi.org/10.3390/bios13010126





