Model of the SARS-CoV-2 Virus for Development of a DNA-Modified, Surface-Enhanced Raman Spectroscopy Sensor with a Novel Hybrid Plasmonic Platform in Sandwich Mode
Abstract
1. Introduction
2. Materials and Methods
2.1. Oligonucleotides and Some Materials
2.2. Synthesis of the Model of SARS-CoV-2 Virions
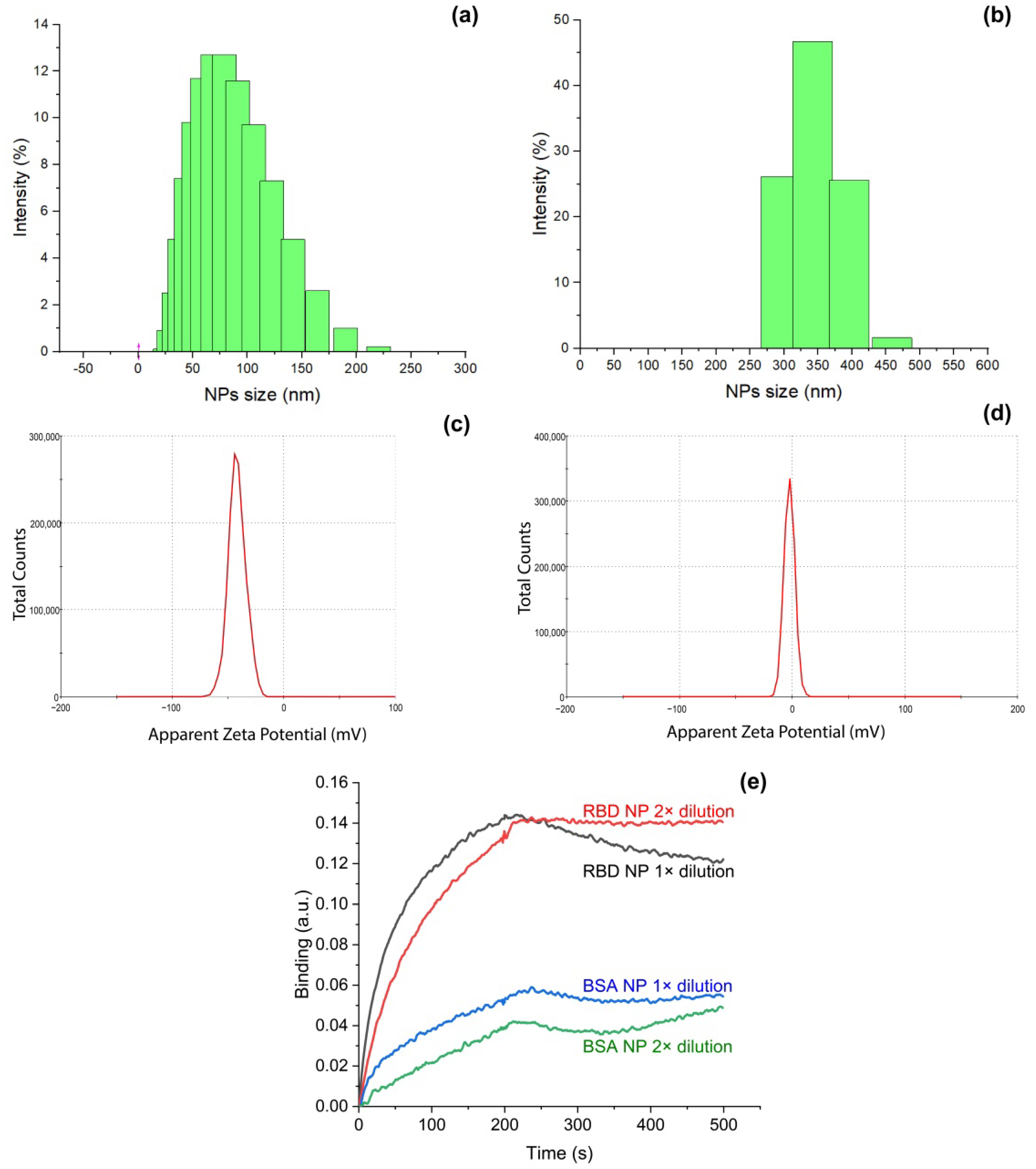
2.3. Characterization of Nanoparticles Coated with RBD Protein
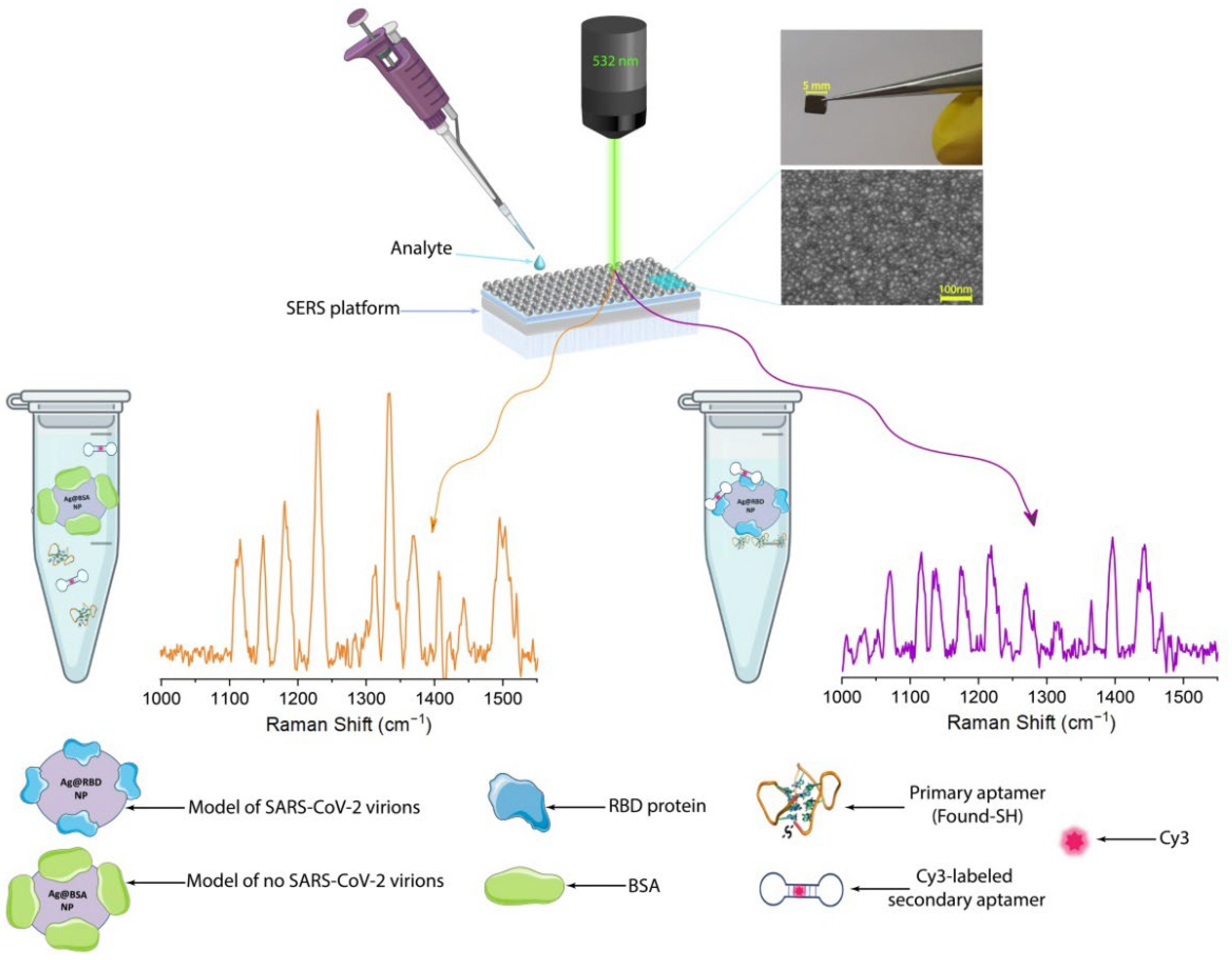
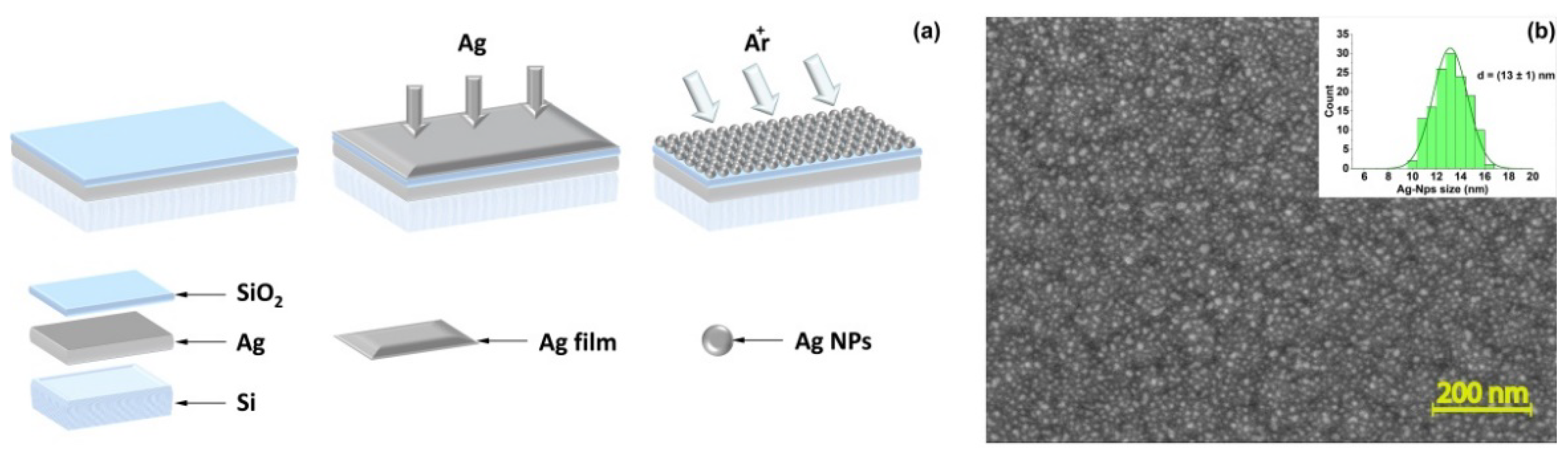
2.4. SERS-Active Aptasensor Platform
2.5. SERS-Based Substrates
2.6. Characterization of SERS-Based Substrates
3. Results and Discussion
3.1. Fabrication and Characterization of Model of SARS-CoV-2 Virions
3.2. Characterization of the Hybrid Plasmonic Sandwiched Structures
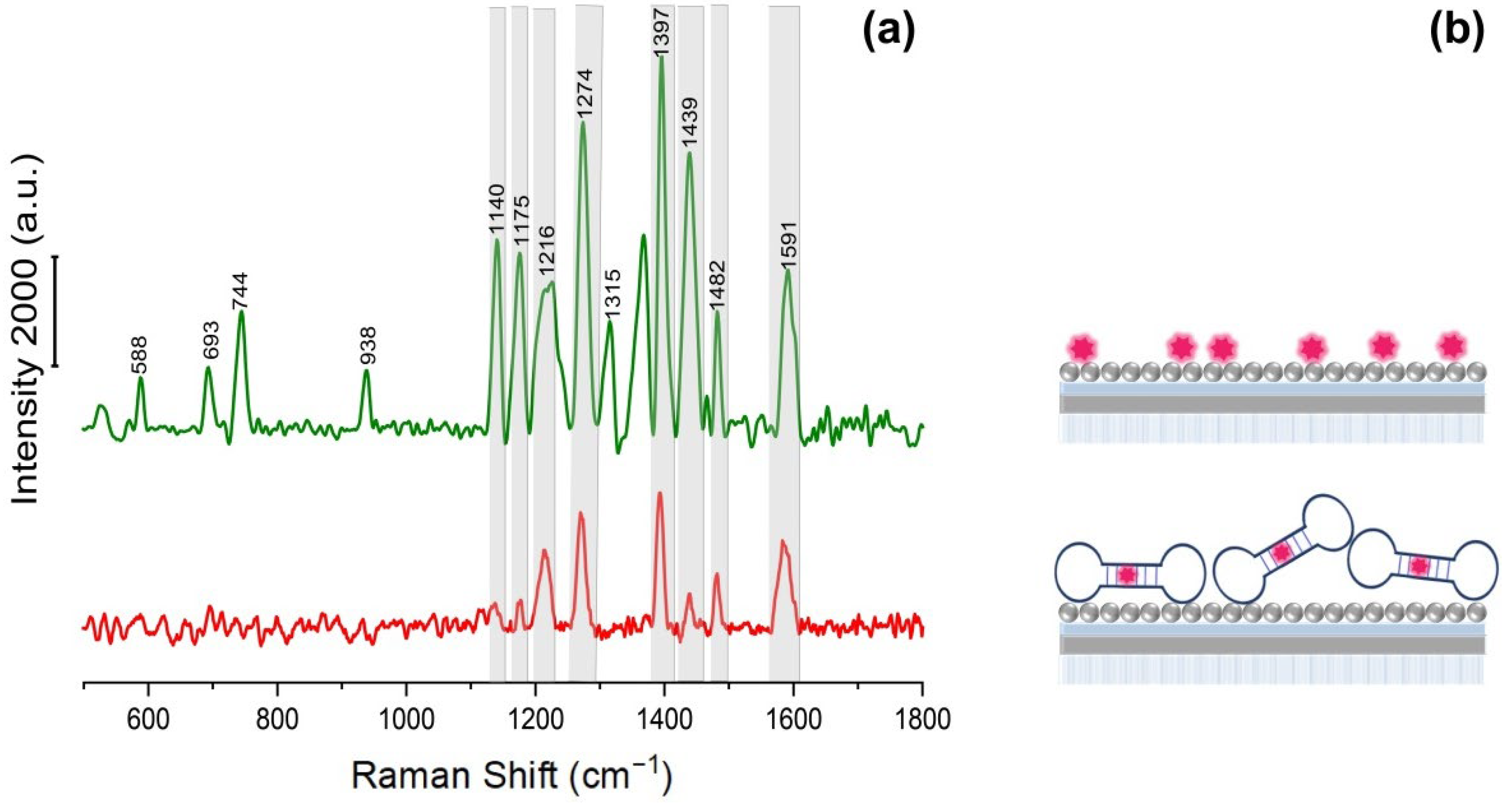
3.3. SERS-Active Aptasensor Platform for the Detection of Model of SARS-CoV-2 Virions Using Hybrid Plasmonic Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Cao, H.; Li, Y.; Sun, Z.; Yang, Y.; Jiao, T. A Novel Natural Surface-Enhanced Fluorescence System Based on Reed Leaf as Substrate for Crystal Violet Trace Detection. Chinese Phys. B 2022. [Google Scholar] [CrossRef]
- Samodelova, M.V.; Kapitanova, O.O.; Evdokimov, P.V.; Eremina, O.E.; Goodilin, E.A.; Veselova, I.A. Plasmonic Features of Free-Standing Chitosan Nanocomposite Film with Silver and Graphene Oxide for SERS Applications. Nanotechnology 2022, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, E.; De Jimenez Aberasturi, D.; Henriksen-Lacey, M.; Piñeiro, P.; Muniz, A.J.; Lahann, J.; Liz-Marzán, L.M. SERS and Fluorescence-Active Multimodal Tessellated Scaffolds for Three-Dimensional Bioimaging. ACS Appl. Mater. Interfaces 2022, 14, 20708–20719. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dong, W.; Chen, Z.; Pors, A.; Wang, Z.; Bozhevolnyi, S.I. Gap-Plasmon Based Broadband Absorbers for Enhanced Hot-Electron and Photocurrent Generation. Sci. Rep. 2016, 6, 30650. [Google Scholar] [CrossRef]
- Nielsen, M.G.; Gramotnev, D.K.; Pors, A.; Albrektsen, O.; Bozhevolnyi, S.I. Continuous Layer Gap Plasmon Resonators. Opt. Express 2011, 19, 19310. [Google Scholar] [CrossRef]
- Tatmyshevskiy, M.K.; Yakubovsky, D.I.; Kapitanova, O.O.; Solovey, V.R.; Vyshnevyy, A.A.; Ermolaev, G.A.; Klishin, Y.A.; Mironov, M.S.; Voronov, A.A.; Arsenin, A.V.; et al. Hybrid Metal-Dielectric-Metal Sandwiches for SERS Applications. Nanomaterials 2021, 11, 3205. [Google Scholar] [CrossRef]
- Novikov, S.M.; Boroviks, S.; Evlyukhin, A.B.; Tatarkin, D.E.; Arsenin, A.V.; Volkov, V.S.; Bozhevolnyi, S.I. Fractal Shaped Periodic Metal Nanostructures Atop Dielectric-Metal Substrates for SERS Applications. ACS Photonics 2020, 7, 1708–1715. [Google Scholar] [CrossRef]
- Kasera, S.; Herrmann, L.O.; Del Barrio, J.; Baumberg, J.J.; Scherman, O.A. Quantitative Multiplexing with Nano-Self-Assemblies in SERS. Sci. Rep. 2014, 4, 6785. [Google Scholar] [CrossRef]
- Pisarev, E.K.; Kapitanova, O.O.; Vesolova, I.A.; Zvereva, M.I. Amplification-Free Identification and Determination of Nucleic Acids by Surface Plasmon Resonance and Surface-Enhanced Raman Spectroscopy. Mosc. Univ. Chem. Bull. 2021, 76, 353–360. [Google Scholar] [CrossRef]
- Tadesse, L.F.; Safir, F.; Ho, C.S.; Hasbach, X.; Khuri-Yakub, B.P.; Jeffrey, S.S.; Saleh, A.A.E.; Dionne, J. Toward Rapid Infectious Disease Diagnosis with Advances in Surface-Enhanced Raman Spectroscopy. J. Chem. Phys. 2020, 152, 240902. [Google Scholar] [CrossRef] [PubMed]
- Saviñon-Flores, F.; Méndez, E.; López-Castaños, M.; Carabarin-Lima, A.; López-Castaños, K.A.; González-Fuentes, M.A.; Méndez-Albores, A. A Review on Sers-Based Detection of Human Virus Infections: Influenza and Coronavirus. Biosensors 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Ambartsumyan, O.; Gribanyov, D.; Kukushkin, V.; Kopylov, A.; Zavyalova, E. SERS-Based Biosensors for Virus Determination with Oligonucleotides as Recognition Elements. Int. J. Mol. Sci. 2020, 21, 3373. [Google Scholar] [CrossRef] [PubMed]
- Paria, D.; Kwok, K.S.; Raj, P.; Zheng, P.; Gracias, D.H.; Barman, I. Label-Free Spectroscopic SARS-CoV-2 Detection on Versatile Nanoimprinted Substrates. Nano Lett. 2022, 22, 3620–3627. [Google Scholar] [CrossRef]
- Payne, T.D.; Klawa, S.J.; Jian, T.; Kim, S.H.; Papanikolas, M.J.; Freeman, R.; Schultz, Z.D. Catching COVID: Engineering Peptide-Modified Surface-Enhanced Raman Spectroscopy Sensors for SARS-CoV-2. ACS Sensors 2021, 6, 3436–3444. [Google Scholar] [CrossRef]
- Abdullah, M.B.; Dab, C.; Almalki, M.; Alnaim, A.; Abuzir, A.; Awada, C. Ultrafast Detection of SARS-CoV-2 Spike Protein (S) and Receptor-Binding Domain (RBD) in Saliva Using Surface-Enhanced Raman Spectroscopy. Appl. Sci. 2022, 12, 5039. [Google Scholar] [CrossRef]
- Sarychev, A.K.; Sukhanova, A.; Ivanov, A.V.; Bykov, I.V.; Bakholdin, N.V.; Vasina, D.V.; Gushchin, V.A.; Tkachuk, A.P.; Nifontova, G.; Samokhvalov, P.S.; et al. Label-Free Detection of the Receptor-Binding Domain of the SARS-CoV-2 Spike Glycoprotein at Physiologically Relevant Concentrations Using Surface-Enhanced Raman Spectroscopy. Biosensors 2022, 12, 300. [Google Scholar] [CrossRef]
- Ye, J.; Yeh, Y.-T.; Xue, Y.; Wang, Z.; Zhang, N.; Liu, H.; Zhang, K.; Ricker, R.; Yu, Z.; Roder, A.; et al. Accurate Virus Identification with Interpretable Raman Signatures by Machine Learning. Proc. Natl. Acad. Sci. USA 2022, 119, e2118836119. [Google Scholar] [CrossRef]
- Shanmukh, S.; Jones, L.; Driskell, J.; Zhao, Y.; Dluhy, R.; Tripp, R.A. Rapid and Sensitive Detection of Respiratory Virus Molecular Signatures Using a Silver Nanorod Array SERS Substrate. Nano Lett. 2006, 6, 2630–2636. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.C.; Oh, B.K.; Choi, J.W. Rapid and Sensitive Determination of HIV-1 Virus Based on Surface Enhanced Raman Spectroscopy. J. Biomed. Nanotechnol. 2015, 11, 2223–2230. [Google Scholar] [CrossRef]
- Jen Lin, Y. A Rapid and Sensitive Early Diagnosis of Influenza Virus Subtype via Surface Enhanced Raman Scattering. J. Biosens. Bioelectron. 2014, 5, 1–5. [Google Scholar] [CrossRef]
- Kukushkin, V.I.; Ivanov, N.M.; Novoseltseva, A.A.; Gambaryan, A.S.; Yaminsky, I.V.; Kopylov, A.M.; Zavyalova, E.G. Highly Sensitive Detection of Influenza Virus with SERS Aptasensor. PLoS ONE 2019, 14, e0216247. [Google Scholar] [CrossRef] [PubMed]
- Ebrem Bilgin, B.; Torun, H.; Ilgü, M.; Yanik, C.; Batur, S.N.; Çelik, S.; Öztürk, M.; Dogan, Ö.; Ergönül, Ö.; Solaroglu, I.; et al. Clinical Validation of SERS Metasurface SARS-CoV-2 Biosensor; SPIE: Bellingham, WA, USA, 2022; Volume 1195708, p. 36. [Google Scholar] [CrossRef]
- Chen, H.; Park, S.G.; Choi, N.; Moon, J.I.; Dang, H.; Das, A.; Lee, S.; Kim, D.G.; Chen, L.; Choo, J. SERS Imaging-Based Aptasensor for Ultrasensitive and Reproducible Detection of Influenza Virus A. Biosens. Bioelectron. 2020, 167, 112496. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Park, S.K.; Joung, Y.; Kang, T.; Lee, M.K.; Choo, J. SERS-Based Dual-Mode DNA Aptasensors for Rapid Classification of SARS-CoV-2 and Influenza A/H1N1 Infection. Sens. Actuators B Chem. 2022, 355, 131324. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Y.; Lan, B.L.; Ramakrishnan, N. Emerging Biosensors to Detect Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Review. Biosensors 2021, 11, 434. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Acedo, P. An Electrochemical Impedance Spectroscopy-Based Aptasensor for the Determination of SARS-CoV-2-RBD Using a Carbon Nanofiber–Gold Nanocomposite Modified Screen-Printed Electrode. Biosensors 2022, 12, 142. [Google Scholar] [CrossRef]
- Qu, J.H.; Leirs, K.; Maes, W.; Imbrechts, M.; Callewaert, N.; Lagrou, K.; Geukens, N.; Lammertyn, J.; Spasic, D. Innovative FO-SPR Label-Free Strategy for Detecting Anti-RBD Antibodies in COVID-19 Patient Serum and Whole Blood. ACS Sens. 2022, 7, 477–487. [Google Scholar] [CrossRef]
- Calvo-Lozano, O.; Sierra, M.; Soler, M.; Estévez, M.C.; Chiscano-Camón, L.; Ruiz-Sanmartin, A.; Ruiz-Rodriguez, J.C.; Ferrer, R.; González-López, J.J.; Esperalba, J.; et al. Label-Free Plasmonic Biosensor for Rapid, Quantitative, and Highly Sensitive COVID-19 Serology: Implementation and Clinical Validation. Anal. Chem. 2022, 94, 975–984. [Google Scholar] [CrossRef]
- Besselink, G.; Schütz-trilling, A.; Veerbeek, J.; Verbruggen, M.; Van Der Meer, A. Asymmetric Mach—Zehnder Interferometric Biosensing for Quantitative and Sensitive Multiplex Detection of Anti-SARS-CoV-2 Antibodies in Human Plasma. Biosensors 2022, 12, 553. [Google Scholar] [CrossRef]
- Svobodova, M.; Skouridou, V.; Jauset-Rubio, M.; Viéitez, I.; Fernández-Villar, A.; Cabrera Alvargonzalez, J.J.; Poveda, E.; Bofill, C.B.; Sans, T.; Bashammakh, A.; et al. Aptamer Sandwich Assay for the Detection of SARS-CoV-2 Spike Protein Antigen. ACS Omega 2021, 6, 35657–35666. [Google Scholar] [CrossRef]
- Chen, H.; Park, S.G.; Choi, N.; Kwon, H.J.; Kang, T.; Lee, M.K.; Choo, J. Sensitive Detection of SARS-CoV-2 Using a SERS-Based Aptasensor. ACS Sens. 2021, 6, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, X.; Ma, R.; Deng, S.; Wang, X.; Wang, X.; Zhang, X.; Huang, X.; Liu, Y.; Li, G.; et al. Ultra-Fast and Onsite Interrogation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Waters via Surface Enhanced Raman Scattering (SERS). Water Res. 2021, 200, 117243. [Google Scholar] [CrossRef] [PubMed]
- Khrenova, M.G.; Nikiforova, L.; Grabovenko, F.; Orlova, N.; Sinegubova, M. In Vitro Selection of an Aptamer Targeting SARS-CoV-2 Spike Protein with Nanopore Sequence Identification Reveals Discrimination Between the Authentic Strain and Omicron. ChemRxiv 2022, 1–23. [Google Scholar] [CrossRef]
- Song, Y.; Song, J.; Wei, X.; Huang, M.; Sun, M.; Zhu, L.; Lin, B.; Shen, H.; Zhu, Z.; Yang, C. Discovery of Aptamers Targeting the Receptor-Binding Domain of the SARS-CoV-2 Spike Glycoprotein. Anal. Chem. 2020, 92, 9895–9900. [Google Scholar] [CrossRef]
- Sinegubova, M.V.; Orlova, N.A.; Kovnir, S.V.; Dayanova, L.K.; Vorobiev, I.I. High-Level Expression of the Monomeric SARS-CoV-2 S Protein RBD 320-537 in Stably Transfected CHO Cells by the EEF1A1-Based Plasmid Vector. PLoS ONE 2021, 16, e0242890. [Google Scholar] [CrossRef]
- Novikov, S.M.; Streletskiy, O.A.; Doroshina, N.V.; Yakubovsky, D.I.; Mironov, M.S.; Sychev, V.V.; Voronov, A.A.; Arsenin, A.V.; Volkov, V.S. Long-Term Stable Structures Formed by Ion-Beam Modification of Silver Film for SERS Applications. J. Phys. Conf. Ser. 2021, 2015, 8–12. [Google Scholar] [CrossRef]
- Waghmare, M.; Khade, B.; Chaudhari, P.; Dongre, P. Multiple Layer Formation of Bovine Serum Albumin on Silver Nanoparticles Revealed by Dynamic Light Scattering and Spectroscopic Techniques. J. Nanoparticle Res. 2018, 20, 185. [Google Scholar] [CrossRef]
- Grabovenko, F.; Nikiforova, L.; Yanenko, B.; Ulitin, A.; Loktyushov, E.; Zatsepin, T.; Zavyalova, E.; Zvereva, M. Glycosylation of Receptor Binding Domain of SARS-CoV-2 S-Protein Influences on Binding to Immobilized DNA Aptamers. Int. J. Mol. Sci. 2022, 23, 557. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoint, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Eremina, O.E.; Zatsepin, T.S.; Farzan, V.M.; Veselova, I.A.; Zvereva, M.I. DNA Detection by Dye Labeled Oligonucleotides Using Surface Enhanced Raman Spectroscopy. Mendeleev Commun. 2020, 30, 18–21. [Google Scholar] [CrossRef]
- Jaworska, A.; Pyrak, E.; Kudelski, A. Comparison of the Efficiency of Generation of Raman Radiation by Various Raman Reporters Connected via DNA Linkers to Different Plasmonic Nano-Structures. Vib. Spectrosc. 2019, 101, 34–39. [Google Scholar] [CrossRef]
- Despres, H.W.; Mills, M.G.; Shirley, D.J.; Schmidt, M.M.; Huang, M.L.; Roychoudhury, P.; Jerome, K.R.; Greninger, A.L.; Bruce, E.A. Measuring Infectious SARS-CoV-2 in Clinical Samples Reveals a Higher Viral Titer:RNA Ratio for Delta and Epsilon vs. Alpha Variants. Proc. Natl. Acad. Sci. USA 2022, 119, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Yoon, S.Y.; Lim, C.S.; Yoon, J. Comparison of Three Molecular Diagnostic Assays for SARS-CoV-2 Detection: Evaluation of Analytical Sensitivity and Clinical Performance. J. Clin. Lab. Anal. 2022, 36, e24242. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, Y.; Zhang, R.; Zhang, R.; Li, J. Comparison of Analytical Sensitivity of SARS-CoV-2 Molecular Detection Kits. Int. J. Infect. Dis. 2021, 111, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Cui, J.; Huang, L.; Du, B.; Chen, L.; Xue, G.; Li, S.; Zhang, W.; Zhao, L.; Sun, Y.; et al. Rapid and Visual Detection of 2019 Novel Coronavirus (SARS-CoV-2) by a Reverse Transcription Loop-Mediated Isothermal Amplification Assay. Clin. Microbiol. Infect. 2020, 26, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; To, K.K.W.; Chan, J.F.W.; Li, C.P.Y.; Chen, H.; Yuen, K.Y. Analytical Sensitivity of Seven Point-of-Care Influenza Virus Detection Tests and Two Molecular Tests for Detection of Avian Origin H7N9 and Swine Origin H3N2 Variant Influenza a Viruses. J. Clin. Microbiol. 2013, 51, 3160–3161. [Google Scholar] [CrossRef]
- Peters, T.R.; Blakeney, E.; Vannoy, L.; Poehling, K.A. Evaluation of the Limit of Detection of the BD VeritorTM System Flu A+B Test and Two Rapid Influenza Detection Tests for Influenza Virus. Diagn. Microbiol. Infect. Dis. 2013, 75, 200–202. [Google Scholar] [CrossRef]
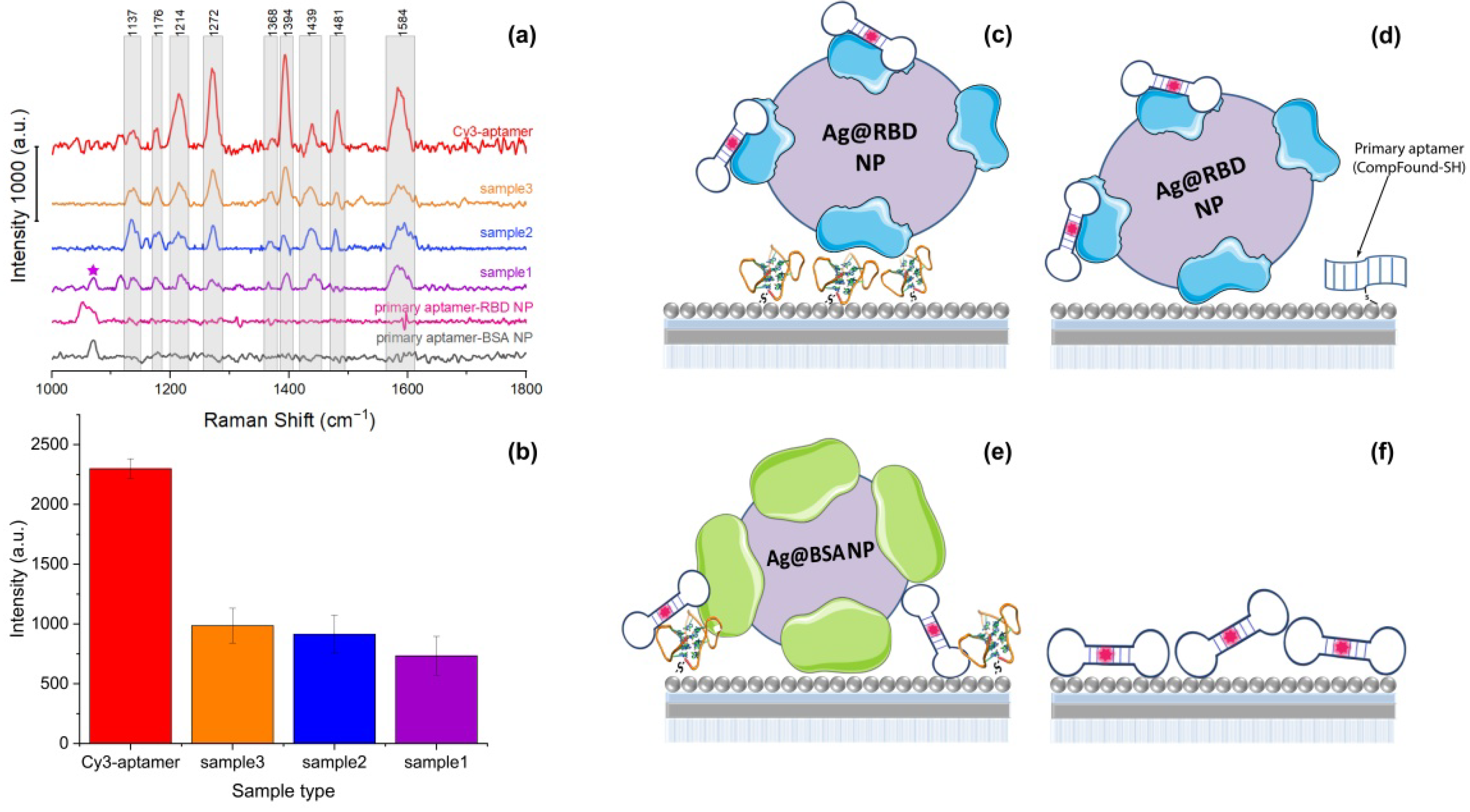
| Primary Aptamer | Nanoparticles | Secondary Aptamer | Comment | |
|---|---|---|---|---|
| Sample 1 | Found-SH | RBD NP | RBD-1C-Cy3 | Experiment |
| Sample 2 | CompFound-SH | RBD NP | RBD-1C-Cy3 | Control with nonspecific primary oligonucleotide |
| Sample 3 | Found-SH | BSA NP | RBD-1C-Cy3 | Control with nonspecific protein instead of RBD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samodelova, M.V.; Kapitanova, O.O.; Meshcheryakova, N.F.; Novikov, S.M.; Yarenkov, N.R.; Streletskii, O.A.; Yakubovsky, D.I.; Grabovenko, F.I.; Zhdanov, G.A.; Arsenin, A.V.; et al. Model of the SARS-CoV-2 Virus for Development of a DNA-Modified, Surface-Enhanced Raman Spectroscopy Sensor with a Novel Hybrid Plasmonic Platform in Sandwich Mode. Biosensors 2022, 12, 768. https://doi.org/10.3390/bios12090768
Samodelova MV, Kapitanova OO, Meshcheryakova NF, Novikov SM, Yarenkov NR, Streletskii OA, Yakubovsky DI, Grabovenko FI, Zhdanov GA, Arsenin AV, et al. Model of the SARS-CoV-2 Virus for Development of a DNA-Modified, Surface-Enhanced Raman Spectroscopy Sensor with a Novel Hybrid Plasmonic Platform in Sandwich Mode. Biosensors. 2022; 12(9):768. https://doi.org/10.3390/bios12090768
Chicago/Turabian StyleSamodelova, Mariia V., Olesya O. Kapitanova, Nadezda F. Meshcheryakova, Sergey. M. Novikov, Nikita R. Yarenkov, Oleg A. Streletskii, Dmitry I. Yakubovsky, Fedor I. Grabovenko, Gleb A. Zhdanov, Aleksey V. Arsenin, and et al. 2022. "Model of the SARS-CoV-2 Virus for Development of a DNA-Modified, Surface-Enhanced Raman Spectroscopy Sensor with a Novel Hybrid Plasmonic Platform in Sandwich Mode" Biosensors 12, no. 9: 768. https://doi.org/10.3390/bios12090768
APA StyleSamodelova, M. V., Kapitanova, O. O., Meshcheryakova, N. F., Novikov, S. M., Yarenkov, N. R., Streletskii, O. A., Yakubovsky, D. I., Grabovenko, F. I., Zhdanov, G. A., Arsenin, A. V., Volkov, V. S., Zavyalova, E. G., Veselova, I. A., & Zvereva, M. I. (2022). Model of the SARS-CoV-2 Virus for Development of a DNA-Modified, Surface-Enhanced Raman Spectroscopy Sensor with a Novel Hybrid Plasmonic Platform in Sandwich Mode. Biosensors, 12(9), 768. https://doi.org/10.3390/bios12090768








