A Highly Integrated and Diminutive Fluorescence Detector for Point-of-Care Testing: Dual Negative Feedback Light-Emitting Diode (LED) Drive and Photoelectric Processing Circuits Design and Implementation
Abstract
:1. Introduction
2. Materials and Methods
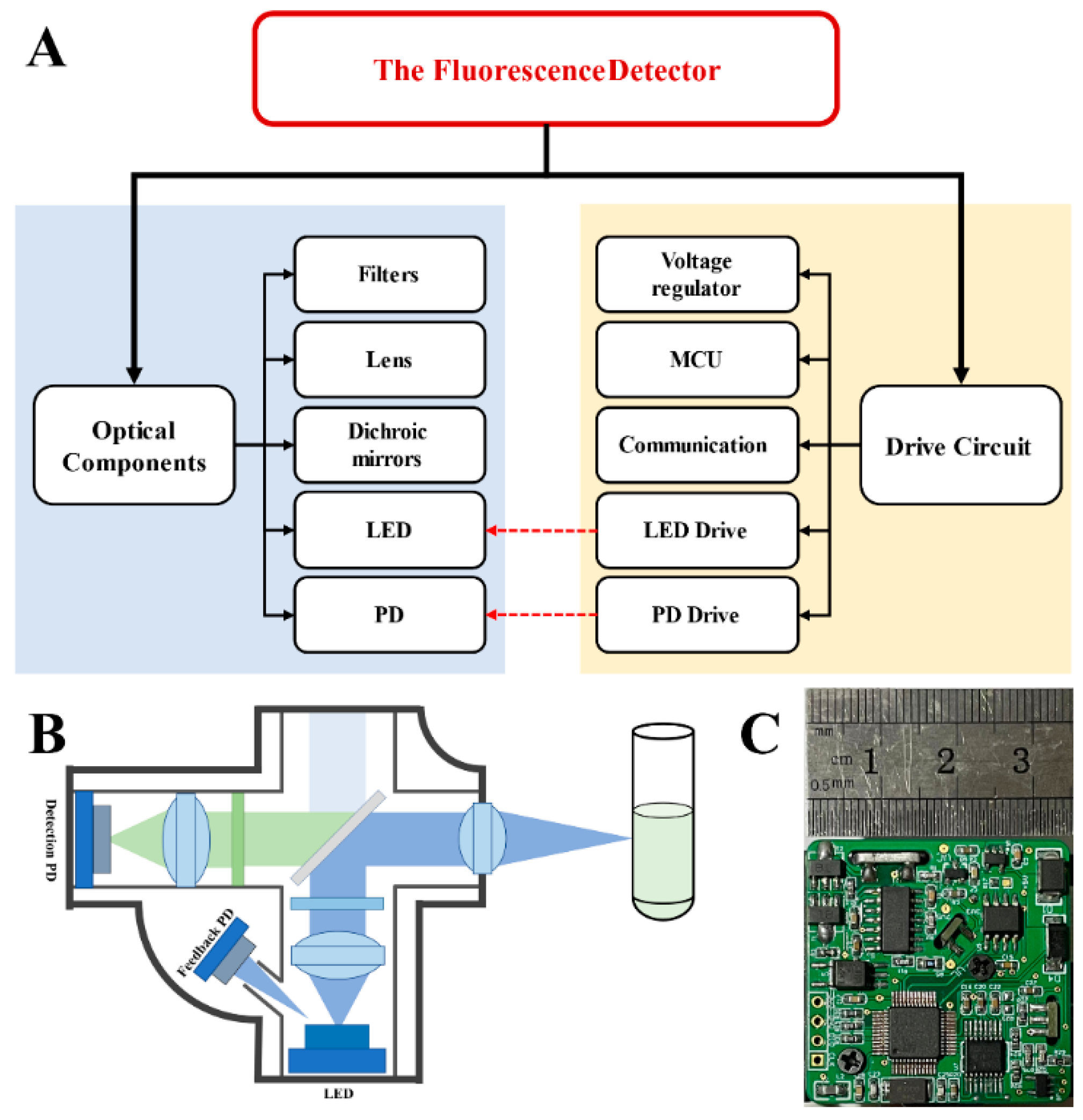
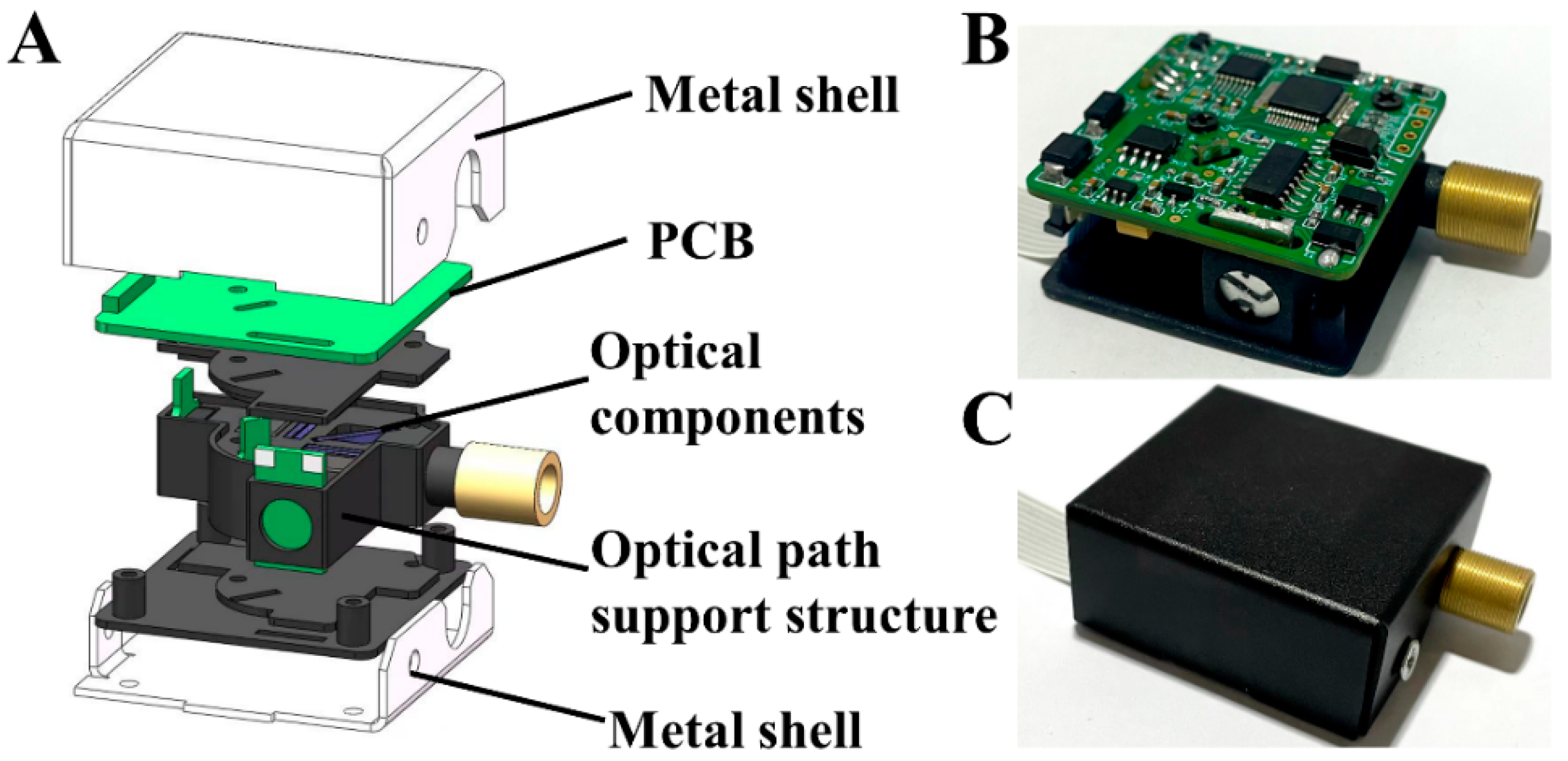
2.1. Structure of the Fluorescence Detector
2.2. Fluorescence Detector Circuit Design
2.2.1. Detector Operating Principle
2.2.2. The Current–Light Dual Negative Feedback LED Drive Circuit
2.2.3. The Photoelectric Processing Circuit
2.3. A Test Platform Based on the Fluorescence Detector
3. Results and Discussion
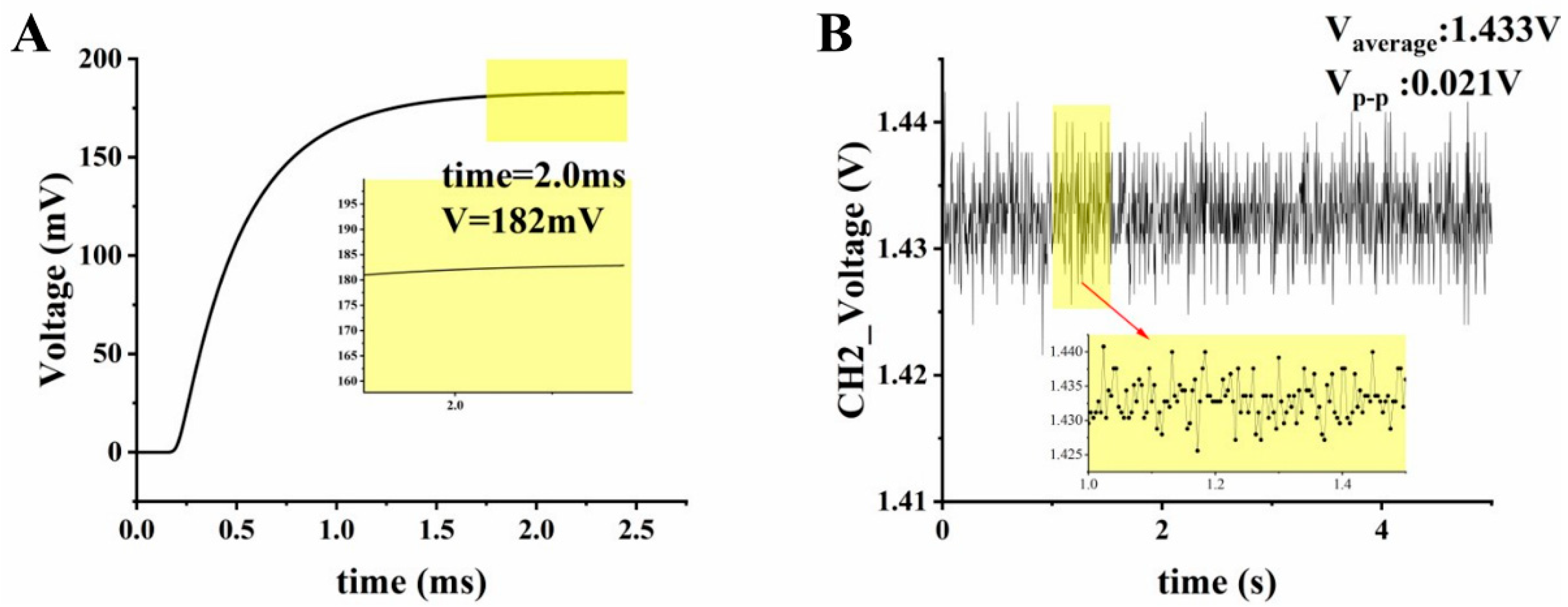
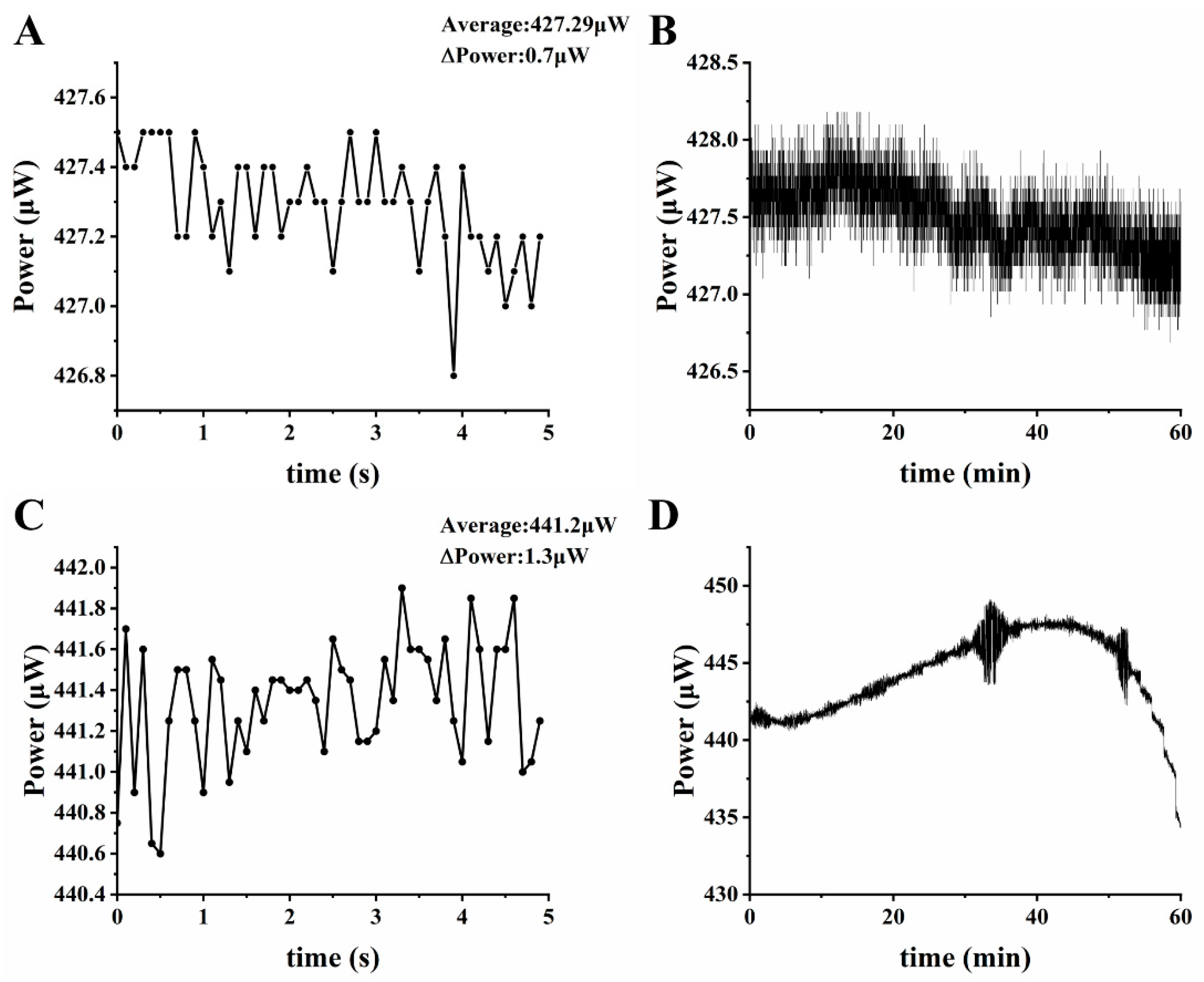
3.1. LED Light Intensity and Stability Testing
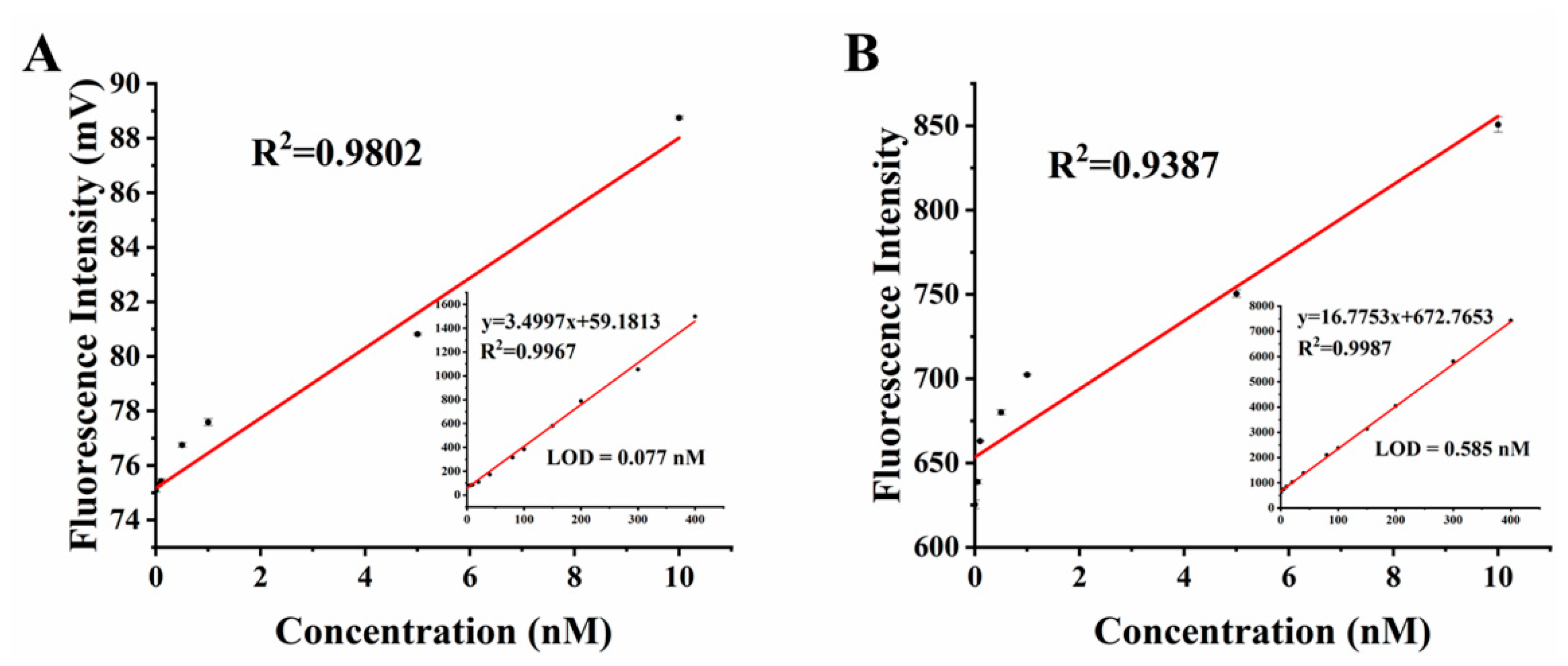
3.2. Fluorescein Sodium Solution Concentration Detection Experiment
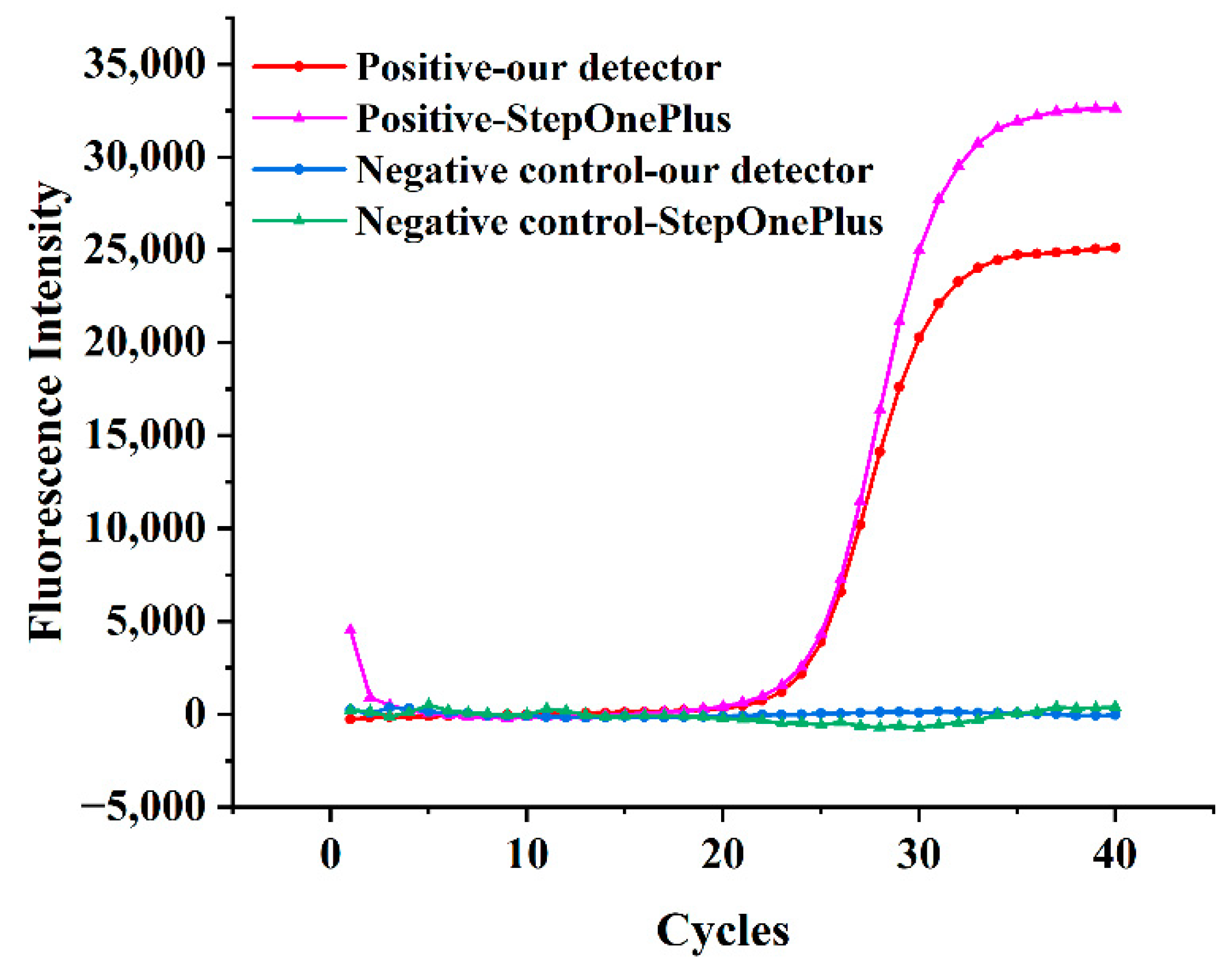
3.3. Real-Time Fluorescence PCR Test for Legionella pneumophila
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, X.X.; Chen, Q.S.; Liu, W.; Li, H.F.; Lin, J.M. A portable microchip for ultrasensitive and high-throughput assay of thrombin by rolling circle amplification and hemin/G-quadruplex system. Biosens. Bioelectron. 2014, 56, 71–76. [Google Scholar] [CrossRef]
- Chin, C.D.; Laksanasopin, T.; Cheung, Y.K.; Steinmiller, D.; Linder, V.; Parsa, H.; Wang, J.; Moore, H.; Rouse, R.; Umviligihozo, G.; et al. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat. Med. 2011, 17, 1015–1019. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, Y. Portable and Quantitative Detection of Protein Biomarkers and Small Molecular Toxins Using Antibodies and Ubiquitous Personal Glucose Meters. Anal. Chem. 2012, 84, 4174–4178. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Y.; Chen, Z.; Mou, X.; Li, Z.; Su, E.; Deng, Y.; He, N. From Sample to Answer: A Low-Cost Disposable Cartridge for Epidemic Detection on Site Based on 3D Printing Technology. Nanosci. Nanotechnol. Lett. 2016, 8, 1118–1126. [Google Scholar] [CrossRef]
- He, Z.; Tong, Z.; Tan, B.; He, X.; Zhang, T.; Guo, Y.; Jin, L.; He, N.; Li, S.; Chen, Z. Rapid Detection of DNA Methylation with a Novel Real-Time Fluorescence Recombinase-Aided Amplification Assay. J. Biomed. Nanotechnol. 2021, 17, 1364–1370. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, T.; Chen, Z.; Jin, L.; Wu, Z.; Yan, J.; Zhao, X.; Cai, L.; Deng, Y.; Guo, Y.; et al. The point-of-care-testing of nucleic acids by chip, cartridge and paper sensors. Chin. Chem. Lett. 2021, 32, 3675–3686. [Google Scholar] [CrossRef]
- Xie, H.; Di, K.; Huang, R.; Khan, A.; Xia, Y.; Xu, H.; Liu, C.; Tan, T.; Tian, X.; Shen, H.; et al. Extracellular vesicles based electrochemical biosensors for detection of cancer cells: A review. Chin. Chem. Lett. 2020, 31, 1737–1745. [Google Scholar] [CrossRef]
- He, L.; Huang, R.; Xiao, P.; Liu, Y.; Jin, L.; Liu, H.; Li, S.; Deng, Y.; Chen, Z.; Li, Z.; et al. Current signal amplification strategies in aptamer-based electrochemical biosensor: A review. Chin. Chem. Lett. 2021, 32, 1593–1602. [Google Scholar] [CrossRef]
- Shin, Y.H.; Gutierrez-Wing, M.T.; Choi, J.W. Review-Recent Progress in Portable Fluorescence Sensors. J. Electrochem. Soc. 2021, 168, 17502. [Google Scholar] [CrossRef]
- Lu, Y.; Ji, H.; Chen, Y.; Li, Z.; Timira, V. A systematic review on the recent advances of wheat allergen detection by mass spectrometry: Future prospects. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, N. Electrochemical Biosensors Based on Micro-fabricated Devices for Point-of-care Testing: A Review. Electroanalysis 2022, 34, 168–183. [Google Scholar] [CrossRef]
- Trotter, M.; Borst, N.; Thewes, R.; von Stetten, F. Review: Electrochemical DNA sensing—Principles, commercial systems, and applications. Biosens. Bioelectron. 2020, 154, 112069. [Google Scholar] [CrossRef] [PubMed]
- Tepeli, Y.; Anik, U. Electrochemical biosensors for influenza virus a detection: The potential of adaptation of these devices to POC systems. Sens. Actuators B-Chem. 2018, 254, 377–384. [Google Scholar] [CrossRef]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab Chip 2020, 20, 9–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R.; Yang, X.; Qi, H.; Zhang, C. Recent advances in electrogenerated chemiluminescence biosensing methods for pharmaceuticals. J. Pharm. Anal. 2019, 9, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Xu, C. Research progress on chemiluminescence immunoassay combined with novel technologies. TrAC Trends Anal. Chem. 2020, 124, 115780. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, J. Chemiluminescence-based aptasensors for various target analytes. Luminescence 2018, 33, 1298–1305. [Google Scholar] [CrossRef]
- He, L.; Yang, H.; Xiao, P.; Singh, R.; He, N.; Liu, B.; Li, Z. Highly Selective, Sensitive and Rapid Detection of Escherichia coli O157: H7 Using Duplex PCR and Magnetic Nanoparticle-Based Chemiluminescence Assay. J. Biomed. Nanotechnol. 2017, 13, 1243–1252. [Google Scholar] [CrossRef]
- Wang, J.; Lu, P.; Yan, J.; Zhang, Y.; Huang, L.; Ali, Z.; Liu, B.; Li, Z.; He, N. Rapid and Sensitive Detection of RNA Viruses Based on Reverse Transcription Loop-Mediated Isothermal Amplification, Magnetic Nanoparticles, and Chemiluminescence. J. Biomed. Nanotechnol. 2016, 12, 710–716. [Google Scholar] [CrossRef]
- Yang, H.; Liang, W.; Si, J.; Li, Z.; He, N. Long Spacer Arm-Functionalized Magnetic Nanoparticle Platform for Enhanced Chemiluminescent Detection of Hepatitis B Virus. J. Biomed. Nanotechnol. 2014, 10, 3610–3619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lin, Q.; Huang, L.; Zhai, Y.; Liu, Y.; Deng, Y.; Su, E.; He, N. Ultrasensitive chemiluminescence immunoassay with enhanced precision for the detection of cTnI amplified by acridinium ester-loaded microspheres and internally calibrated by magnetic fluorescent nanoparticles. Nanoscale 2021, 13, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, H.; Wang, L.; Cao, L.; Sui, J.; Wang, K. Optical sensing techniques for rapid detection of agrochemicals: Strategies, challenges, and perspectives. Sci. Total Environ. 2022, 838, 156515. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, J.; Wang, X.; Peng, H.; Xiong, H.; Chen, L. Strategies of molecular imprinting-based fluorescence sensors for chemical and biological analysis. Biosens. Bioelectron. 2018, 112, 54–71. [Google Scholar] [CrossRef]
- Ramachandran, A.; Huyke, D.A.; Sharma, E.; Sahoo, M.K.; Huang, C.H.; Banaei, N.; Pinsky, B.A.; Santiago, J.G. Electric field-driven microfluidics for rapid CRISPR-based diagnostics and its application to detection of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 29518–29525. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, B.; Zhang, H.; Liu, Y.; Xie, P. Participation of fluorescence technology in the cross-disciplinary detection of microcystins. Coord. Chem. Rev. 2022, 457, 214416. [Google Scholar] [CrossRef]
- Fu, L.; Qian, Y.; Zhou, J.; Zheng, L.; Wang, Y. Fluorescence-based quantitative platform for ultrasensitive food allergen detection: From immunoassays to DNA sensors. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3343–3364. [Google Scholar] [CrossRef]
- Zou, Z.; Deng, Y.; Hu, J.; Jiang, X.; Hou, X. Recent trends in atomic fluorescence spectrometry towards miniaturized instrumentation-A review. Anal. Chim. Acta 2018, 1019, 25–37. [Google Scholar] [CrossRef]
- Lambert, P. A literature review of portable fluorescence-based oil-in-water monitors. J. Hazard. Mater. 2003, 102, 39–55. [Google Scholar] [CrossRef]
- Wilkes, T.C.; McGonigle, A.J.S.; Willmott, J.R.; Pering, T.D.; Cook, J.M. Low-cost 3D printed 1 nm resolution smartphone sensor-based spectrometer: Instrument design and application in ultraviolet spectroscopy. Opt. Lett. 2017, 42, 4323–4326. [Google Scholar] [CrossRef]
- Shin, Y.-H.; Barnett, J.Z.; Gutierrez-Wing, M.T.; Rusch, K.A.; Choi, J.-W. A hand-held fluorescent sensor platform for selectively estimating green algae and cyanobacteria biomass. Sens. Actuators B-Chem. 2018, 262, 938–946. [Google Scholar] [CrossRef]
- Kozma, P.; Lehmann, A.; Wunderlich, K.; Michel, D.; Schumacher, S.; Ehrentreich-Foerster, E.; Bier, F.F. A novel handheld fluorescent microarray reader for point-of-care diagnostic. Biosens. Bioelectron. 2013, 47, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Katzmeier, F.; Aufinger, L.; Dupin, A.; Quintero, J.; Lenz, M.; Bauer, L.; Klumpe, S.; Sherpa, D.; Durr, B.; Honemann, M.; et al. A low-cost fluorescence reader for in vitro transcription and nucleic acid detection with Cas13a. PLoS ONE 2019, 14, e0220091. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liao, P.; Li, S.; He, N.; Chen, Z.; Chen, H.; Wu, Y.; Su, E.; Wang, Z. Design and Implementation of the Control Circuit and Driver for a Cassette-Based Fully Automated Nucleic Acid Analyzer. Nanosci. Nanotechnol. Lett. 2017, 9, 650–659. [Google Scholar] [CrossRef]
- Spibey, C.A.; Jackson, P.; Herick, K. A unique charge-coupled device/xenon arc lamp based imaging system for the accurate detection and quantitation of multicolour fluorescence. Electrophoresis 2001, 22, 829–836. [Google Scholar] [CrossRef]
- Mukunda, D.C.; Joshi, V.K.; Mahato, K.K. Light emitting diodes (LEDs) in fluorescence-based analytical applications: A review. Appl. Spectrosc. Rev. 2022, 57, 1–38. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Q.; Hong, Y.; Zheng, C. Design of high-power buck-boost LED constant current drive circuit. In Proceedings of the 11th IEEE Conference on Industrial Electronics and Applications, ICIEA 2016, Hefei, China, 5–7 June 2016; pp. 2223–2226. [Google Scholar]
- Dai, G.; Yu, F.; Wang, X.; Li, W. Design and implementation of a high precision and wide range adjustable LED drive controller. J. Semicond. 2010, 31, 76–81. [Google Scholar] [CrossRef]
- Nishikawa, M.; Ishizuka, Y.; Matsuo, H.; Shigernatsu, K. An LED Drive Circuit with Constant Output Current Control and Constant Luminance Control. In Proceedings of the 28th International Telecommunications Energy Conference, Providence, RI, USA, 10–14 September 2006. [Google Scholar]
- Murari, K.; Semwal, G.; Vashisth, P. Photodiode Trans-Impedance Configuration with DC Compensation. In Proceedings of the International Conference on Optics and Electro-optics, ICOL 2019, Dehradun, India, 19–22 October 2019; pp. 127–129. [Google Scholar]
- Sun, D. Optimized design of photodiode amplifier circuit and its application in gas sensing. In Proceedings of the 2020 Applied Optics and Photonics China: Optical Sensing and Imaging Technology, AOPC 2020, Xiamen, China, 25–27 August 2020. [Google Scholar]
- Yubo, L.; Zhen, P. Design of Photodiode Circuit Based on Signal Acquisition. Opt. Mem. Neural Netw. 2019, 28, 50–57. [Google Scholar] [CrossRef]
- Spahic, L.; Ilic, S.; Andelkovic, M.; Palma, A.J.; Ristic, G.S. Application of Transimpedance Amplifiers in PIN Photodiode Dosimetry. In Proceedings of the 32nd IEEE International Conference on Microelectronics, MIEL 2021, Nis, Serbia, 12–14 September 2021; pp. 317–320. [Google Scholar]
- Beguni, C.; Cailean, A.-M.; Avatamanitei, S.-A.; Dimian, M. Photodiode Amplifier with Transimpedance and Differential Stages for Automotive Visible Light Applications. In Proceedings of the 15th International Conference on Development and Application Systems, DAS 2020, Suceava, Romania, 21–23 May 2020; pp. 127–132. [Google Scholar]
- Lowry, M.; Fakayode, S.O.; Geng, M.L.; Baker, G.A.; Wang, L.; McCarroll, M.E.; Patonay, G.; Warner, I.M. Molecular fluorescence, phosphorescence, and chemiluminescence spectrometry. Anal. Chem. 2008, 80, 4551–4574. [Google Scholar] [CrossRef]
- Renz, M. Fluorescence microscopy-A historical and technical perspective. Cytom. Part A 2013, 83, 767–779. [Google Scholar] [CrossRef]



| Manufacturer | PD | Spectral Response Range (nm) | Peak Sensitivity Wavelength (nm) λ | Photosensitivity (A/W) λ |
|---|---|---|---|---|
| Hamamatsu Photonics | S1337-33BR | 340 to 1100 | 960 | 0.62 |
| S1133-14 | 320 to 1000 | 720 | 0.40 | |
| S1227-33BR | 190 to 1000 | 720 | 0.43 | |
| S2387-33R | 340 to 1100 | 960 | 0.58 | |
| OSI Optoelectronics | PIN-13DSB | 350 to 1100 | 960 | 0.52 |
| First Sensor | PS7-5B-TO5 | 350 to 1000 | 750 | 0.42 |
| PS11.9-5-TO5 | 400 to 1100 | 800 | 0.52 |
| Reagents | Volume |
|---|---|
| LP Nucleic Acid Fluorescent PCR Assay Mix | 36 μL |
| Enzyme (Taq + UNG) | 0.4 μL |
| LP Positive Control | 2 μL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Fang, Y.; Liu, H.; Su, X.; Chen, Z.; Li, S.; He, N. A Highly Integrated and Diminutive Fluorescence Detector for Point-of-Care Testing: Dual Negative Feedback Light-Emitting Diode (LED) Drive and Photoelectric Processing Circuits Design and Implementation. Biosensors 2022, 12, 764. https://doi.org/10.3390/bios12090764
Wang Y, Fang Y, Liu H, Su X, Chen Z, Li S, He N. A Highly Integrated and Diminutive Fluorescence Detector for Point-of-Care Testing: Dual Negative Feedback Light-Emitting Diode (LED) Drive and Photoelectric Processing Circuits Design and Implementation. Biosensors. 2022; 12(9):764. https://doi.org/10.3390/bios12090764
Chicago/Turabian StyleWang, Yue, Yile Fang, Haoran Liu, Xiangyi Su, Zhu Chen, Song Li, and Nongyue He. 2022. "A Highly Integrated and Diminutive Fluorescence Detector for Point-of-Care Testing: Dual Negative Feedback Light-Emitting Diode (LED) Drive and Photoelectric Processing Circuits Design and Implementation" Biosensors 12, no. 9: 764. https://doi.org/10.3390/bios12090764
APA StyleWang, Y., Fang, Y., Liu, H., Su, X., Chen, Z., Li, S., & He, N. (2022). A Highly Integrated and Diminutive Fluorescence Detector for Point-of-Care Testing: Dual Negative Feedback Light-Emitting Diode (LED) Drive and Photoelectric Processing Circuits Design and Implementation. Biosensors, 12(9), 764. https://doi.org/10.3390/bios12090764







