Carbon Nanotube and Its Derived Nanomaterials Based High Performance Biosensing Platform
Abstract
1. Introduction
2. Preparation of Carbon Nanotubes (CNTs)
2.1. Electric-Arc Discharge
2.2. Laser Ablation
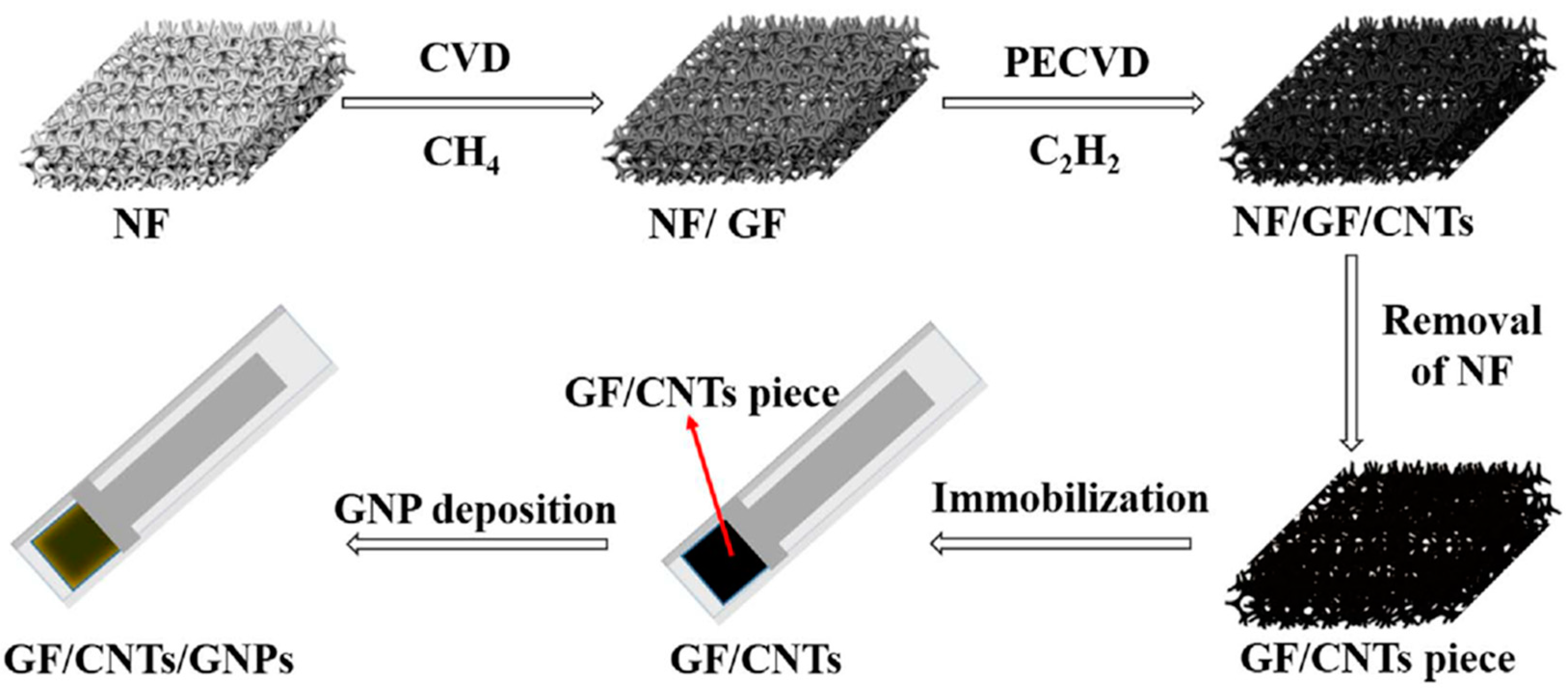
2.3. Chemical Vapor Deposition (CVD)
2.4. Others
3. Mechanism of Sensing
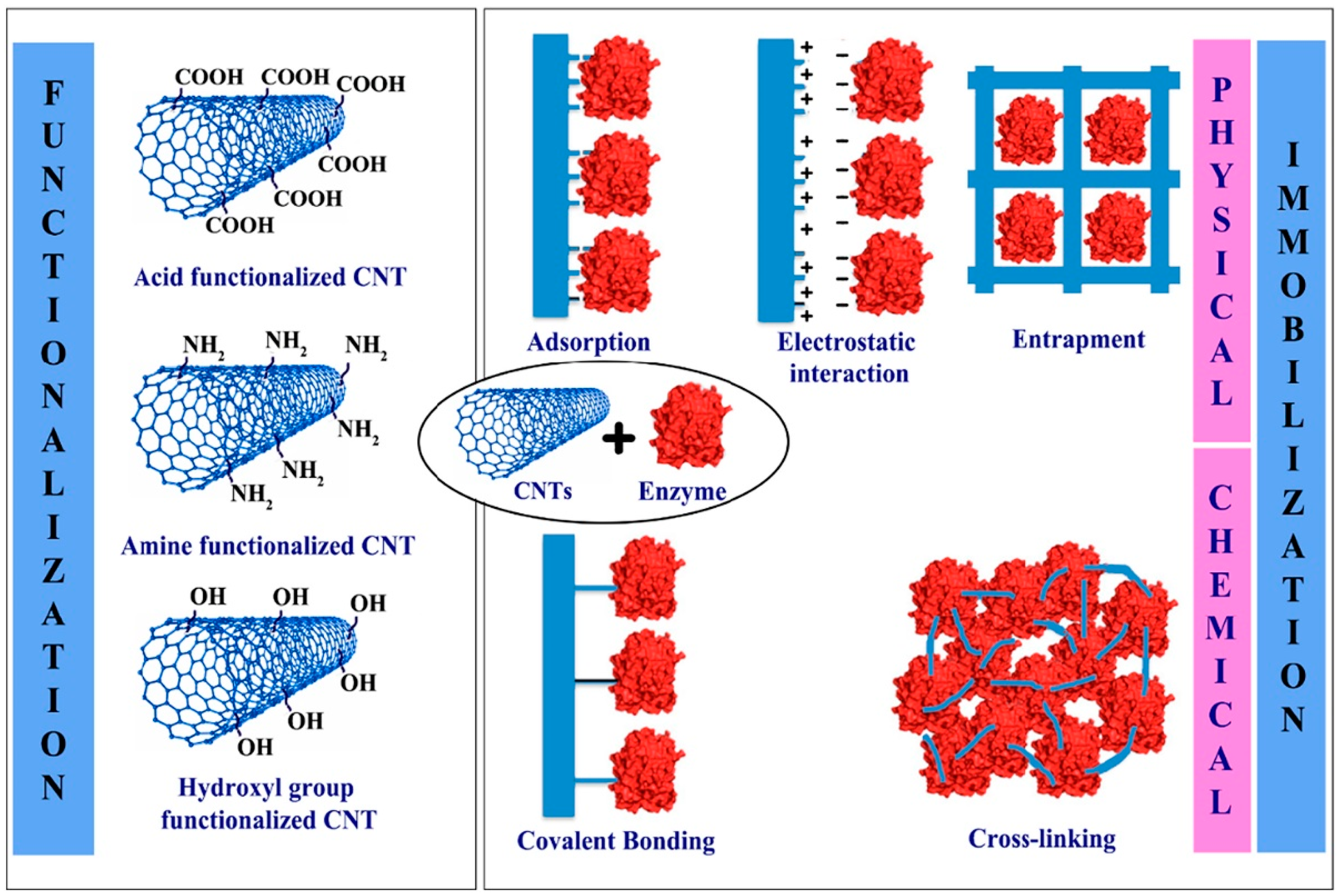
3.1. Physical Sensing
3.2. Chemical Sensing
4. Toxicology Profile of Carbon Nanotubes
5. Applications of Carbon Nanotubes as Biosensors
5.1. Carbon Nanotube-Based Sensors for Detection of Cancer
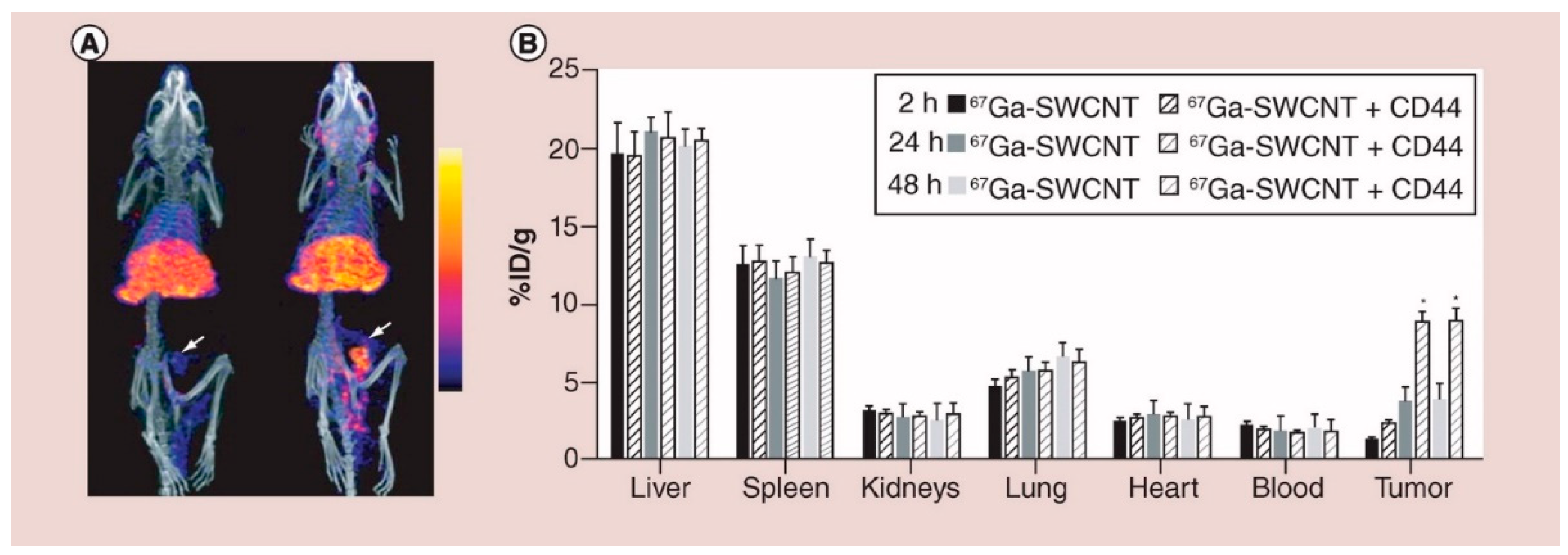
5.1.1. CD44 Expressing Cancer Cell
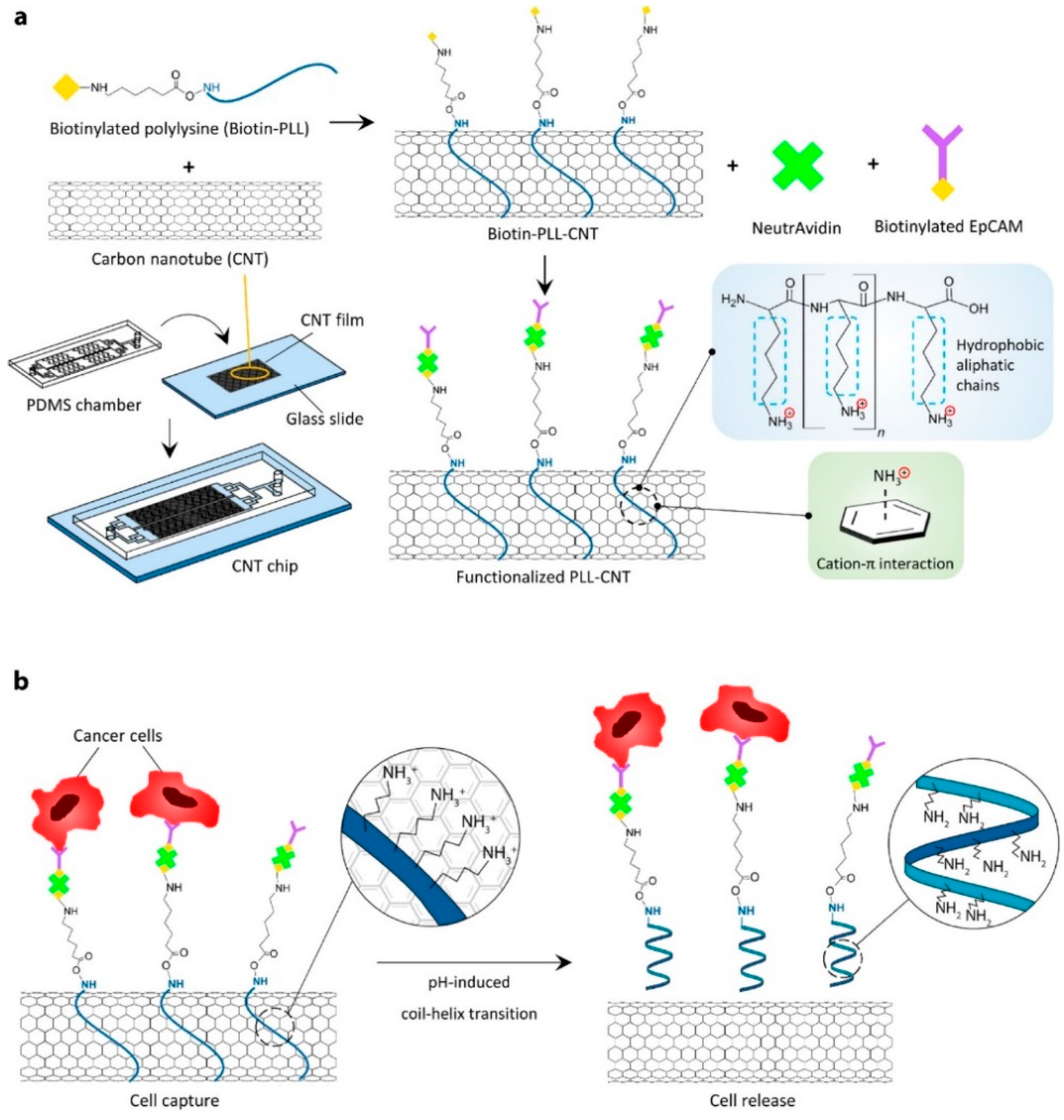
5.1.2. EpCAM Expressing Cancer Cell
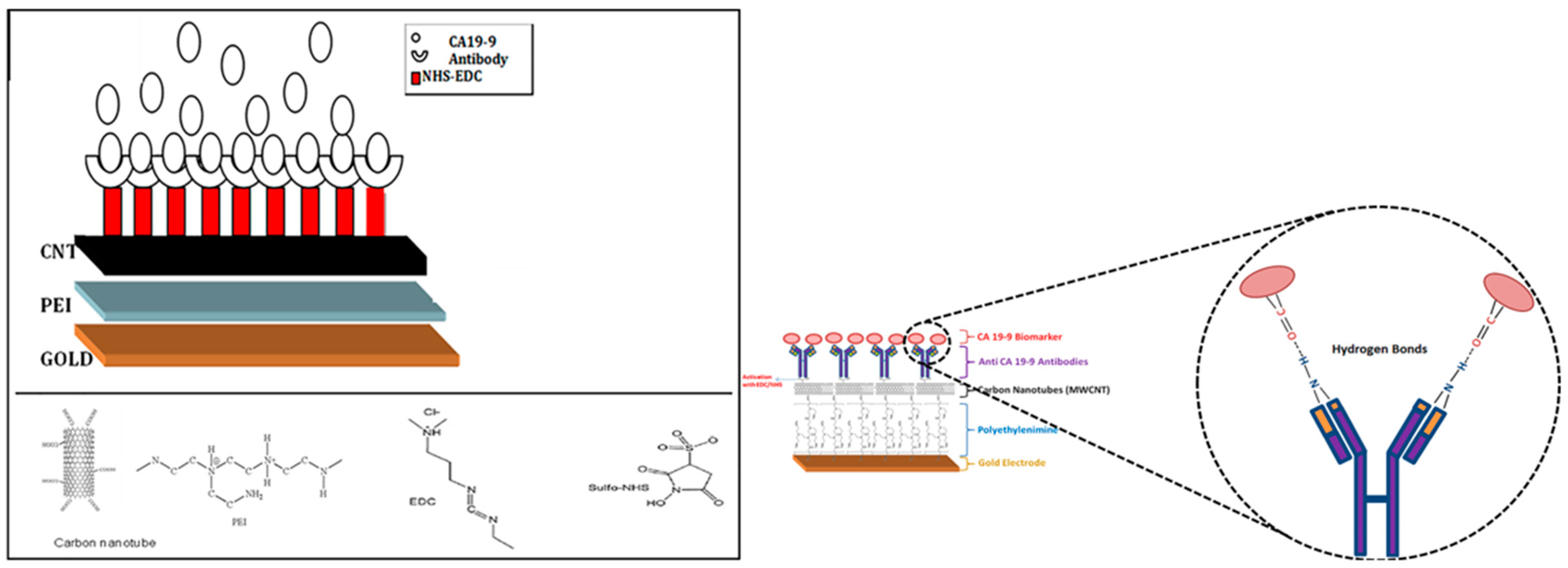
5.1.3. CA19-9 Expressing Cancer Cell
5.1.4. VEGF Expressing Cancer Cell
5.1.5. MUC−1 Expressing Cancer Cell
5.2. Carbon Nanotube-Based Sensors for Detection of Diabetes
5.2.1. SWCNTs Based Nanosensors
5.2.2. MWCNTs Based Nanosensors
5.3. Carbon Nanotube for Biological Components Detection
5.4. Carbon Nanotube for Bacteria and Virus Detection
6. Commercialization of Carbon Nanotube as Sensors
7. Conclusions
8. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Justino, C.I.L.; Rocha-Santos, T.A.; Duarte, A.C.; Rocha-Santos, T.A. Review of Analytical Figures of Merit of Sensors and Biosensors in Clinical Applications. TrAC Trends Anal. Chem. 2010, 29, 1172–1183. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Biomolecule-Nanoparticle Hybrids for Electrochemical Biosensors. TrAC Trends Anal. Chem. 2009, 28, 96–109. [Google Scholar] [CrossRef]
- Chen, X.; Van Pée, K.-H. Catalytic Mechanisms, Basic Roles, and Biotechnological and Environmental Significance of Halogenating Enzymes. Acta Biochim. Biophys. Sin. 2008, 40, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Cornell, B.A.; Braach-Maksvytis, V.L.B.; King, L.G.; Osman, P.D.J.; Raguse, B.; Wieczorek, L.; Pace, R.J. A Biosensor That Uses Ion-Channel Switches. Nature 1997, 387, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Luong, J.H.T.; Male, K.B.; Glennon, J.D. Biosensor Technology: Technology Push versus Market Pull. Biotechnol. Adv. 2008, 26, 492–500. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Anal. Lett. 2001, 34, 635–659. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.-H.; Mondal, J.; Hwang, J.; Kim, H.S.; Kumar, V.; Raj, A.; Hwang, S.R.; Lee, Y.-K. Magnetofluoro-Immunosensing Platform Based on Binary Nanoparticle-Decorated Graphene for Detection of Cancer Cell-Derived Exosomes. Int. J. Mol. Sci. 2022, 23, 9619. [Google Scholar] [CrossRef]
- Sireesha, M.; Jagadeesh Babu, V.; Kranthi Kiran, A.S.; Ramakrishna, S. A Review on Carbon Nanotubes in Biosensor Devices and Their Applications in Medicine. Nanocomposites 2018, 4, 36–57. [Google Scholar] [CrossRef]
- Kundu, M.; Krishnan, P.; Kotnala, R.K.; Sumana, G. Recent Developments in Biosensors to Combat Agricultural Challenges and Their Future Prospects. Trends Food Sci. Technol. 2019, 88, 157–178. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Recent Advances in Nanomaterial-Mediated Bio and Immune Sensors for Detection of Aflatoxin in Food Products. TrAC Trends Anal. Chem. 2017, 87, 112–128. [Google Scholar] [CrossRef]
- Theuer, L.; Randek, J.; Junne, S.; Neubauer, P.; Mandenius, C.-F.; Beni, V. Single-Use Printed Biosensor for L-Lactate and Its Application in Bioprocess Monitoring. Processes 2020, 8, 321. [Google Scholar] [CrossRef]
- Cennamo, N.; Zeni, L.; Tortora, P.; Regonesi, M.E.; Giusti, A.; Staiano, M.; D’Auria, S.; Varriale, A. A High Sensitivity Biosensor to Detect the Presence of Perfluorinated Compounds in Environment. Talanta 2018, 178, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Meshram, B.D.; Agrawal, A.K.; Adil, S.; Ranvir, S.; Sande, K.K. Biosensor and Its Application in Food and Dairy Industry: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3305–3324. [Google Scholar] [CrossRef]
- Peng, B.; Locascio, M.; Zapol, P.; Li, S.; Mielke, S.L.; Schatz, G.C.; Espinosa, H.D. Measurements of Near-Ultimate Strength for Multiwalled Carbon Nanotubes and Irradiation-Induced Crosslinking Improvements. Nat. Nanotechnol. 2008, 3, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Carbon-Nanotube Based Electrochemical Biosensors: A Review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Yun, Y.; Dong, Z.; Shanov, V.; Heineman, W.R.; Halsall, H.B.; Bhattacharya, A.; Conforti, L.; Narayan, R.K.; Ball, W.S.; Schulz, M.J. Nanotube Electrodes and Biosensors. Nano Today 2007, 2, 30–37. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Kubendhiran, S.; Chen, S.-M.; Lin, K.-Y. Highly Sensitive Electrochemical Detection of Palmatine Using a Biocompatible Multiwalled Carbon Nanotube/Poly-L-Lysine Composite. J. Colloid Interface Sci. 2017, 498, 144–152. [Google Scholar] [CrossRef]
- Singh, S.; Vardharajula, S.; Tiwari, P.; Eroğlu, E.; Vig, K.; Dennis, V. Ali Functionalized Carbon Nanotubes: Biomedical Applications. Int. J. Nanomed. 2012, 7, 5361. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Partidos, C.D.; Prato, M. Biomedical Applications of Functionalised Carbon Nanotubes. Chem. Commun. 2005, 5, 571. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.S.; Liu, Z.; Dai, H. Functionalization of Carbon Nanotubes via Cleavable Disulfide Bonds for Efficient Intracellular Delivery of SiRNA and Potent Gene Silencing. J. Am. Chem. Soc. 2005, 127, 12492–12493. [Google Scholar] [CrossRef] [PubMed]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon Nanotube Biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Ali, M.A. Nanomaterials in Biosensors. In Nanomaterials for Biosensors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–74. [Google Scholar]
- Balasubramanian, K.; Burghard, M. Biosensors Based on Carbon Nanotubes. Anal. Bioanal. Chem. 2006, 385, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Review: Carbon Nanotube Based Electrochemical Sensors for Biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef]
- Jin, Q.; Dai, M.; Zhan, X.; Wang, S.; He, Z. Carbon Nanotubes and Graphene Composites Used in Cr(VI) Detection Techniques: A Review. J. Alloys Compd. 2022, 922, 166268. [Google Scholar] [CrossRef]
- Zamzami, M.A.; Rabbani, G.; Ahmad, A.; Basalah, A.A.; Al-Sabban, W.H.; Nate Ahn, S.; Choudhry, H. Carbon Nanotube Field-Effect Transistor (CNT-FET)-Based Biosensor for Rapid Detection of SARS-CoV-2 (COVID-19) Surface Spike Protein S1. Bioelectrochemistry 2022, 143, 107982. [Google Scholar] [CrossRef]
- Liu, M.; Ding, X.; Yang, Q.; Wang, Y.; Zhao, G.; Yang, N. A PM Leveled Photoelectrochemical Sensor for Microcystin-LR Based on Surface Molecularly Imprinted TiO2@CNTs Nanostructure. J. Hazard. Mater. 2017, 331, 309–320. [Google Scholar] [CrossRef]
- Aasi, A.; Aasi, E.; Mehdi Aghaei, S.; Panchapakesan, B. CNT Biodevices for Early Liver Cancer Diagnosis Based on Biomarkers Detection- a Promising Platform. J. Mol. Graph. Model. 2022, 114, 108208. [Google Scholar] [CrossRef]
- Ahmadian, E.; Janas, D.; Eftekhari, A.; Zare, N. Application of Carbon Nanotubes in Sensing/Monitoring of Pancreas and Liver Cancer. Chemosphere 2022, 302, 134826. [Google Scholar] [CrossRef]
- Lin, M.-H.; Gupta, S.; Chang, C.; Lee, C.-Y.; Tai, N.-H. Carbon Nanotubes/Polyethylenimine/Glucose Oxidase as a Non-Invasive Electrochemical Biosensor Performs High Sensitivity for Detecting Glucose in Saliva. Microchem. J. 2022, 180, 107547. [Google Scholar] [CrossRef]
- Anand, U.; Chandel, A.K.S.; Oleksak, P.; Mishra, A.; Krejcar, O.; Raval, I.H.; Dey, A.; Kuca, K. Recent Advances in the Potential Applications of Luminescence-Based, SPR-Based, and Carbon-Based Biosensors. Appl. Microbiol. Biotechnol. 2022, 106, 2827–2853. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-Shell Carbon Nanotubes of 1-Nm Diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-Catalysed Growth of Carbon Nanotubes with Single-Atomic-Layer Walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N.N. Arc Discharge Synthesis of Carbon Nanotubes: Comprehensive Review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Gamaly, E.G.; Ebbesen, T.W. Mechanism of Carbon Nanotube Formation in the Arc Discharge. Phys. Rev. B 1995, 52, 2083–2089. [Google Scholar] [CrossRef]
- De Heer, W.A.; Poncharal, P.; Berger, C.; Gezo, J.; Song, Z.; Bettini, J.; Ugarte, D. Liquid Carbon, Carbon-Glass Beads, and the Crystallization of Carbon Nanotubes. Science 2005, 307, 907–910. [Google Scholar] [CrossRef]
- Ugarte, D. High-Temperature Behaviour of “Fullerene Black”. Carbon N. Y. 1994, 32, 1245–1248. [Google Scholar] [CrossRef]
- Zhou, D.; Chow, L. Complex Structure of Carbon Nanotubes and Their Implications for Formation Mechanism. J. Appl. Phys. 2003, 93, 9972–9976. [Google Scholar] [CrossRef]
- Doherty, S.P.; Chang, R.P.H. Synthesis of Multiwalled Carbon Nanotubes from Carbon Black. Appl. Phys. Lett. 2002, 81, 2466–2468. [Google Scholar] [CrossRef]
- Doherty, S.P.; Buchholz, D.B.; Li, B.-J.; Chang, R.P.H. Solid-State Synthesis of Multiwalled Carbon Nanotubes. J. Mater. Res. 2003, 18, 941–949. [Google Scholar] [CrossRef]
- Doherty, S.P.; Buchholz, D.B.; Chang, R.P.H. Semi-Continuous Production of Multiwalled Carbon Nanotubes Using Magnetic Field Assisted Arc Furnace. Carbon N. Y. 2006, 44, 1511–1517. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Li, F.; Ren, W.-C.; Cong, H.; Liu, C.; Lu, G.Q.; Cheng, H.-M. Double-Walled Carbon Nanotubes Synthesized Using Carbon Black as the Dot Carbon Source. Nanotechnology 2006, 17, 3100–3104. [Google Scholar] [CrossRef]
- Sano, N.; Nakano, J.; Kanki, T. Synthesis of Single-Walled Carbon Nanotubes with Nanohorns by Arc in Liquid Nitrogen. Carbon N. Y. 2004, 42, 686–688. [Google Scholar] [CrossRef]
- Shang, H.; Xie, H.; Zhu, H.; Dai, F.; Wu, D.; Wang, W.; Fang, Y. Investigation of Strain in Individual Multi-Walled Carbon Nanotube by a Novel Moiré Method. J. Mater. Process. Technol. 2005, 170, 108–111. [Google Scholar] [CrossRef]
- Tripathi, G.; Tripathi, B.; Sharma, M.K.; Vijay, Y.K.; Chandra, A.; Jain, I.P. A Comparative Study of Arc Discharge and Chemical Vapor Deposition Synthesized Carbon Nanotubes. Int. J. Hydrogen Energy 2012, 37, 3833–3838. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, L.; Yang, Z.; Zhang, Y. Continuous and Low-Cost Synthesis of High-Quality Multi-Walled Carbon Nanotubes by Arc Discharge in Air. Phys. E Low-Dimens. Syst. Nanostruct. 2012, 44, 1639–1643. [Google Scholar] [CrossRef]
- Keidar, M.; Levchenko, I.; Arbel, T.; Alexander, M.; Waas, A.M.; Ostrikov, K. (Ken) Increasing the Length of Single-Wall Carbon Nanotubes in a Magnetically Enhanced Arc Discharge. Appl. Phys. Lett. 2008, 92, 043129. [Google Scholar] [CrossRef]
- Keidar, M.; Levchenko, I.; Arbel, T.; Alexander, M.; Waas, A.M.; Ostrikov, K.K. Magnetic-Field-Enhanced Synthesis of Single-Wall Carbon Nanotubes in Arc Discharge. J. Appl. Phys. 2008, 103, 094318. [Google Scholar] [CrossRef]
- Tang, D.; Xie, S.; Zhou, W.; Liu, Z.; Ci, L.; Yan, X.; Yuan, H.; Zhou, Z.; Liang, Y.; Liu, D.; et al. Effect of Cupped Cathode on Microstructures of Carbon Nanotubes in Arc Discharge. Carbon N. Y. 2002, 40, 1609–1613. [Google Scholar] [CrossRef]
- Shimotani, K.; Anazawa, K.; Watanabe, H.; Shimizu, M. New Synthesis of Multi-Walled Carbon Nanotubes Using an Arc Discharge Technique under Organic Molecular Atmospheres. Appl. Phys. A 2001, 73, 451–454. [Google Scholar] [CrossRef]
- Yousef, S.; Khattab, A.; Osman, T.A.; Zaki, M. Effects of Increasing Electrodes on CNTs Yield Synthesized by Using Arc-Discharge Technique. J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Roch, A.; Jost, O.; Schultrich, B.; Beyer, E. High-Yield Synthesis of Single-Walled Carbon Nanotubes with a Pulsed Arc-Discharge Technique. Phys. Status Solidi 2007, 244, 3907–3910. [Google Scholar] [CrossRef]
- Okada, T.; Kaneko, T.; Hatakeyama, R. Conversion of Toluene into Carbon Nanotubes Using Arc Discharge Plasmas in Solution. Thin Solid Film. 2007, 515, 4262–4265. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Chiu, S.-C.; Lin, K.-M.; Li, Y.-Y. Formation of Carbon Nanotubes from Polyvinyl Alcohol Using Arc-Discharge Method. Carbon N. Y. 2004, 42, 2535–2541. [Google Scholar] [CrossRef]
- Horváth, Z.E.; Kertész, K.; Pethő, L.; Koós, A.A.; Tapasztó, L.; Vértesy, Z.; Osváth, Z.; Darabont, A.; Nemes-Incze, P.; Sárközi, Z.; et al. Inexpensive, Upscalable Nanotube Growth Methods. Curr. Appl. Phys. 2006, 6, 135–140. [Google Scholar] [CrossRef]
- Ren, F.; Kanaan, S.A.; Majewska, M.M.; Keskar, G.D.; Azoz, S.; Wang, H.; Wang, X.; Haller, G.L.; Chen, Y.; Pfefferle, L.D. Increase in the Yield of (and Selective Synthesis of Large-Diameter) Single-Walled Carbon Nanotubes through Water-Assisted Ethanol Pyrolysis. J. Catal. 2014, 309, 419–427. [Google Scholar] [CrossRef]
- Ando, Y.; Zhao, X.; Sugai, T.; Kumar, M. Growing Carbon Nanotubes. Mater. Today 2004, 7, 22–29. [Google Scholar] [CrossRef]
- ChandraKishore, S.; Pandurangan, A. Electrophoretic Deposition of Cobalt Catalyst Layer over Stainless Steel for the High Yield Synthesis of Carbon Nanotubes. Appl. Surf. Sci. 2012, 258, 7936–7942. [Google Scholar] [CrossRef]
- Ahmad, S.; Liao, Y.; Hussain, A.; Zhang, Q.; Ding, E.-X.; Jiang, H.; Kauppinen, E.I. Systematic Investigation of the Catalyst Composition Effects on Single-Walled Carbon Nanotubes Synthesis in Floating-Catalyst CVD. Carbon N. Y. 2019, 149, 318–327. [Google Scholar] [CrossRef]
- Hoecker, C.; Smail, F.; Pick, M.; Boies, A. The Influence of Carbon Source and Catalyst Nanoparticles on CVD Synthesis of CNT Aerogel. Chem. Eng. J. 2017, 314, 388–395. [Google Scholar] [CrossRef]
- Barnard, J.S.; Paukner, C.; Koziol, K.K. The Role of Carbon Precursor on Carbon Nanotube Chirality in Floating Catalyst Chemical Vapour Deposition. Nanoscale 2016, 8, 17262–17270. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, H.B.; Irmawati, R.; Ismail, I.; Yusof, N.A. Utilization of Waste Engine Oil for Carbon Nanotube Aerogel Production Using Floating Catalyst Chemical Vapor Deposition. J. Clean. Prod. 2020, 261, 121188. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, H.; Teng, Y.; Xiong, Y.; Chen, Z.; You, L.; Xiao, D. Recent Advances in Magnetic Carbon Nanotubes: Synthesis, Challenges and Highlighted Applications. J. Mater. Chem. B 2021, 9, 9076–9099. [Google Scholar] [CrossRef]
- Rangreez, T.A.; Mobin, R.; Chisti, H.T.N. Potentiometric Determination of Mercury Ions by Sol-Gel Synthesized Multi-Walled Carbon Nanotubes Zr (IV) Phosphate Composite Fabricated Membrane Electrode. Curr. Anal. Chem. 2022, 18, 466–474. [Google Scholar] [CrossRef]
- Safaei, B.; How, H.C.; Scribano, G. A Computational Study on Synthesis of Carbon Nanotubes in a Sooty Inverse Diffusion Flame. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 2006, 102, 29–45. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, J.; Li, L.; Wang, Z.; O’Neill, W.; Estrela, P. Carbon Nanostructure-Based Field-Effect Transistors for Label-Free Chemical/Biological Sensors. Sensors 2010, 10, 5133–5159. [Google Scholar] [CrossRef]
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon Nanotube Based Biosensors. Sens. Actuators B Chem. 2015, 207, 690–715. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Silvera-Batista, C.A.; Wang, R.K.; Weinberg, P.; Ziegler, K.J. Solvatochromic Shifts of Single-Walled Carbon Nanotubes in Nonpolar Microenvironments. Phys. Chem. Chem. Phys. 2010, 12, 6990. [Google Scholar] [CrossRef] [PubMed]
- Satishkumar, B.C.; Brown, L.O.; Gao, Y.; Wang, C.-C.; Wang, H.-L.; Doorn, S.K. Reversible Fluorescence Quenching in Carbon Nanotubes for Biomolecular Sensing. Nat. Nanotechnol. 2007, 2, 560–564. [Google Scholar] [CrossRef]
- Lee, A.J.; Wang, X.; Carlson, L.J.; Smyder, J.A.; Loesch, B.; Tu, X.; Zheng, M.; Krauss, T.D. Bright Fluorescence from Individual Single-Walled Carbon Nanotubes. Nano Lett. 2011, 11, 1636–1640. [Google Scholar] [CrossRef]
- Ghosh, S.; Bachilo, S.M.; Simonette, R.A.; Beckingham, K.M.; Weisman, R.B. Oxygen Doping Modifies Near-Infrared Band Gaps in Fluorescent Single-Walled Carbon Nanotubes. Science 2010, 330, 1656–1659. [Google Scholar] [CrossRef]
- Chiu, C.F.; Barth, B.A.; Kotchey, G.P.; Zhao, Y.; Gogick, K.A.; Saidi, W.A.; Petoud, S.; Star, A. Enzyme-Catalyzed Oxidation Facilitates the Return of Fluorescence for Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2013, 135, 13356–13364. [Google Scholar] [CrossRef]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon Nanotubes as Optical Biomedical Sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but Strong: A Review of the Mechanical Properties of Carbon Nanotube–Polymer Composites. Carbon N. Y. 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Helbling, T.; Roman, C.; Hierold, C. Signal-to-Noise Ratio in Carbon Nanotube Electromechanical Piezoresistive Sensors. Nano Lett. 2010, 10, 3350–3354. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification1International Union of Pure and Applied Chemistry: Physical Chemistry Division, Commission I.7 (Biophysical Chemistry); Analytical Chemistry Division, Commission V.5 (Electroanalytical. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Meng, L.; Wu, P.; Chen, G.; Cai, C.; Sun, Y.; Yuan, Z. Low Potential Detection of Glutamate Based on the Electrocatalytic Oxidation of NADH at Thionine/Single-Walled Carbon Nanotubes Composite Modified Electrode. Biosens. Bioelectron. 2009, 24, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Magner, E. Trends in Electrochemical Biosensors. Analyst 1998, 123, 1967–1970. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Tsoufis, T.; Enotiadis, A.; Gournis, D.; Stamatis, H. Functionalized Multi-Wall Carbon Nanotubes for Lipase Immobilization. Adv. Eng. Mater. 2010, 12, B179–B183. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, L.; Tan, L.; Tan, H.; Yang, S.; Yao, S. Direct Electrochemistry of Cholesterol Oxidase Immobilized on Gold Nanoparticles-Decorated Multiwalled Carbon Nanotubes and Cholesterol Sensing. Talanta 2013, 106, 192–199. [Google Scholar] [CrossRef]
- Özmen, E.N.; Kartal, E.; Turan, M.B.; Yazıcıoğlu, A.; Niazi, J.H.; Qureshi, A. Graphene and Carbon Nanotubes Interfaced Electrochemical Nanobiosensors for the Detection of SARS-CoV-2 (COVID-19) and Other Respiratory Viral Infections: A Review. Mater. Sci. Eng. C 2021, 129, 112356. [Google Scholar] [CrossRef] [PubMed]
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive Biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Donaldson, K. Nanotoxicology. Occup. Environ. Med. 2004, 61, 727–728. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon Nanotubes: Evaluation of Toxicity at Biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
- An, J.M.; Shahriar, S.M.S.; Hasan, M.N.; Cho, S.; Lee, Y. Carboxymethyl Cellulose, Pluronic, and Pullulan-Based Compositions Efficiently Enhance Antiadhesion and Tissue Regeneration Properties without Using Any Drug Molecules. ACS Appl. Mater. Interfaces 2021, 13, 15992–16006. [Google Scholar] [CrossRef]
- An, J.M.; Shahriar, S.M.S.; Lee, D.Y.; Hwang, S.R.; Lee, Y. Pore Size-Dependent Stereoscopic Hydrogels Enhance the Therapeutic Efficiency of Botulinum Toxin for the Treatment of Nerve-Related Diseases. ACS Appl. Mater. Interfaces 2022, 14, 19139–19153. [Google Scholar] [CrossRef] [PubMed]
- Duke, K.S.; Bonner, J.C. Mechanisms of Carbon Nanotube-induced Pulmonary Fibrosis: A Physicochemical Characteristic Perspective. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1498. [Google Scholar] [CrossRef] [PubMed]
- Pauluhn, J. Subchronic 13-Week Inhalation Exposure of Rats to Multiwalled Carbon Nanotubes: Toxic Effects Are Determined by Density of Agglomerate Structures, Not Fibrillar Structures. Toxicol. Sci. 2010, 113, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Irianto, J.; Xia, Y.; Pfeifer, C.R.; Athirasala, A.; Ji, J.; Alvey, C.; Tewari, M.; Bennett, R.R.; Harding, S.M.; Liu, A.J.; et al. DNA Damage Follows Repair Factor Depletion and Portends Genome Variation in Cancer Cells after Pore Migration. Curr. Biol. 2017, 27, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.; Siivola, K.M.; Nymark, P.; Lindberg, H.; Suhonen, S.; Järventaus, H.; Koivisto, A.J.; Moreno, C.; Vanhala, E.; Wolff, H.; et al. In Vitro and in Vivo Genotoxic Effects of Straight versus Tangled Multi-Walled Carbon Nanotubes. Nanotoxicology 2016, 10, 794–806. [Google Scholar] [CrossRef]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.-F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory Toxicity of Multi-Wall Carbon Nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. In Comprehensive Physiology; Wiley: Amsterdam, The Netherlands, 2014; pp. 177–197. [Google Scholar]
- Buratti, S.; Lavine, J.E. Drugs and the Liver: Advances in Metabolism, Toxicity, and Therapeutics. Curr. Opin. Pediatr. 2002, 14, 601–607. [Google Scholar] [CrossRef]
- An, J.M.; Shahriar, S.M.S.; Hwang, Y.H.; Hwang, S.R.; Lee, D.Y.; Cho, S.; Lee, Y. Oral Delivery of Parathyroid Hormone Using a Triple-Padlock Nanocarrier for Osteoporosis via an Enterohepatic Circulation Pathway. ACS Appl. Mater. Interfaces 2021, 13, 23314–23327. [Google Scholar] [CrossRef]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug Metabolism in the Liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Al-Badri, A.M.; Bargooth, A.F.; Al-Jebori, J.G.; Zegyer, E.A.K. Identification of Carbon Nanotube Particles in Liver Tissue and Its Effects on Apoptosis of Birds Exposed to Air Pollution. Vet. World 2019, 12, 1372–1377. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, D.; Li, L.; Shen, X.; Deng, X.; Dong, L.; Wu, M.; Liu, Y. The Hepatotoxicity of Multi-Walled Carbon Nanotubes in Mice. Nanotechnology 2009, 20, 445101. [Google Scholar] [CrossRef]
- Sticova, E. New Insights in Bilirubin Metabolism and Their Clinical Implications. World J. Gastroenterol. 2013, 19, 6398. [Google Scholar] [CrossRef] [PubMed]
- Scharf, C.; Liebchen, U.; Paal, M.; Becker-Pennrich, A.; Irlbeck, M.; Zoller, M.; Schroeder, I. Successful Elimination of Bilirubin in Critically Ill Patients with Acute Liver Dysfunction Using a Cytokine Adsorber and Albumin Dialysis: A Pilot Study. Sci. Rep. 2021, 11, 10190. [Google Scholar] [CrossRef] [PubMed]
- Tameda, M. Aspartate Aminotransferase-Immunoglobulin Complexes in Patients with Chronic Liver Disease. World J. Gastroenterol. 2005, 11, 1529. [Google Scholar] [CrossRef] [PubMed]
- Kamimoto, Y.; Horiuchi, S.; Tanase, S.; Morino, Y. Plasma Clearance of Intravenously Injected Aspartate Aminotransferase Isozymes: Evidence for Preferential Uptake by Sinusoidal Liver Cells. Hepatology 1985, 5, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Carnicer-Lombarte, A.; Chen, S.-T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 622524. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Yoshioka, N.; Nakata, K.; Nishizawa, T.; Inagawa, H.; Kohchi, C.; Soma, G.-I. Mechanism for Maintaining Homeostasis in the Immune System of the Intestine. Anticancer. Res. 2009, 29, 4855–4860. [Google Scholar]
- Masereeuw, R.; Russel, F.G.M. Mechanisms and Clinical Implications of Renal Drug Excretion. Drug Metab. Rev. 2001, 33, 299–351. [Google Scholar] [CrossRef]
- Zamani, F.; Samiei, F.; Mousavi, Z.; Azari, M.R.; Seydi, E.; Pourahmad, J. Apigenin Ameliorates Oxidative Stress and Mitochondrial Damage Induced by Multiwall Carbon Nanotubes in Rat Kidney Mitochondria. J. Biochem. Mol. Toxicol. 2021, 35, 1–7. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, W.; Zhao, J.; Sun, W.; Yang, Q.; Chen, C.; Xia, P.; Zhu, J.; Zhou, Y.; Huang, G.; et al. Apigenin Ameliorates Doxorubicin-Induced Renal Injury via Inhibition of Oxidative Stress and Inflammation. Biomed. Pharmacother. 2021, 137, 111308. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and Naringenin Regulate Glucose and Lipid Metabolism, and Ameliorate Vascular Dysfunction in Type 2 Diabetic Rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Rutter, J.; Winge, D.R.; Schiffman, J.D. Succinate Dehydrogenase—Assembly, Regulation and Role in Human Disease. Mitochondrion 2010, 10, 393–401. [Google Scholar] [CrossRef]
- Rustin, P.; Munnich, A.; Rötig, A. Succinate Dehydrogenase and Human Diseases: New Insights into a Well-Known Enzyme. Eur. J. Hum. Genet. 2002, 10, 289–291. [Google Scholar] [CrossRef]
- Chertkova, R.V.; Brazhe, N.A.; Bryantseva, T.V.; Nekrasov, A.N.; Dolgikh, D.A.; Yusipovich, A.I.; Sosnovtseva, O.; Maksimov, G.V.; Rubin, A.B.; Kirpichnikov, M.P. New Insight into the Mechanism of Mitochondrial Cytochrome c Function. PLoS ONE 2017, 12, e0178280. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of Cytochrome c Release from Mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal Structure, Function, and Histology of the Spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef]
- Bronte, V.; Pittet, M.J. The Spleen in Local and Systemic Regulation of Immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- Chadburn, A. The Spleen: Anatomy and Anatomical Function. Semin. Hematol. 2000, 37, 13–21. [Google Scholar] [CrossRef]
- Khang, D.; Lee, S.; Kim, S.-H. High Dispersity of Carbon Nanotubes Diminishes Immunotoxicity in Spleen. Int. J. Nanomed. 2015, 10, 2697–2710. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clichici, S.; Biris, A.R.; Catoi, C.; Filip, A.; Tabaran, F. Short-Term Splenic Impact of Single-Strand DNA Functionalized Multi-Walled Carbon Nanotubes Intraperitoneally Injected in Rats. J. Appl. Toxicol. 2014, 34, 332–344. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the Mechanism of IL-1β Secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.L.; Nakayama-Ratchford, N.; Davis, C.R.; Kam, N.W.S.; Chu, P.; Liu, Z.; Sun, X.; Dai, H.; Gambhir, S.S. A Pilot Toxicology Study of Single-Walled Carbon Nanotubes in a Small Sample of Mice. Nat. Nanotechnol. 2008, 3, 216–221. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of Pegylation on Pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Peng, D. Hydroxylation of Multi-Walled Carbon Nanotubes Reduces Their Cytotoxicity by Limiting the Activation of Mitochondrial Mediated Apoptotic Pathway. J. Mater. Sci. Mater. Med. 2014, 25, 1033–1044. [Google Scholar] [CrossRef]
- Sweeney, S.; Hu, S.; Ruenraroengsak, P.; Chen, S.; Gow, A.; Schwander, S.; Zhang, J.; Chung, K.F.; Ryan, M.P.; Porter, A.E.; et al. Carboxylation of Multiwalled Carbon Nanotubes Reduces Their Toxicity in Primary Human Alveolar Macrophages. Environ. Sci. Nano 2016, 3, 1340–1350. [Google Scholar] [CrossRef]
- Taylor-Just, A.J.; Ihrie, M.D.; Duke, K.S.; Lee, H.Y.; You, D.J.; Hussain, S.; Kodali, V.K.; Ziemann, C.; Creutzenberg, O.; Vulpoi, A.; et al. The Pulmonary Toxicity of Carboxylated or Aminated Multi-Walled Carbon Nanotubes in Mice Is Determined by the Prior Purification Method. Part. Fibre Toxicol. 2020, 17, 60. [Google Scholar] [CrossRef]
- Yao, M.-Z.; Hu, Y.-L.; Sheng, X.-X.; Lin, J.; Ling, D.; Gao, J.-Q. Toxicity Analysis of Various Pluronic F-68-Coated Carbon Nanotubes on Mesenchymal Stem Cells. Chem. Biol. Interact. 2016, 250, 47–58. [Google Scholar] [CrossRef]
- Wu, H.; Shi, H.; Zhang, H.; Wang, X.; Yang, Y.; Yu, C.; Hao, C.; Du, J.; Hu, H.; Yang, S. Prostate Stem Cell Antigen Antibody-Conjugated Multiwalled Carbon Nanotubes for Targeted Ultrasound Imaging and Drug Delivery. Biomaterials 2014, 35, 5369–5380. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, Caspase-3 and Caspase-7 Have Distinct Roles during Intrinsic Apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Al Faraj, A.; Shaik, A.S.; Al Sayed, B.; Halwani, R.; Al Jammaz, I. Specific Targeting and Noninvasive Imaging of Breast Cancer Stem Cells Using Single-Walled Carbon Nanotubes as Novel Multimodality Nanoprobes. Nanomedicine 2016, 11, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Neoh, K.H.; Cheng, S.K.S.; Wu, H.; Chen, A.; Sun, Y.; Li, B.; Cao, A.; Han, R.P.S. PH-Responsive Carbon Nanotube Film-Based Microfluidic Chip for Efficient Capture and Release of Cancer Cells. ACS Appl. Nano Mater. 2022, 5, 6911–6924. [Google Scholar] [CrossRef]
- Lee, T.; Teng, T.Z.J.; Shelat, V.G. Carbohydrate Antigen 19-9—Tumor Marker: Past, Present, and Future. World J. Gastrointest. Surg. 2020, 12, 468–490. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Soares, A.C.; Soares, J.C.; Awan, I.T.; Volpati, D.; Melendez, M.E.; Fregnani, J.H.T.G.; Carvalho, A.L.; Oliveira, O.N. Carbon Nanotube Matrix for Highly Sensitive Biosensors To Detect Pancreatic Cancer Biomarker CA19-9. ACS Appl. Mater. Interfaces 2017, 9, 25878–25886. [Google Scholar] [CrossRef] [PubMed]
- Będkowska, G.E.; Gacuta, E.; Zbucka-Krętowska, M.; Ławicki, P.; Szmitkowski, M.; Lemancewicz, A.; Motyka, J.; Kobus, A.; Chorąży, M.; Paniczko, M.; et al. Plasma Levels and Diagnostic Utility of VEGF in a Three-Year Follow-Up of Patients with Breast Cancer. J. Clin. Med. 2021, 10, 5452. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Hong, M.-S.; Lee, W.-H.; Kim, J.-G.; Kim, K. Highly Sensitive Electrochemical Aptasensor for Detecting the VEGF165 Tumor Marker with PANI/CNT Nanocomposites. Biosensors 2021, 11, 114. [Google Scholar] [CrossRef]
- Rashid, S.; Nawaz, M.H.; Rehman, I.U.; Hayat, A.; Marty, J.L. Dopamine/Mucin-1 Functionalized Electro-Active Carbon Nanotubes as a Probe for Direct Competitive Electrochemical Immunosensing of Breast Cancer Biomarker. Sens. Actuators B Chem. 2021, 330, 129351. [Google Scholar] [CrossRef]
- Li, T.; Liang, Y.; Li, J.; Yu, Y.; Xiao, M.-M.; Ni, W.; Zhang, Z.; Zhang, G.-J. Carbon Nanotube Field-Effect Transistor Biosensor for Ultrasensitive and Label-Free Detection of Breast Cancer Exosomal MiRNA21. Anal. Chem. 2021, 93, 15501–15507. [Google Scholar] [CrossRef]
- Zhang, R.; Rejeeth, C.; Xu, W.; Zhu, C.; Liu, X.; Wan, J.; Jiang, M.; Qian, K. Label-Free Electrochemical Sensor for CD44 by Ligand-Protein Interaction. Anal. Chem. 2019, 91, 7078–7085. [Google Scholar] [CrossRef]
- Williams, R.M.; Lee, C.; Heller, D.A. A Fluorescent Carbon Nanotube Sensor Detects the Metastatic Prostate Cancer Biomarker UPA. ACS Sens. 2018, 3, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Polo, E.; Nitka, T.T.; Neubert, E.; Erpenbeck, L.; Vuković, L.; Kruss, S. Control of Integrin Affinity by Confining RGD Peptides on Fluorescent Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 17693–17703. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Yang, L.; Deng, S.; Hao, Y.; Zhang, K.; Wang, X.; Liu, Y.; Liu, H.; Chen, Y.; Xie, M. Development of Nanosensor by Bioorthogonal Reaction for Multi-Detection of the Biomarkers of Hepatocellular Carcinoma. Sens. Actuators B Chem. 2021, 334, 129653. [Google Scholar] [CrossRef]
- Cash, K.J.; Clark, H.A. Nanosensors and Nanomaterials for Monitoring Glucose in Diabetes. Trends Mol. Med. 2010, 16, 584–593. [Google Scholar] [CrossRef]
- Taguchi, M.; Ptitsyn, A.; McLamore, E.S.; Claussen, J.C. Nanomaterial-Mediated Biosensors for Monitoring Glucose. J. Diabetes Sci. Technol. 2014, 8, 403–411. [Google Scholar] [CrossRef]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical Glucose Sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef]
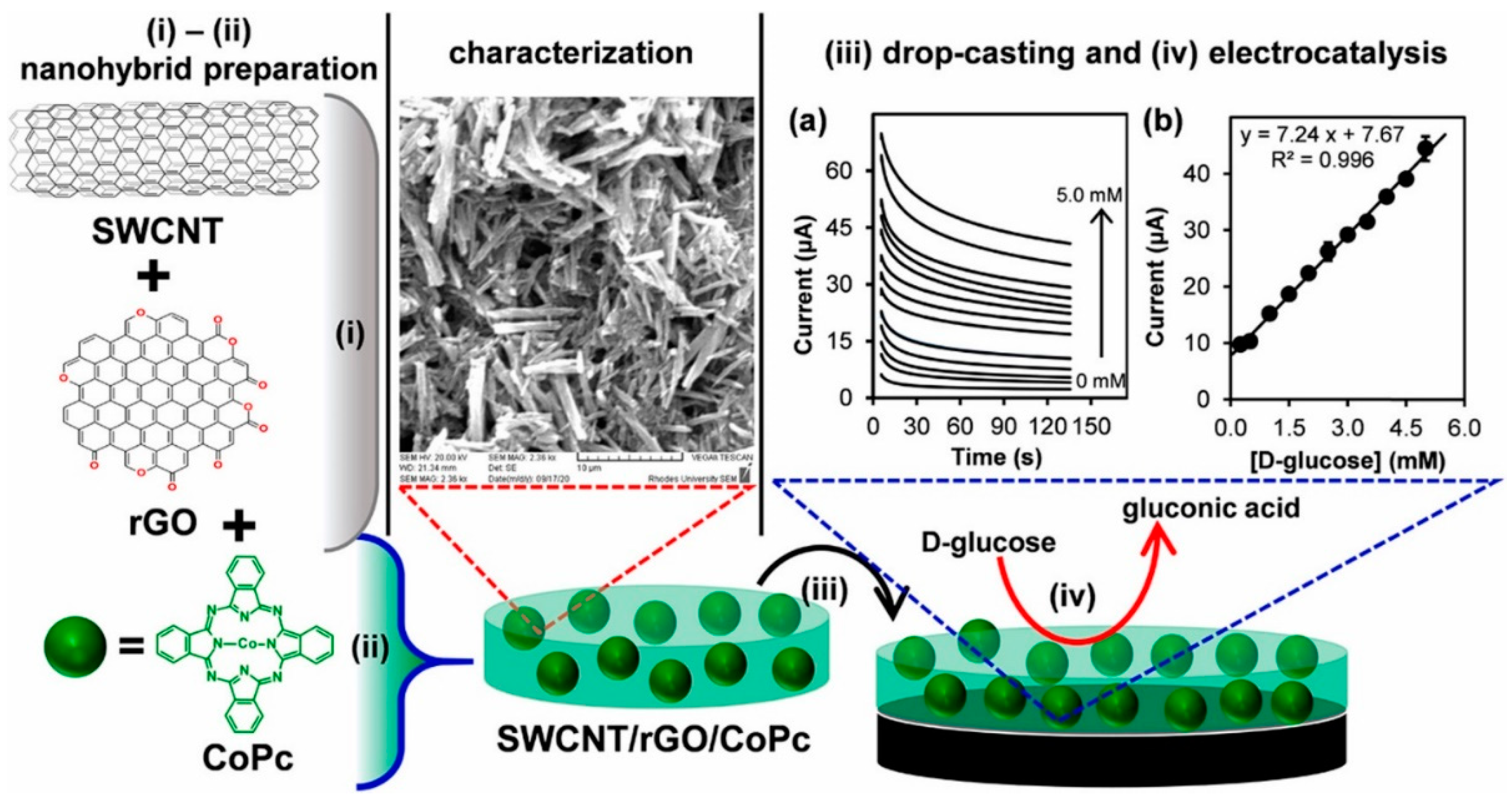
- Adeniyi, O.; Nwahara, N.; Mwanza, D.; Nyokong, T.; Mashazi, P. Nanohybrid Electrocatalyst Based on Cobalt Phthalocyanine-Carbon Nanotube-Reduced Graphene Oxide for Ultrasensitive Detection of Glucose in Human Saliva. Sens. Actuators B Chem. 2021, 348, 130723. [Google Scholar] [CrossRef]
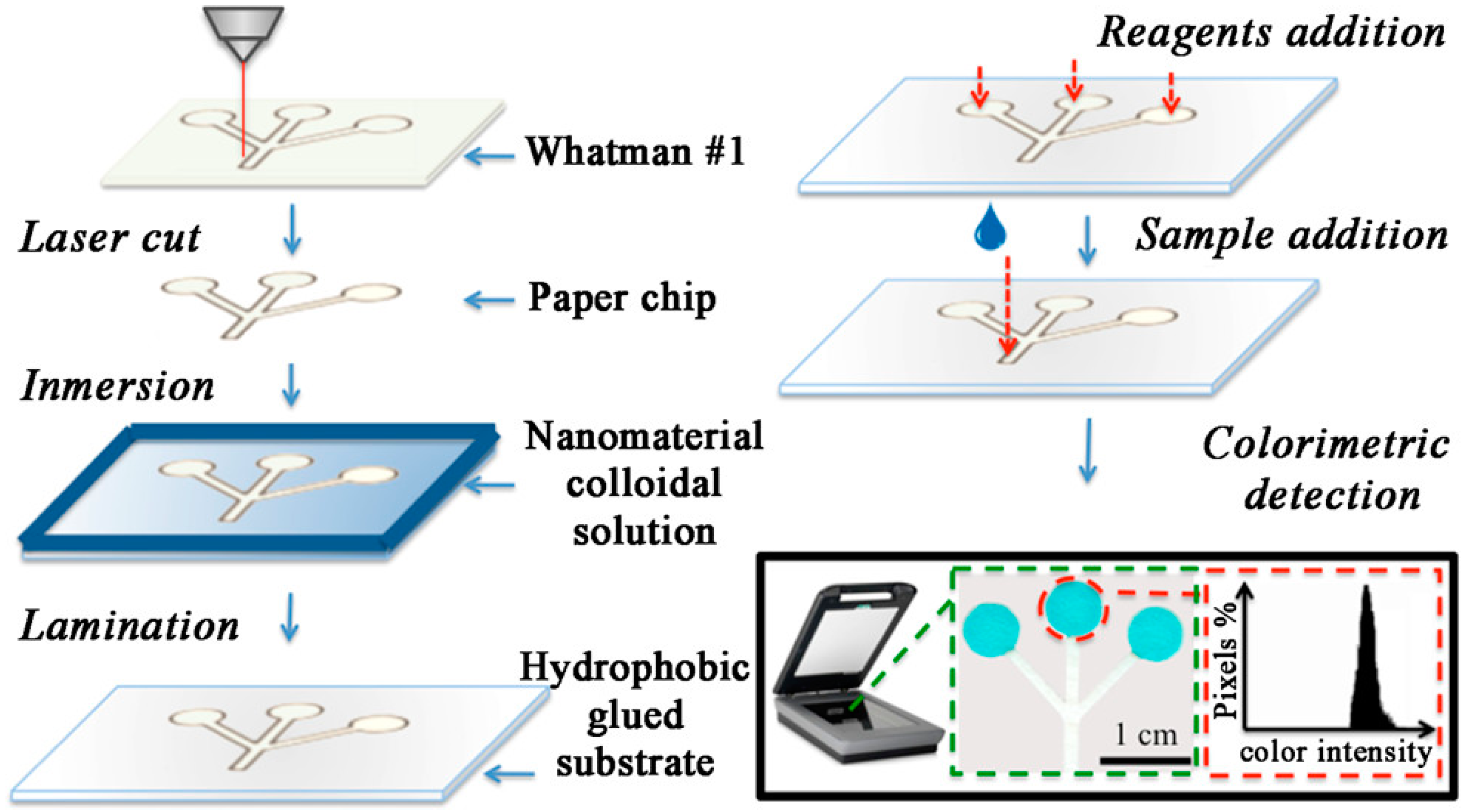
- Figueredo, F.; Garcia, P.T.; Cortón, E.; Coltro, W.K.T. Enhanced Analytical Performance of Paper Microfluidic Devices by Using Fe 3 O 4 Nanoparticles, MWCNT, and Graphene Oxide. ACS Appl. Mater. Interfaces 2016, 8, 11–15. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, H.; Wang, X. Thermal Self-Regulatory Intelligent Biosensor Based on Carbon-Nanotubes-Decorated Phase-Change Microcapsules for Enhancement of Glucose Detection. Biosens. Bioelectron. 2022, 195, 113586. [Google Scholar] [CrossRef]
- Rasmussen, J.; Langerman, H. Alzheimer’s Disease—Why We Need Early Diagnosis. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 123–130. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; Wang, Z.; Zhu, Y.; Nassery, N.; Saber Tehrani, A.S.; Schaffer, A.C.; Yu-Moe, C.W.; Clemens, G.D.; Fanai, M.; Siegal, D. Rate of Diagnostic Errors and Serious Misdiagnosis-Related Harms for Major Vascular Events, Infections, and Cancers: Toward a National Incidence Estimate Using the “Big Three”. Diagnosis 2021, 8, 67–84. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, H.; Gui, Y.; Tang, J. Mechanism and Application of Carbon Nanotube Sensors in SF6 Decomposed Production Detection: A Review. Nanoscale Res. Lett. 2017, 12, 177. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Carbon Nanotube-Based Fluorescence Sensors. J. Photochem. Photobiol. C Photochem. Rev. 2014, 19, 20–34. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Ngah Demon, S.Z.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon Nanotubes: Functionalisation and Their Application in Chemical Sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Kohn, J. A rapid method of estimating blood-glucose ranges. Lancet 1957, 270, 119–121. [Google Scholar] [CrossRef]
- Free, A.H.; Free, H.M. Self Testing, an Emerging Component of Clinical Chemistry. Clin. Chem. 1984, 30, 829–838. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Tan, R.; Wang, Q.; Zhang, Z. Colorimetric Method for Glucose Detection with Enhanced Signal Intensity Using ZnFe2O4–Carbon Nanotube–Glucose Oxidase Composite Material. Analyst 2019, 144, 1831–1839. [Google Scholar] [CrossRef]
- Kumari, A.; Rajeev, R.; Benny, L.; Sudhakar, Y.N.; Varghese, A.; Hegde, G. Recent Advances in Carbon Nanotubes-Based Biocatalysts and Their Applications. Adv. Colloid Interface Sci. 2021, 297, 102542. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Tan, R. Preparation of Enzyme-Functionalized Carbon Nanotubes and Their Application in Glucose and Fe2+ Detection through “Turn on” and “Turn off” Approaches. Analyst 2018, 143, 4118–4127. [Google Scholar] [CrossRef]
- Wilson, T.A.; Musameh, M.; Kyratzis, I.L.; Zhang, J.; Bond, A.M.; Hearn, M.T.W. Enhanced NADH Oxidation Using Polytyramine/Carbon Nanotube Modified Electrodes for Ethanol Biosensing. Electroanalysis 2017, 29, 1985–1993. [Google Scholar] [CrossRef]
- Nishikimi, M.; Yagi, K. Molecular Basis for the Deficiency in Humans of Gulonolactone Oxidase, a Key Enzyme for Ascorbic Acid Biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1203S–1208S. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.; Adams, W.; Williams, K. Is Vitamin C Enough? A Case Report of Scurvy in a Five-Year-Old Girl and Review of the Literature. BMC Pediatr. 2019, 19, 74. [Google Scholar] [CrossRef]
- Yimcharoen, M.; Kittikunnathum, S.; Suknikorn, C.; Nak-on, W.; Yeethong, P.; Anthony, T.G.; Bunpo, P. Effects of Ascorbic Acid Supplementation on Oxidative Stress Markers in Healthy Women Following a Single Bout of Exercise. J. Int. Soc. Sports Nutr. 2019, 16, 2. [Google Scholar] [CrossRef]
- Carr, A.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-Van Straaten, H.M. Vitamin C. Curr. Opin. Crit. Care 2018, 24, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Al-Niaimi, F.; Chiang, N.Y.Z. Topical Vitamin C and the Skin: Mechanisms of Action and Clinical Applications. J. Clin. Aesthet. Dermatol. 2017, 10, 14–17. [Google Scholar]
- Travica, N.; Ried, K.; Sali, A.; Scholey, A.; Hudson, I.; Pipingas, A. Vitamin C Status and Cognitive Function: A Systematic Review. Nutrients 2017, 9, 960. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, J.; Xu, H.; Gao, S.; Jiang, T.; Zhang, S.; Jin, J. Gold Nanorods Decorated with Graphene Oxide and Multi-Walled Carbon Nanotubes for Trace Level Voltammetric Determination of Ascorbic Acid. Microchim. Acta 2019, 186, 17. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Nien, P.-C.; Hu, C.-W.; Ho, K.-C. Detection of Uric Acid Based on Multi-Walled Carbon Nanotubes Polymerized with a Layer of Molecularly Imprinted PMAA. Sens. Actuators B Chem. 2010, 146, 466–471. [Google Scholar] [CrossRef]
- Hatefi-Mehrjardi, A.; Karimi, M.A.; Soleymanzadeh, M.; Barani, A. Highly Sensitive Detection of Dopamine, Ascorbic and Uric Acid with a Nanostructure of Dianix Yellow/Multi-Walled Carbon Nanotubes Modified Electrode. Measurement 2020, 163, 107893. [Google Scholar] [CrossRef]
- Jarošová, R.; Mcclure, S.E.; Gajda, M.; Jović, M.; Girault, H.H.; Lesch, A.; Maiden, M.; Waters, C.; Swain, G.M. Inkjet-Printed Carbon Nanotube Electrodes for Measuring Pyocyanin and Uric Acid in a Wound Fluid Simulant and Culture Media. Anal. Chem. 2019, 91, 8835–8844. [Google Scholar] [CrossRef]
- Gliozzi, M.; Malara, N.; Muscoli, S.; Mollace, V. The Treatment of Hyperuricemia. Int. J. Cardiol. 2016, 213, 23–27. [Google Scholar] [CrossRef]
- Ruggiero, C.; Cherubini, A.; Ble, A.; Bos, A.J.G.; Maggio, M.; Dixit, V.D.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Uric Acid and Inflammatory Markers. Eur. Heart J. 2006, 27, 1174–1181. [Google Scholar] [CrossRef]
- Prasad Sah, O.S.; Qing, Y.X. Associations between Hyperuricemia and Chronic Kidney Disease: A Review. Nephrourol. Mon. 2015, 7, e27233. [Google Scholar] [CrossRef]
- Huang, B.; Liu, J.; Lai, L.; Yu, F.; Ying, X.; Ye, B.-C.; Li, Y. A Free-Standing Electrochemical Sensor Based on Graphene Foam-Carbon Nanotube Composite Coupled with Gold Nanoparticles and Its Sensing Application for Electrochemical Determination of Dopamine and Uric Acid. J. Electroanal. Chem. 2017, 801, 129–134. [Google Scholar] [CrossRef]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking Pathophysiological Processes in Alzheimer’s Disease: An Updated Hypothetical Model of Dynamic Biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef]
- Chen, G.; Xu, T.; Yan, Y.; Zhou, Y.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Oh, J.; Yoo, G.; Chang, Y.W.; Kim, H.J.; Jose, J.; Kim, E.; Pyun, J.-C.; Yoo, K.-H. A Carbon Nanotube Metal Semiconductor Field Effect Transistor-Based Biosensor for Detection of Amyloid-Beta in Human Serum. Biosens. Bioelectron. 2013, 50, 345–350. [Google Scholar] [CrossRef]
- Borissoff, J.I.; Spronk, H.M.H.; Heeneman, S.; ten Cate, H. Is Thrombin a Key Player in the “coagulation-Atherogenesis” Maze? Cardiovasc. Res. 2009, 82, 392–403. [Google Scholar] [CrossRef]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How It All Starts: Initiation of the Clotting Cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xu, X.; Xu, H.; Zhang, Y.; Li, C.; Ma, Y.; Song, D.; Xie, Q. Amperometric Thrombin Aptasensor Using a Glassy Carbon Electrode Modified with Polyaniline and Multiwalled Carbon Nanotubes Tethered with a Thiolated Aptamer. Microchim. Acta 2017, 184, 1677–1682. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Machhi, J.; Herskovitz, J.; Oleynikov, M.D.; Blomberg, W.R.; Bajwa, N.; Soni, D.; Das, S.; Hasan, M.; Patel, M.; et al. Diagnostics for SARS-CoV-2 Infections. Nat. Mater. 2021, 20, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, L.; Wymant, C.; Kendall, M.; Zhao, L.; Nurtay, A.; Abeler-Dörner, L.; Parker, M.; Bonsall, D.; Fraser, C. Quantifying SARS-CoV-2 Transmission Suggests Epidemic Control with Digital Contact Tracing. Science 2020, 368, eabb6936. [Google Scholar] [CrossRef] [PubMed]
- Pinals, R.L.; Ledesma, F.; Yang, D.; Navarro, N.; Jeong, S.; Pak, J.E.; Kuo, L.; Chuang, Y.-C.; Cheng, Y.-W.; Sun, H.-Y.; et al. Rapid SARS-CoV-2 Spike Protein Detection by Carbon Nanotube-Based Near-Infrared Nanosensors. Nano Lett. 2021, 21, 2272–2280. [Google Scholar] [CrossRef]
- Yang, J.; Petitjean, S.J.L.; Koehler, M.; Zhang, Q.; Dumitru, A.C.; Chen, W.; Derclaye, S.; Vincent, S.P.; Soumillion, P.; Alsteens, D. Molecular Interaction and Inhibition of SARS-CoV-2 Binding to the ACE2 Receptor. Nat. Commun. 2020, 11, 4541. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Kato, Y.; Toyosato, R.; Nishizawa, M. Fluid-Permeable Enzymatic Lactate Sensors for Micro-Volume Specimen. Analyst 2018, 143, 5545–5551. [Google Scholar] [CrossRef]
- Bhushan, P.; Umasankar, Y.; RoyChoudhury, S.; Hirt, P.A.; MacQuhaec, F.E.; Borda, L.J.; Lev-Tov, H.A.; Kirsner, R.S.; Bhansali, S. Biosensor for Monitoring Uric Acid in Wound and Its Proximity: A Potential Wound Diagnostic Tool. J. Electrochem. Soc. 2019, 166, B830–B836. [Google Scholar] [CrossRef]
- Zappi, D.; Caminiti, R.; Ingo, G.M.; Sadun, C.; Tortolini, C.; Antonelli, M.L. Biologically Friendly Room Temperature Ionic Liquids and Nanomaterials for the Development of Innovative Enzymatic Biosensors. Talanta 2017, 175, 566–572. [Google Scholar] [CrossRef]
- Chen, C.; Ran, R.; Yang, Z.; Lv, R.; Shen, W.; Kang, F.; Huang, Z.-H. An Efficient Flexible Electrochemical Glucose Sensor Based on Carbon Nanotubes/Carbonized Silk Fabrics Decorated with Pt Microspheres. Sens. Actuators B Chem. 2018, 256, 63–70. [Google Scholar] [CrossRef]
- Madhurantakam, S.; Jayanth Babu, K.; Balaguru Rayappan, J.B.; Krishnan, U.M. Fabrication of Mediator-Free Hybrid Nano-Interfaced Electrochemical Biosensor for Monitoring Cancer Cell Proliferation. Biosens. Bioelectron. 2017, 87, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, M.; Verma, N. Electrochemical Preparation of Fe3O4/MWCNT-Polyaniline Nanocomposite Film for Development of Urea Biosensor and Its Application in Milk Sample. J. Food Meas. Charact. 2020, 14, 163–175. [Google Scholar] [CrossRef]
- Magar, H.S.; Ghica, M.E.; Abbas, M.N.; Brett, C.M.A. A Novel Sensitive Amperometric Choline Biosensor Based on Multiwalled Carbon Nanotubes and Gold Nanoparticles. Talanta 2017, 167, 462–469. [Google Scholar] [CrossRef]
- Alizadeh, T.; Nayeri, S. An Enzyme-Free Sensing Platform Based on Molecularly Imprinted Polymer/MWCNT Composite for Sub-Micromolar-Level Determination of Pyruvic Acid as a Cancer Biomarker. Anal. Bioanal. Chem. 2020, 412, 657–667. [Google Scholar] [CrossRef]
- Arkan, E.; Saber, R.; Karimi, Z.; Shamsipur, M. A Novel Antibody–Antigen Based Impedimetric Immunosensor for Low Level Detection of HER2 in Serum Samples of Breast Cancer Patients via Modification of a Gold Nanoparticles Decorated Multiwall Carbon Nanotube-Ionic Liquid Electrode. Anal. Chim. Acta 2015, 874, 66–74. [Google Scholar] [CrossRef]
- Nawaz, M.A.H.; Majdinasab, M.; Latif, U.; Nasir, M.; Gokce, G.; Anwar, M.W.; Hayat, A. Development of a Disposable Electrochemical Sensor for Detection of Cholesterol Using Differential Pulse Voltammetry. J. Pharm. Biomed. Anal. 2018, 159, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Nguyen, D.M.; Lee, M.S.; Kim, H.G.; Ko, S.C.; Kwac, L.K. N-Doped Graphene-Carbon Nanotube Hybrid Networks Attaching with Gold Nanoparticles for Glucose Non-Enzymatic Sensor. Mater. Sci. Eng. C 2018, 90, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Riberi, W.I.; Tarditto, L.V.; Zon, M.A.; Arévalo, F.J.; Fernández, H. Development of an Electrochemical Immunosensor to Determine Zearalenone in Maize Using Carbon Screen Printed Electrodes Modified with Multi-Walled Carbon Nanotubes/Polyethyleneimine Dispersions. Sens. Actuators B Chem. 2018, 254, 1271–1277. [Google Scholar] [CrossRef]
- Chen, M.; Wu, D.; Tu, S.; Yang, C.; Chen, D.; Xu, Y. A Novel Biosensor for the Ultrasensitive Detection of the LncRNA Biomarker MALAT1 in Non-Small Cell Lung Cancer. Sci. Rep. 2021, 11, 3666. [Google Scholar] [CrossRef]
- Sabahi, A.; Salahandish, R.; Ghaffarinejad, A.; Omidinia, E. Electrochemical Nano-Genosensor for Highly Sensitive Detection of MiR-21 Biomarker Based on SWCNT-Grafted Dendritic Au Nanostructure for Early Detection of Prostate Cancer. Talanta 2020, 209, 120595. [Google Scholar] [CrossRef]
- Rostamabadi, P.F.; Heydari-Bafrooei, E. Impedimetric Aptasensing of the Breast Cancer Biomarker HER2 Using a Glassy Carbon Electrode Modified with Gold Nanoparticles in a Composite Consisting of Electrochemically Reduced Graphene Oxide and Single-Walled Carbon Nanotubes. Microchim. Acta 2019, 186, 495. [Google Scholar] [CrossRef] [PubMed]
- Phonklam, K.; Wannapob, R.; Sriwimol, W.; Thavarungkul, P.; Phairatana, T. A Novel Molecularly Imprinted Polymer PMB/MWCNTs Sensor for Highly-Sensitive Cardiac Troponin T Detection. Sens. Actuators B Chem. 2020, 308, 127630. [Google Scholar] [CrossRef]
- Kumar, T.H.V.; Sundramoorthy, A.K. Non-Enzymatic Electrochemical Detection of Urea on Silver Nanoparticles Anchored Nitrogen-Doped Single-Walled Carbon Nanotube Modified Electrode. J. Electrochem. Soc. 2018, 165, B3006–B3016. [Google Scholar] [CrossRef]
- Qian, Q.; Hu, Q.; Li, L.; Shi, P.; Zhou, J.; Kong, J.; Zhang, X.; Sun, G.; Huang, W. Sensitive Fiber Microelectrode Made of Nickel Hydroxide Nanosheets Embedded in Highly-Aligned Carbon Nanotube Scaffold for Nonenzymatic Glucose Determination. Sens. Actuators B Chem. 2018, 257, 23–28. [Google Scholar] [CrossRef]
- Palve, Y.P.; Jha, N. A Novel Bilayer of Copper Nanowire and Carbon Nanotube Electrode for Highly Sensitive Enzyme Free Glucose Detection. Mater. Chem. Phys. 2020, 240, 122086. [Google Scholar] [CrossRef]
- Tan, C.; Dutta, G.; Yin, H.; Siddiqui, S.; Arumugam, P.U. Detection of Neurochemicals with Enhanced Sensitivity and Selectivity via Hybrid Multiwall Carbon Nanotube-Ultrananocrystalline Diamond Microelectrodes. Sens. Actuators B Chem. 2018, 258, 193–203. [Google Scholar] [CrossRef]
- Zhang, S.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, S.; Hui, X.; Chandra Barman, S.; Sharma, S.; Yoon, H.S.; Park, C.; Park, J.Y. A Wearable Battery-Free Wireless and Skin-Interfaced Microfluidics Integrated Electrochemical Sensing Patch for on-Site Biomarkers Monitoring in Human Perspiration. Biosens. Bioelectron. 2021, 175, 112844. [Google Scholar] [CrossRef]
- Safaee, M.M.; Gravely, M.; Roxbury, D. A Wearable Optical Microfibrous Biomaterial with Encapsulated Nanosensors Enables Wireless Monitoring of Oxidative Stress. Adv. Funct. Mater. 2021, 31, 2006254. [Google Scholar] [CrossRef]
- Ma, H.; Xue, N.; Li, Z.; Xing, K.; Miao, X. Ultrasensitive Detection of MiRNA-155 Using Multi-Walled Carbon Nanotube-Gold Nanocomposites as a Novel Fluorescence Quenching Platform. Sens. Actuators B Chem. 2018, 266, 221–227. [Google Scholar] [CrossRef]
- Elmizadeh, H.; Faridbod, F.; Soleimani, M.; Ganjali, M.R.; Bardajee, G.R. Fluorescent Apta-Nanobiosensors for Fast and Sensitive Detection of Digoxin in Biological Fluids Using RGQDs: Comparison of Two Approaches for Immobilization of Aptamer. Sens. Actuators B Chem. 2020, 302, 127133. [Google Scholar] [CrossRef]
- Ghrera, A.S.; Pandey, C.M.; Malhotra, B.D. Multiwalled Carbon Nanotube Modified Microfluidic-Based Biosensor Chip for Nucleic Acid Detection. Sens. Actuators B Chem. 2018, 266, 329–336. [Google Scholar] [CrossRef]
- Nandeshwar, R.; Tallur, S. Integrated Low Cost Optical Biosensor for High Resolution Sensing of Myeloperoxidase (MPO) Activity Through Carbon Nanotube Degradation. IEEE Sens. J. 2021, 21, 1236–1243. [Google Scholar] [CrossRef]
- Shao, W.; Shurin, M.R.; Wheeler, S.E.; He, X.; Star, A. Rapid Detection of SARS-CoV-2 Antigens Using High-Purity Semiconducting Single-Walled Carbon Nanotube-Based Field-Effect Transistors. ACS Appl. Mater. Interfaces 2021, 13, 10321–10327. [Google Scholar] [CrossRef] [PubMed]
- Alfaifi, S.Y.M.; Hussain, M.M.; Asiri, A.M.; Rahman, M.M. Glassy Carbon Electrodes Decorated with HgO/CNT Nanocomposite and Modified with a Conducting Polymer Matrix for Enzyme-Free Ascorbic Acid Detection. ChemistrySelect 2022, 7, e202200086. [Google Scholar] [CrossRef]
- Lee, J.; Adegoke, O.; Park, E.Y. High-Performance Biosensing Systems Based on Various Nanomaterials as Signal Transducers. Biotechnol. J. 2019, 14, 1800249. [Google Scholar] [CrossRef]
- Lee, J.; Takemura, K.; Park, E. Plasmonic Nanomaterial-Based Optical Biosensing Platforms for Virus Detection. Sensors 2017, 17, 2332. [Google Scholar] [CrossRef]
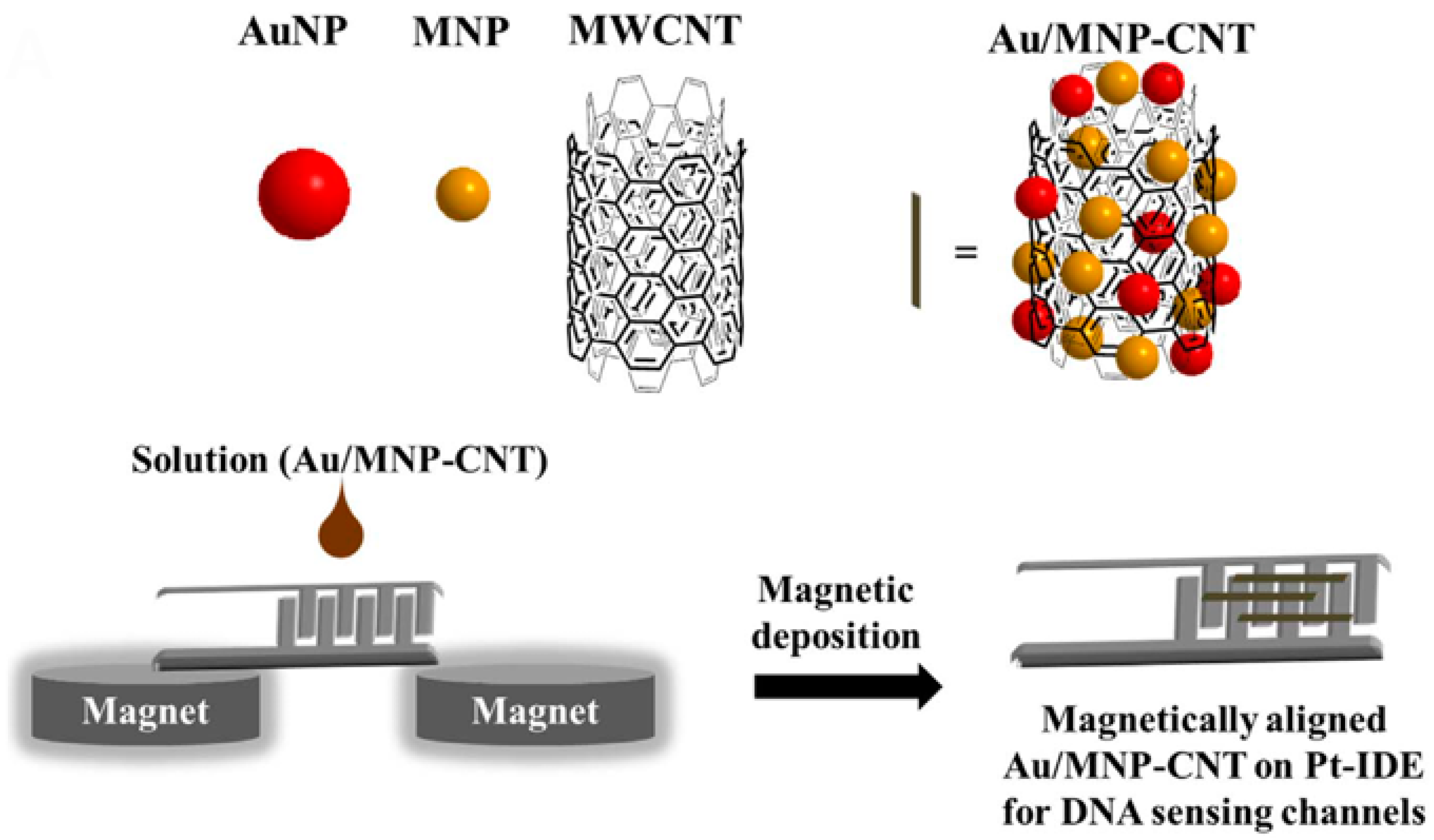
- Lee, J.; Morita, M.; Takemura, K.; Park, E.Y. A Multi-Functional Gold/Iron-Oxide Nanoparticle-CNT Hybrid Nanomaterial as Virus DNA Sensing Platform. Biosens. Bioelectron. 2018, 102, 425–431. [Google Scholar] [CrossRef]
- Lee, J.; Ahmed, S.R.; Oh, S.; Kim, J.; Suzuki, T.; Parmar, K.; Park, S.S.; Lee, J.; Park, E.Y. A Plasmon-Assisted Fluoro-Immunoassay Using Gold Nanoparticle-Decorated Carbon Nanotubes for Monitoring the Influenza Virus. Biosens. Bioelectron. 2015, 64, 311–317. [Google Scholar] [CrossRef]
- Wasik, D.; Mulchandani, A.; Yates, M.V. A Heparin-Functionalized Carbon Nanotube-Based Affinity Biosensor for Dengue Virus. Biosens. Bioelectron. 2017, 91, 811–816. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, W.; Han, E.; Wang, S.; Shen, J. Hybrid Nanostructure-Based Immunosensing for Electrochemical Assay of Escherichia Coli as Indicator Bacteria Relevant to the Recycling of Urban Sludge. Electrochim. Acta 2014, 141, 384–390. [Google Scholar] [CrossRef]
- Li, T.; Zhu, F.; Guo, W.; Gu, H.; Zhao, J.; Yan, M.; Liu, S. Selective Capture and Rapid Identification of E. Coli O157:H7 by Carbon Nanotube Multilayer Biosensors and Microfluidic Chip-Based LAMP. RSC Adv. 2017, 7, 30446–30452. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, J.; Ma, H.; Jiang, Y.; Huang, C.; Han, E.; Yao, B.; He, Y. Optimized Dendrimer-Encapsulated Gold Nanoparticles and Enhanced Carbon Nanotube Nanoprobes for Amplified Electrochemical Immunoassay of E. coli in Dairy Product Based on Enzymatically Induced Deposition of Polyaniline. Biosens. Bioelectron. 2016, 80, 666–673. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Cai, Y.; Yang, Z.; Li, P.; Lei, H.; Liu, W.; Liu, Y. A Dual-Signal Readout Enzyme-Free Immunosensor Based on Hybridization Chain Reaction-Assisted Formation of Copper Nanoparticles for the Detection of Microcystin-LR. Biosens. Bioelectron. 2019, 126, 151–159. [Google Scholar] [CrossRef]
- Han, C.; Doepke, A.; Cho, W.; Likodimos, V.; de la Cruz, A.A.; Back, T.; Heineman, W.R.; Halsall, H.B.; Shanov, V.N.; Schulz, M.J.; et al. A Multiwalled-Carbon-Nanotube-Based Biosensor for Monitoring Microcystin-LR in Sources of Drinking Water Supplies. Adv. Funct. Mater. 2013, 23, 1807–1816. [Google Scholar] [CrossRef]
- Queirós, R.B.; Noronha, J.P.; Marques, P.V.S.; Sales, M.G.F. Label-Free Detection of Microcystin-LR in Waters Using Real-Time Potentiometric Biosensors Based on Single-Walled Carbon Nanotubes Imprinted Polymers. Procedia Eng. 2012, 47, 758–761. [Google Scholar] [CrossRef][Green Version]
- Viswanathan, S.; Wu, L.; Huang, M.-R.; Ho, J.A. Electrochemical Immunosensor for Cholera Toxin Using Liposomes and Poly(3,4-Ethylenedioxythiophene)-Coated Carbon Nanotubes. Anal. Chem. 2006, 78, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Palomar, Q.; Gondran, C.; Holzinger, M.; Marks, R.; Cosnier, S. Controlled Carbon Nanotube Layers for Impedimetric Immunosensors: High Performance Label Free Detection and Quantification of Anti-Cholera Toxin Antibody. Biosens. Bioelectron. 2017, 97, 177–183. [Google Scholar] [CrossRef]
- Yamada, K.; Choi, W.; Lee, I.; Cho, B.-K.; Jun, S. Rapid Detection of Multiple Foodborne Pathogens Using a Nanoparticle-Functionalized Multi-Junction Biosensor. Biosens. Bioelectron. 2016, 77, 137–143. [Google Scholar] [CrossRef]
- Kangkamano, T.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. Chitosan Cryogel with Embedded Gold Nanoparticles Decorated Multiwalled Carbon Nanotubes Modified Electrode for Highly Sensitive Flow Based Non-Enzymatic Glucose Sensor. Sens. Actuators B Chem. 2017, 246, 854–863. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Tang, X.; Xu, L.; Min, L.; Xue, Y.; Hu, X.; Yang, Z. Multiwalled Carbon Nanotubes Coated with Cobalt(II) Sulfide Nanoparticles for Electrochemical Sensing of Glucose via Direct Electron Transfer to Glucose Oxidase. Microchim. Acta 2020, 187, 80. [Google Scholar] [CrossRef]
- Dervisevic, M.; Custiuc, E.; Çevik, E.; Şenel, M. Construction of Novel Xanthine Biosensor by Using Polymeric Mediator/MWCNT Nanocomposite Layer for Fish Freshness Detection. Food Chem. 2015, 181, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhu, H.; Sun, S.; Zhu, Z.; Hao, J.; Lu, S.; Cai, Y.; Zhang, M.; Du, M. Synergistic Integration of Au Nanoparticles, Co-MOF and MWCNT as Biosensors for Sensitive Detection of Low-Concentration Nitrite. Electrochim. Acta 2021, 365, 137375. [Google Scholar] [CrossRef]
- Tabatabaei, M.K.; Fard, H.G.; Koohsorkhi, J.; Mohammadnejad Arough, J. High-performance Immunosensor for Urine Albumin Using Hybrid Architectures of ZnO Nanowire/Carbon Nanotube. IET Nanobiotechnol. 2020, 14, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Alagappan, M.; Immanuel, S.; Sivasubramanian, R.; Kandaswamy, A. Development of Cholesterol Biosensor Using Au Nanoparticles Decorated F-MWCNT Covered with Polypyrrole Network. Arab. J. Chem. 2020, 13, 2001–2010. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ahn, J.-H.; Barone, P.W.; Jin, H.; Zhang, J.; Heller, D.A.; Strano, M.S. A Luciferase/Single-Walled Carbon Nanotube Conjugate for Near-Infrared Fluorescent Detection of Cellular ATP. Angew. Chemie Int. Ed. 2010, 49, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kruss, S.; Hilmer, A.J.; Shimizu, S.; Schmois, Z.; De La Cruz, F.; Barone, P.W.; Reuel, N.F.; Heller, D.A.; Strano, M.S. A Rapid, Direct, Quantitative, and Label-Free Detector of Cardiac Biomarker Troponin T Using Near-Infrared Fluorescent Single-Walled Carbon Nanotube Sensors. Adv. Healthc. Mater. 2014, 3, 412–423. [Google Scholar] [CrossRef]
- Shumeiko, V.; Malach, E.; Helman, Y.; Paltiel, Y.; Bisker, G.; Hayouka, Z.; Shoseyov, O. A Nanoscale Optical Biosensor Based on Peptide Encapsulated SWCNTs for Detection of Acetic Acid in the Gaseous Phase. Sens. Actuators B Chem. 2021, 327, 128832. [Google Scholar] [CrossRef]
- Puri, N.; Niazi, A.; Biradar, A.M.; Mulchandani, A. Rajesh Conducting Polymer Functionalized Single-Walled Carbon Nanotube Based Chemiresistive Biosensor for the Detection of Human Cardiac Myoglobin. Appl. Phys. Lett. 2014, 105, 153701. [Google Scholar] [CrossRef]
- Paul, K.B.; Panigrahi, A.K.; Singh, V.; Singh, S.G. A Multi-Walled Carbon Nanotube–Zinc Oxide Nanofiber Based Flexible Chemiresistive Biosensor for Malaria Biomarker Detection. Analyst 2017, 142, 2128–2135. [Google Scholar] [CrossRef]
- Fu, Y.; Romay, V.; Liu, Y.; Ibarlucea, B.; Baraban, L.; Khavrus, V.; Oswald, S.; Bachmatiuk, A.; Ibrahim, I.; Rümmeli, M.; et al. Chemiresistive Biosensors Based on Carbon Nanotubes for Label-Free Detection of DNA Sequences Derived from Avian Influenza Virus H5N1. Sens. Actuators B Chem. 2017, 249, 691–699. [Google Scholar] [CrossRef]
- Tan, F.; Saucedo, N.M.; Ramnani, P.; Mulchandani, A. Label-Free Electrical Immunosensor for Highly Sensitive and Specific Detection of Microcystin-LR in Water Samples. Environ. Sci. Technol. 2015, 49, 9256–9263. [Google Scholar] [CrossRef] [PubMed]
- Ben Messaoud, N.; Ghica, M.E.; Dridi, C.; Ben Ali, M.; Brett, C.M.A. Electrochemical Sensor Based on Multiwalled Carbon Nanotube and Gold Nanoparticle Modified Electrode for the Sensitive Detection of Bisphenol A. Sens. Actuators B Chem. 2017, 253, 513–522. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Tong, L.; Tong, Q.-X. Graphene Quantum Dots/Multiwalled Carbon Nanotubes Composite-Based Electrochemical Sensor for Detecting Dopamine Release from Living Cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650. [Google Scholar] [CrossRef]
- Chen, M.; Hou, C.; Huo, D.; Yang, M.; Fa, H. An Ultrasensitive Electrochemical DNA Biosensor Based on a Copper Oxide Nanowires/Single-Walled Carbon Nanotubes Nanocomposite. Appl. Surf. Sci. 2016, 364, 703–709. [Google Scholar] [CrossRef]
- Liu, X.; Shuai, H.-L.; Liu, Y.-J.; Huang, K.-J. An Electrochemical Biosensor for DNA Detection Based on Tungsten Disulfide/Multi-Walled Carbon Nanotube Composites and Hybridization Chain Reaction Amplification. Sens. Actuators B Chem. 2016, 235, 603–613. [Google Scholar] [CrossRef]
- Winiarski, J.P.; Rampanelli, R.; Bassani, J.C.; Mezalira, D.Z.; Jost, C.L. Multi-Walled Carbon Nanotubes/Nickel Hydroxide Composite Applied as Electrochemical Sensor for Folic Acid (Vitamin B9) in Food Samples. J. Food Compos. Anal. 2020, 92, 103511. [Google Scholar] [CrossRef]
- Palomar, Q.; Xu, X.; Selegård, R.; Aili, D.; Zhang, Z. Peptide Decorated Gold Nanoparticle/Carbon Nanotube Electrochemical Sensor for Ultrasensitive Detection of Matrix Metalloproteinase-7. Sens. Actuators B Chem. 2020, 325, 128789. [Google Scholar] [CrossRef]
- Alizadeh, M.; Azar, P.A.; Mozaffari, S.A.; Karimi-Maleh, H.; Tamaddon, A.-M. Evaluation of Pt,Pd-Doped, NiO-Decorated, Single-Wall Carbon Nanotube-Ionic Liquid Carbon Paste Chemically Modified Electrode: An Ultrasensitive Anticancer Drug Sensor for the Determination of Daunorubicin in the Presence of Tamoxifen. Front. Chem. 2020, 8, 677. [Google Scholar] [CrossRef]
- Ozkan-Ariksoysal, D.; Kayran, Y.U.; Yilmaz, F.F.; Ciucu, A.A.; David, I.G.; David, V.; Hosgor-Limoncu, M.; Ozsoz, M. DNA-Wrapped Multi-Walled Carbon Nanotube Modified Electrochemical Biosensor for the Detection of Escherichia Coli from Real Samples. Talanta 2017, 166, 27–35. [Google Scholar] [CrossRef]
- Asadian, E.; Shahrokhian, S.; Iraji Zad, A.; Ghorbani-Bidkorbeh, F. Glassy Carbon Electrode Modified with 3D Graphene–Carbon Nanotube Network for Sensitive Electrochemical Determination of Methotrexate. Sens. Actuators B Chem. 2017, 239, 617–627. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Devarakonda, S.; Kumar, S.; Jang, J. Development of a Paper-Based Electrochemical Immunosensor Using an Antibody-Single Walled Carbon Nanotubes Bio-Conjugate Modified Electrode for Label-Free Detection of Foodborne Pathogens. Sens. Actuators B Chem. 2017, 253, 115–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, G.; Liu, H.; Fu, H.; Fan, J.; Wang, K.; Chen, Y.; Li, B.; Zhang, C.; Zhi, X.; et al. Identification of Volatile Biomarkers of Gastric Cancer Cells and Ultrasensitive Electrochemical Detection Based on Sensing Interface of Au-Ag Alloy Coated MWCNTs. Theranostics 2014, 4, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, D.; Zhou, Q.; Zhao, J.; Pan, N.; Zhang, Y.; Wang, L.; Shen, Y. An Enzyme Cascade-Based Electrochemical Immunoassay Using a Polydopamine–Carbon Nanotube Nanocomposite for Signal Amplification. J. Mater. Chem. B 2018, 6, 8180–8187. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Tang, Y.; Sang, Y.; Liu, W.; Wang, S.; Wang, X. Preparation of a Carboxylated Single-Walled Carbon-Nanotube-Chitosan Functional Layer and Its Application to a Molecularly Imprinted Electrochemical Sensor to Quantify Semicarbazide. Food Chem. 2020, 333, 127524. [Google Scholar] [CrossRef]
- Gayathri, C.H.; Mayuri, P.; Sankaran, K.; Kumar, A.S. An Electrochemical Immunosensor for Efficient Detection of Uropathogenic E. Coli Based on Thionine Dye Immobilized Chitosan/Functionalized-MWCNT Modified Electrode. Biosens. Bioelectron. 2016, 82, 71–77. [Google Scholar] [CrossRef]
- Ghanavati, M.; Tadayon, F.; Bagheri, H. A Novel Label-Free Impedimetric Immunosensor for Sensitive Detection of Prostate Specific Antigen Using Au Nanoparticles/MWCNTs- Graphene Quantum Dots Nanocomposite. Microchem. J. 2020, 159, 105301. [Google Scholar] [CrossRef]
- Fusco, G.; Gallo, F.; Tortolini, C.; Bollella, P.; Ietto, F.; De Mico, A.; D’Annibale, A.; Antiochia, R.; Favero, G.; Mazzei, F. AuNPs-Functionalized PANABA-MWCNTs Nanocomposite-Based Impedimetric Immunosensor for 2,4-Dichlorophenoxy Acetic Acid Detection. Biosens. Bioelectron. 2017, 93, 52–56. [Google Scholar] [CrossRef]
- Majd, S.M.; Salimi, A.; Ghasemi, F. An Ultrasensitive Detection of MiRNA-155 in Breast Cancer via Direct Hybridization Assay Using Two-Dimensional Molybdenum Disulfide Field-Effect Transistor Biosensor. Biosens. Bioelectron. 2018, 105, 6–13. [Google Scholar] [CrossRef]
- Pandey, R.R.; Liang, J.; Cakiroglu, D.; Charlot, B.; Todri-Sanial, A. Electrochemical Glucose Sensor Using Single-Wall Carbon Nanotube Field Effect Transistor. arXiv 2020. [Google Scholar] [CrossRef]
- Tran, T.L.; Nguyen, T.T.; Huyen Tran, T.T.; Chu, V.T.; Thinh Tran, Q.; Tuan Mai, A. Detection of Influenza A Virus Using Carbon Nanotubes Field Effect Transistor Based DNA Sensor. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 93, 83–86. [Google Scholar] [CrossRef]
- Fatin, M.F.; Rahim Ruslinda, A.; Gopinath, S.C.B.; Arshad, M.K.M. High-Performance Interactive Analysis of Split Aptamer and HIV-1 Tat on Multiwall Carbon Nanotube-Modified Field-Effect Transistor. Int. J. Biol. Macromol. 2019, 125, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, Y.; Zhang, J.; Wang, X.; Yin, F.; Li, Z.; Zhang, M. Universal DNA Detection Realized by Peptide Based Carbon Nanotube Biosensors. Nanoscale Adv. 2020, 2, 717–723. [Google Scholar] [CrossRef]
- Star, A.; Han, T.-R.; Joshi, V.; Gabriel, J.-C.P.; Grüner, G. Nanoelectronic Carbon Dioxide Sensors. Adv. Mater. 2004, 16, 2049–2052. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G.; Aversa, P.; Cusano, A.; Cutolo, A.; Giordano, M.; Nicolais, L. Carbon Nanotube Acoustic and Optical Sensors for Volatile Organic Compound Detection. Nanotechnology 2005, 16, 2536–2547. [Google Scholar] [CrossRef]
- Lu, Y.; Partridge, C.; Meyyappan, M.; Li, J. A Carbon Nanotube Sensor Array for Sensitive Gas Discrimination Using Principal Component Analysis. J. Electroanal. Chem. 2006, 593, 105–110. [Google Scholar] [CrossRef]
- Donaldson, K.; Tran, C.L. An Introduction to the Short-Term Toxicology of Respirable Industrial Fibres. Mutat. Res. Mol. Mech. Mutagen. 2004, 553, 5–9. [Google Scholar] [CrossRef]
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon Nanotubes: A Review of Their Properties in Relation to Pulmonary Toxicology and Workplace Safety. Toxicol. Sci. 2006, 92, 5–22. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon Nanotubes Introduced into the Abdominal Cavity of Mice Show Asbestos-like Pathogenicity in a Pilot Study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Wörle-Knirsch, J.M.; Pulskamp, K.; Krug, H.F. Oops They Did It Again! Carbon Nanotubes Hoax Scientists in Viability Assays. Nano Lett. 2006, 6, 1261–1268. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon Nanotubes: Properties, Synthesis, Purification, and Medical Applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef]
- Shahriar, S.; Mondal, J.; Hasan, M.; Revuri, V.; Lee, D.; Lee, Y.-K. Electrospinning Nanofibers for Therapeutics Delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Revuri, V.; Choochana, P.; Ganesan, P.; Kang, W.J.; Lee, Y. Sulfur and Nitrogen Doped Carbon Quantum Dots for Detection of Glutathione and Reduction of Cellular Nitric Oxide in Microglial Cells. J. Pharm. Investig. 2020, 50, 209–218. [Google Scholar] [CrossRef]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A Critical Review of Glucose Biosensors Based on Carbon Nanomaterials: Carbon Nanotubes and Graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [PubMed]

| Functionalization of CNTs | Sizes | Diagnosis Methods | Type of Cancer | Ref. |
|---|---|---|---|---|
| PEG-conjugated SWCNT functionalized with anti-CD44 antibody | 1–2/200 nm | MRI/SPECT/NIR fluorescence imaging with nanoprobe | Breast cancer stem cells by monitoring iron content. | [134] |
| High-purity CNT films with gold nanoparticles (AuNPs) | Thin film layer | Field-effect transistor (FET) biosensor | Breast cancer based on detection of exosomal miRNA (miR-FET) | [141] |
| CNT chip coated with thin film with anti-EpCAM antibody | CNT film (10 mm × 10 mm) | pH responsive CNT film based microfluidic device capturing EpCAM expressing CTC | Various EpCAM-expressing circulating tumor cells (CTCs) | [135] |
| Functionalized multi-walled carbon nanotubes (MWCNTs) with anti-CA19-9 antibody | - | PEI-CNT film immobilized with antibodies (anti-CA19-9) was studied using the PM-IRRAS technique | Colon cancer cells (HT-29) expressing CA19-9 | [137] |
| Hyaluronic acid (HA)-conjugated MWCNTs with anti-CD44 antibody | ITO substrate with standard size [7.5 (width) × 25 (height) mm] | Ligand–protein recognition [hyaluronic acid (HA)-CD44] assay. Electrochemistry can convert target analytes into the signal output. | Lung and breast cancer cells expressing CD44 | [142] |
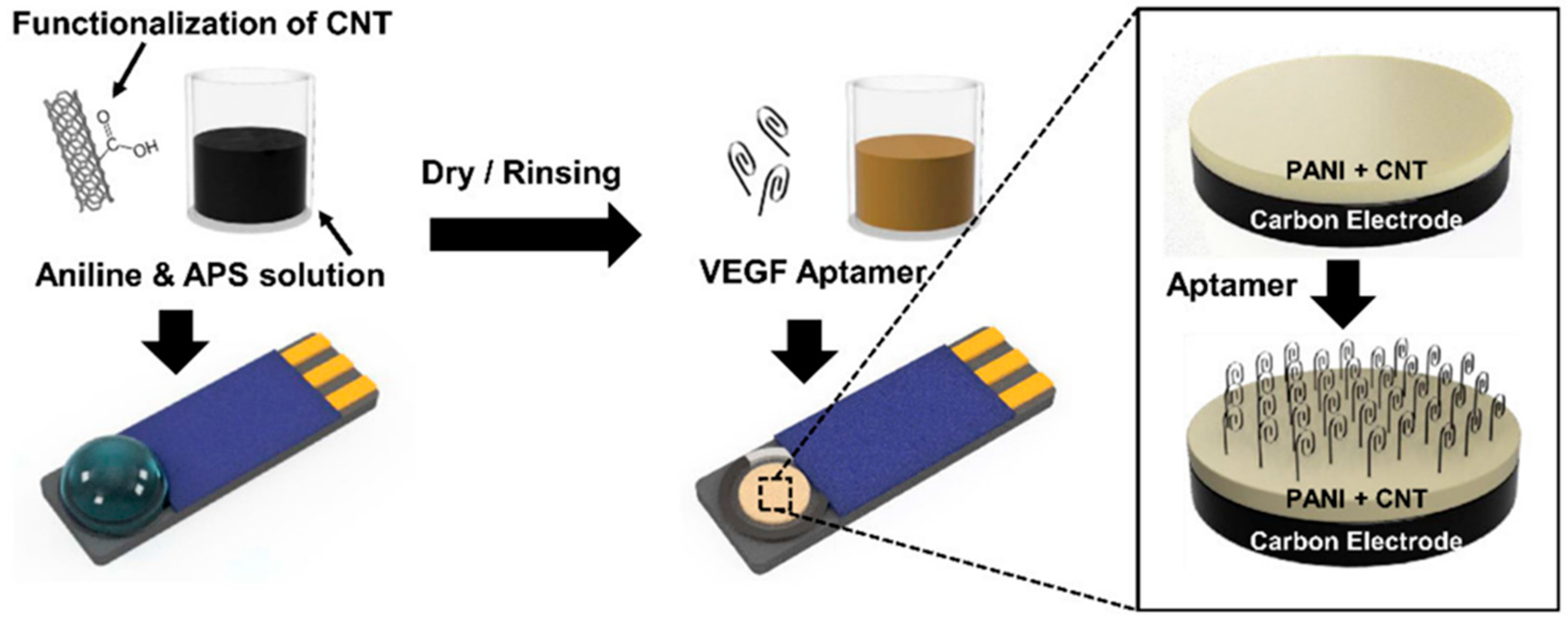
| Nanocomposite containing polyaniline (PANI) and CNT with anti-VEGF RNA aptamer on tumor cells. | CNTs diameter 1.5 nm and length = 1–5 µm) | VEGF detection by electrochemical sensor using RNA aptamer on screen-printed carbon electrode (SPCE) | VEGF-expressing cancer cells | [139] |
| Photo-luminescent SWCNTs engineered to respond metastatic prostate cancer biomarker (uPA). | - | Act by modulating the optical bandgap upon interaction with analyte | Metastatic prostate cancer biomarker (uPA)-expressing cell line | [143] |
| DNA/RGD-peptide/SWCNTS as nIR labels | Length 350 nm | ssDNA-peptide non-covalently adsorbed on SWCNTs and recognize cell surface receptors like integrins. | Integrin receptor-expressing cancer cells | [144] |
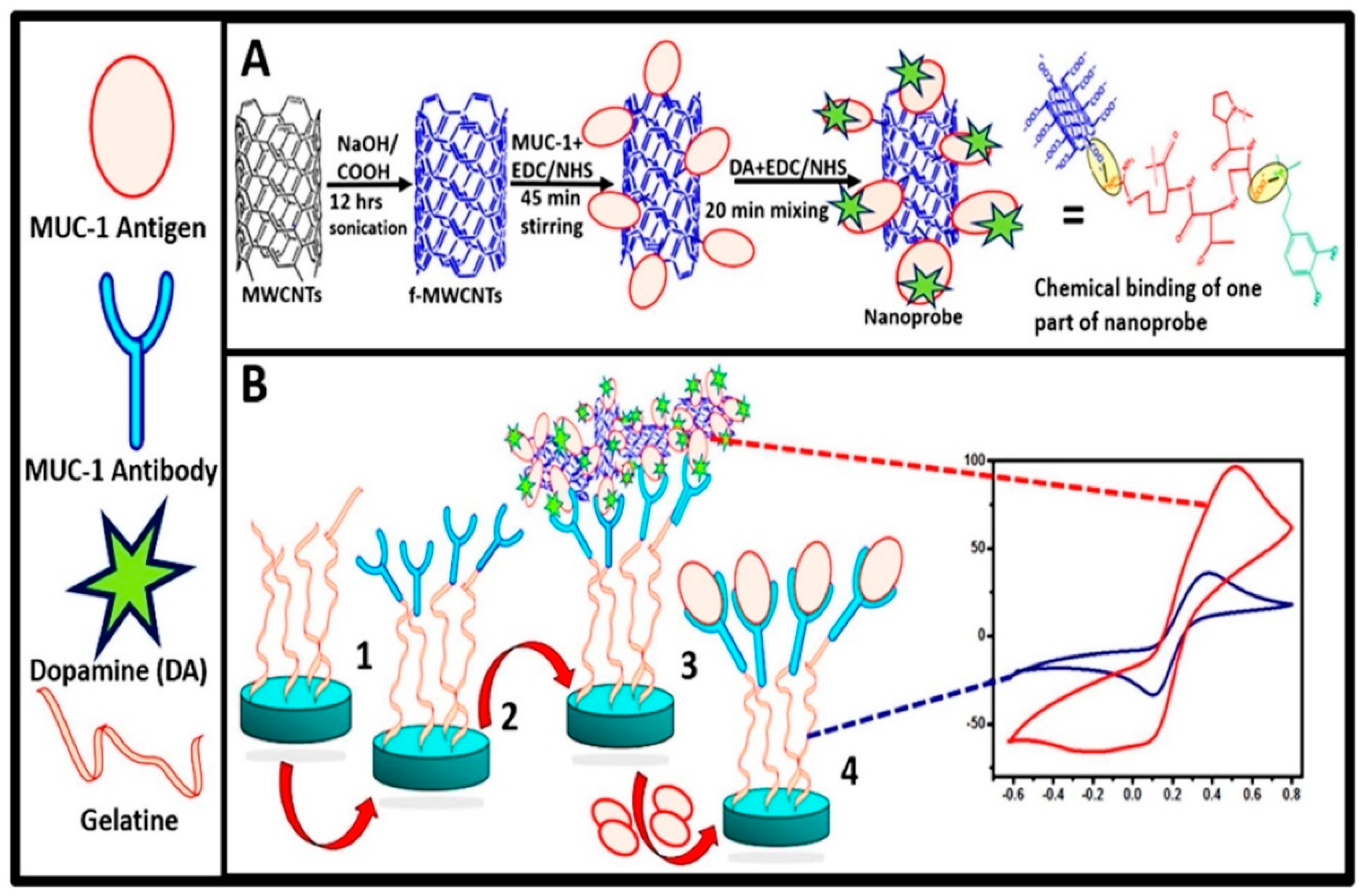
| DA-coated MUC1-functionalized MWCNTs | MWCNTs (diameter: 4–5 nm, length: 0.5–1.5 μm) | Electrochemical immunosensor based on dopamine coated MUC1 functionalized multi-walled carbon nanotubes sensing MUC1 biomarker | MUC1-expressing cancer cells | [140] |
| Enzyme aggregate-conjugated CNTs. | - | Emitted chemiluminescence by nanosensor catalyzed substrates will be detected in different time windows | Hepatocellular carcinoma | [145] |
| Methods | Analytes | Limit of Detection | Detection Range | Ref. |
|---|---|---|---|---|
| Enzymatic | Lactate | Not reported | 5–20 nM | [149] |
| Uric acid | 9.91 μM | 50 to 650 μM | [150] | |
| Glucose | 0.58 μM | 0.8 to 250 μM | [151] | |
| Glucose | 3 × 10−4 M | (1–15) × 10−3 M | [152] | |
| Glucose | 5 × 10−5 M | (0–5) × 10−3 M | [153] | |
| Glucose | 2.99 × 10−6 M | (3–14) × 10−3 M | [154] | |
| Ethanol | 1 × 10−5 M | (1–5) × 10−4 M | [155] | |
| Urease | 67 μM | 1.0–25.0 mM | [156] | |
| Alcohol dehydrogenase | 10 μM | 0.1 to 0.5 μM | [152] | |
| Choline | 0.6 μM | 3–120 μM | [157] | |
| Non-enzymatic | Pyruvic acid | 0.048 μM | 0.1–200 μM | [158] |
| Human epidermal growth factor receptor 2 | 7400 pg/mL | 10–110 ng mL−1 | [159] | |
| Cholesterol | 0.5 nM | 0.001–3 μM | [160] | |
| glucose | 500 nM | 2–19,600 μM | [161] | |
| Zearalenone | 0.15 pg mL−1 | 0.001–0.1 | [162] | |
| Long non-coding RNAs | 42.8 fM | 10−14–10−7 M | [163] | |
| MicroRNA 21 | 0.01 fM | 10−17–10−6 M | [164] | |
| Thrombin | 0.08 pM | 0.001–4 nM | [165] | |
| Human epidermal growth factor receptor 2 | 50 fg mL−1 | 0.1 pg mL−1–1 ng mL−1 | [166] | |
| Cardiac troponin T | 0.04 pg mL−1 | 0.1–8 pg mL−1 | [167] | |
| Urea | 4.7 nM | 0.066–20,600 µM | [168] | |
| Ascorbic acid | 0.85 nM | 0.001–8000 µM | [169] | |
| Glucose | 645 nM | 20–10,500 µM | [170] | |
| Glucose | 0.33 nM | 10–2000 µM | [171] | |
| Dopamine | 9.5 nM | 0.033–1 µM | [172] | |
| Potassium ions | Not reported | 1000–32,000 µM | [173] | |
| Hydrogen peroxide | Not Reported | 5 × 10−6–5 × 10−3 M | [174] | |
| MicroRNA 155 | 3.34 × 10−14 M | 1 × 10−13–1 × 10−9 M | [175] | |
| Digoxin | 7.95 × 10−12 M | 2.65 × 10−11–6.8 × 10−10 M | [176] | |
| Sequence specific to chronic myelogenous leukemia | 1 fM | 10−15–10−6 M | [177] | |
| Myeloperoxidase | 327 ng mL−1 | Not reported | [178] | |
| SARS-CoV-2 spike protein | 35 mg L−1 | Not reported | [179] | |
| SARS-CoV-2 spike protein | 0.55 fg mL−1 | 0.0055–5.5 pg mL−1 | [180] | |
| Ascorbic acid | 76.5 pM | 100 pM to 1 mM | [181] |
| Methods | Analytes | Limit of Detection | Detection Range | Ref. |
|---|---|---|---|---|
| Amperometric | Zearalenone | 0.15 pg mL−1 | 0.001–0.1 ng mL−1 | [162] |
| Polyclonal anti-Staphylococcus aureus | 100 CFU mL−1 | 102–105 CFU mL−1 | [230] | |
| Glucose | 645 nM | 20–10,500 µM | [170] | |
| Glucose | 0.33 nM | 10–2000 µM | [171] | |
| Glucose | 500 nM | 1–1000 µM | [231] | |
| Glucose | 3 × 10−4 M | (1–15) × 10−5 M | [152] | |
| Glucose | 5 × 10−5 M | (0–5) × 10−3 M | [153] | |
| Glucose | 2.99 × 10−6 M | (3–14) × 10−3 M | [154] | |
| Glucose | 5 μM | 8 μM–1.5 mM | [232] | |
| Alcohols | 3.3 × 10−3 M | (12.5–100) × 10−3 M | [152] | |
| Ethanol | 1 × 10−5 M | (1–5) × 10−4 M | [155] | |
| Xanthine | 1.2 × 10−7 M | (2–86) × 10−6 M | [233] | |
| Choline | 6 × 10−7 M | (3–120) × 10−6 M | [157] | |
| Nitrite | 0.4 μM | 1–1000 μM | [234] | |
| Urine albumin | 4.96 × 10−8 mol L−1 | 3.3 ng μL−1–3.3 mg μL−1 | [235] | |
| Cholesterol | 0.1 × 10−3 | 2–8 × 10−3 M | [236] | |
| Fluorescence | Adenosine triphosphate | 2.4 × 10−7 M | Not Reported | [237] |
| Troponin T | 2.5 × 10−9 M | Not Reported | [238] | |
| Acetic acid | 0.05% (v/v) | 0.05–3.2% (v/v) | [239] | |
| SARS-CoV-2 spike protein | 35 mg L−1 | Not reported | [179] | |
| Hydrogen peroxide | Not Reported | 5 × 10−6–5 × 10−3 M | [174] | |
| MicroRNA 155 | 3.34 × 10−14 M | 1 × 10−13–1 × 10−9 M | [175] | |
| Digoxin | 7.95 × 10−12 M | 2.65 × 10−11–6.8 × 10−10 M | [176] | |
| Chemiresistive | E. coli O157:H7 | 105 colony-forming units (CFU) mL−1 (whole cell); 103 CFU mL−1 (lysates) | 103–107 CFU mL−1 | [240] |
| Cardiac myoglobin | 1 ng mL−1 | 1–1000 ng mL−1 | [241] | |
| histidine rich protein II (HRP2) | 0.97 fg mL−1 | 10 fg mL−1–10 ng mL−1 | [242] | |
| Avian influenza virus (H5N1) DNA sequence | Not Reported | 2–200 pM | [243] | |
| Microcystin-LR | 0.6 pg mL−1 | 0.001–1 ng mL−1 | [244] | |
| Differential pulse voltammetry (DPV) | Bisphenol A | 4 nM | 0.01–0.7 µM | [245] |
| Ascorbic acid | 0.85 nM | 0.001–8000 µM | [169] | |
| Dopamine | 1.36 nM | 0.1–48 µM | [207] | |
| Dopamine | 0.87 nM | 0.005–100.0 μM | [246] | |
| Uric acid | 33.03 nM | 0.5–60 µM | [207] | |
| Anthrax lethal factor | 3.5 fM | 10−14–10−10 M | [247] | |
| Hepatitis B virus genomic DNA | 2.5 fM | 10−14–10−8 M | [248] | |
| Folic acid (vitamin B9) | 0.095 μmol L−1 | 0.5–26 μmol L−1 | [249] | |
| Matrix metalloproteinase-7 (MMP-7) | 6 pg mL−1 | 1 × 10−2–1 × 103 ng mL−1 | [250] | |
| Daunorubicin Tamoxifen | 3.0 nM 0.1 μM | 0.008–350 μM 0.5–330 μM | [251] | |
| Long non-coding RNAs | 42.8 fM | 10−14–10−7 M | [163] | |
| Sequence specific to E. coli | 17 × 106 fM | Not reported | [252] | |
| MicroRNA 21 | 0.01 fM | 10−17–10−6 M | [164] | |
| Thrombin | 0.08 pM | 0.001–4 nM | [165] | |
| Methotrexate | 70 nM | 0.7–100 µM | [253] | |
| Staphylococcus aureus | 15 CFU mL−1 | 10–107 CFU mL−1 | [254] | |
| Cyclic voltammetry (CV) | Methotrexate | 70 nM | 0.7–100 µM | [253] |
| Urea | 4.7 nM | 0.066–20,600 µM | [168] | |
| 3-ocatnone | 0.3 ppb | 0–0.0025% (v/v) | [255] | |
| Butanone | 0.5 ppb | 0–0.055% (v/v) | [255] | |
| Carcinoembryonic antigen | 8.39 pg mL−1 | 10 pg mL−1 to 10 ng mL−1 | [256] | |
| Semicarbazide | 0.025 ng mL−1 | 0.04–7.6 ng mL−1 | [257] | |
| Escherichia coli | 50 CFU mL−1 | 102−109 CFU of UPEC mL−1 | [258] | |
| Dopamine | 9.5 nM | 0.033–1 µM | [172] | |
| Impedance spectroscopy | Human epidermal growth factor receptor 2 | 7400 pg mL−1 | 10–110 ng mL−1 | [159] |
| CA19-9 | 0.35 U mL−1 | Not reported | [137] | |
| Prostate-specific antigen | 0.48 pg mL−1 | 1–10,000 pg mL−1 | [259] | |
| Sequence specific to chronic myelogenous leukaemia | 1 fM | 10−15–10−6 M | [177] | |
| 2,4-dichlorophenoxy acetic acid | 0.3 ppb | 1–100 ppb | [260] | |
| Transistor | SARS-CoV-2 spike protein | 0.55 fg mL−1 | 0.0055–5.5 pg mL−1 | [180] |
| SARS-CoV-2 nucleocapsid protein | 0.016 fg mL−1 | 0.016–16 pg mL−1 | [180] | |
| Microcystin-LR | 0.6 pg mL−1 | 0.001–1 ng mL−1 | [244] | |
| miRNA-15 | 0.03 fM | 0.1 fM–10 nM | [261] | |
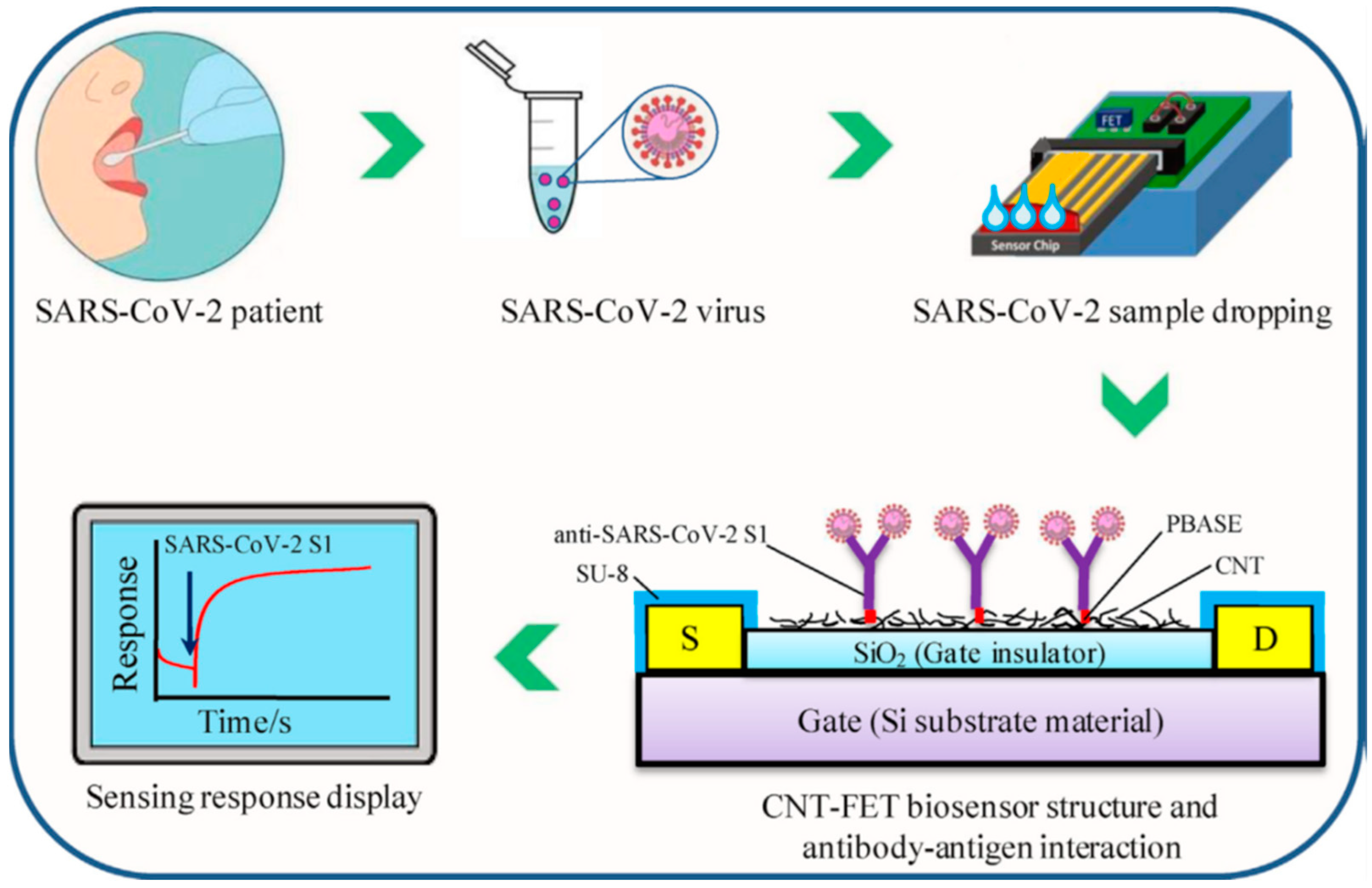
| SARS-CoV-2 S1 antigen | 4.12 fg mL−1 | 0.1 fg mL−1–5.0 pg mL−1 | [28] | |
| Glucose | 0.01 mM | 0.01–2 mM | [262] | |
| influenza A virus DNA | 1 pM | 1 pM to 10 nM | [263] | |
| HIV-1 Tat protein | 600 pM | 0.2 nM–1μM | [264] | |
| cDNA | 0.88 μg L−1 | 1.6 × 10−4–5 μmol L−1 | [265] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, J.; An, J.M.; Surwase, S.S.; Chakraborty, K.; Sutradhar, S.C.; Hwang, J.; Lee, J.; Lee, Y.-K. Carbon Nanotube and Its Derived Nanomaterials Based High Performance Biosensing Platform. Biosensors 2022, 12, 731. https://doi.org/10.3390/bios12090731
Mondal J, An JM, Surwase SS, Chakraborty K, Sutradhar SC, Hwang J, Lee J, Lee Y-K. Carbon Nanotube and Its Derived Nanomaterials Based High Performance Biosensing Platform. Biosensors. 2022; 12(9):731. https://doi.org/10.3390/bios12090731
Chicago/Turabian StyleMondal, Jagannath, Jeong Man An, Sachin S. Surwase, Kushal Chakraborty, Sabuj Chandra Sutradhar, Joon Hwang, Jaewook Lee, and Yong-Kyu Lee. 2022. "Carbon Nanotube and Its Derived Nanomaterials Based High Performance Biosensing Platform" Biosensors 12, no. 9: 731. https://doi.org/10.3390/bios12090731
APA StyleMondal, J., An, J. M., Surwase, S. S., Chakraborty, K., Sutradhar, S. C., Hwang, J., Lee, J., & Lee, Y.-K. (2022). Carbon Nanotube and Its Derived Nanomaterials Based High Performance Biosensing Platform. Biosensors, 12(9), 731. https://doi.org/10.3390/bios12090731








