An Improved Method for Quick Quantification of Unsaturated Transferrin
Abstract
1. Introduction
2. Materials and Methods
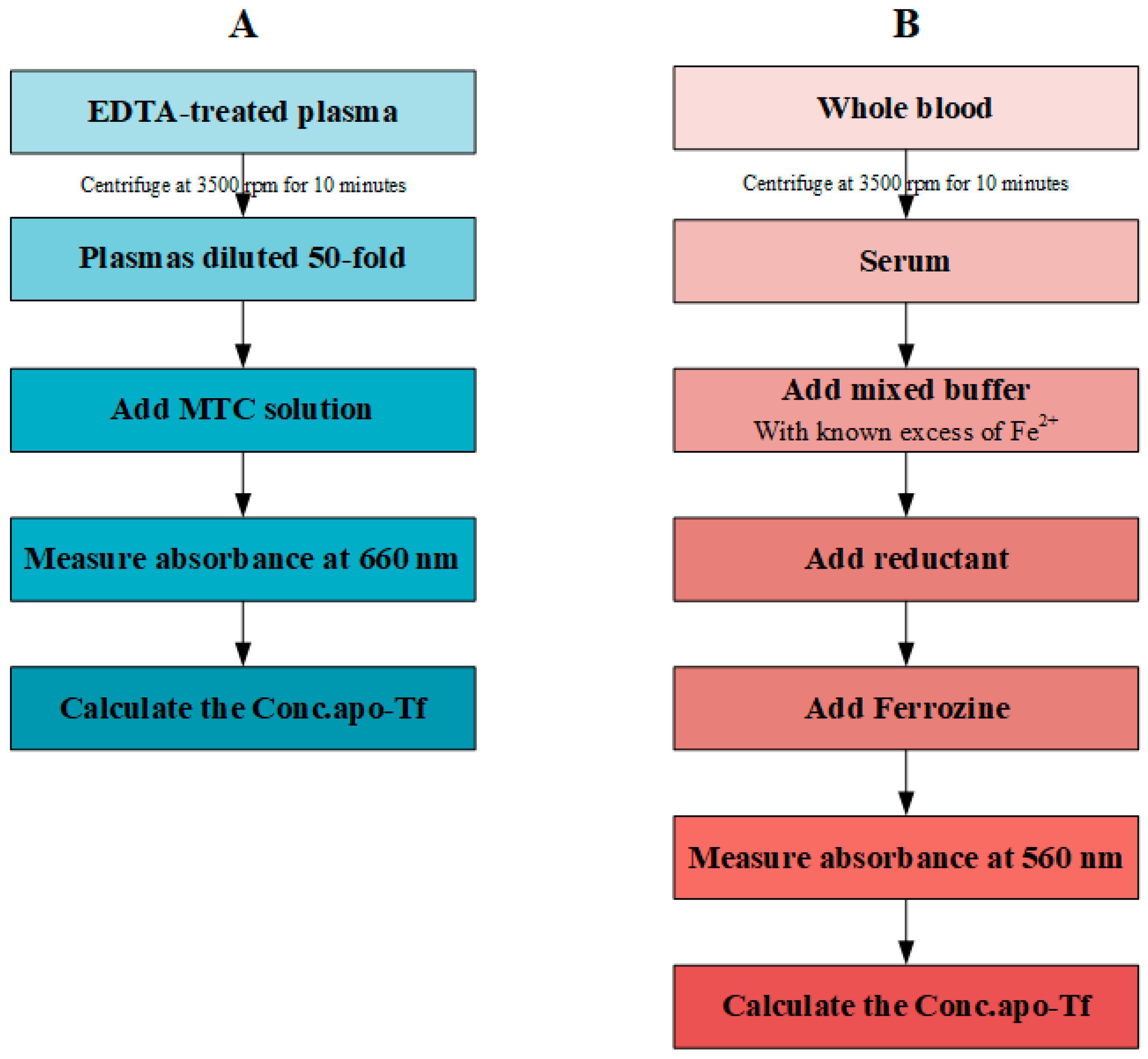
2.1. Sample Collection and Preparation
2.2. MTC Test
2.3. UIBC Test
2.4. Result Analysis
3. Results
3.1. Inconsistency in the UIBC Measurement
3.2. Establishing a Linear Correlation between A660 and Concentration of Apotransferrin in Non-Biological Solutions
3.3. The Extension of MTC Method to Blood Plasma
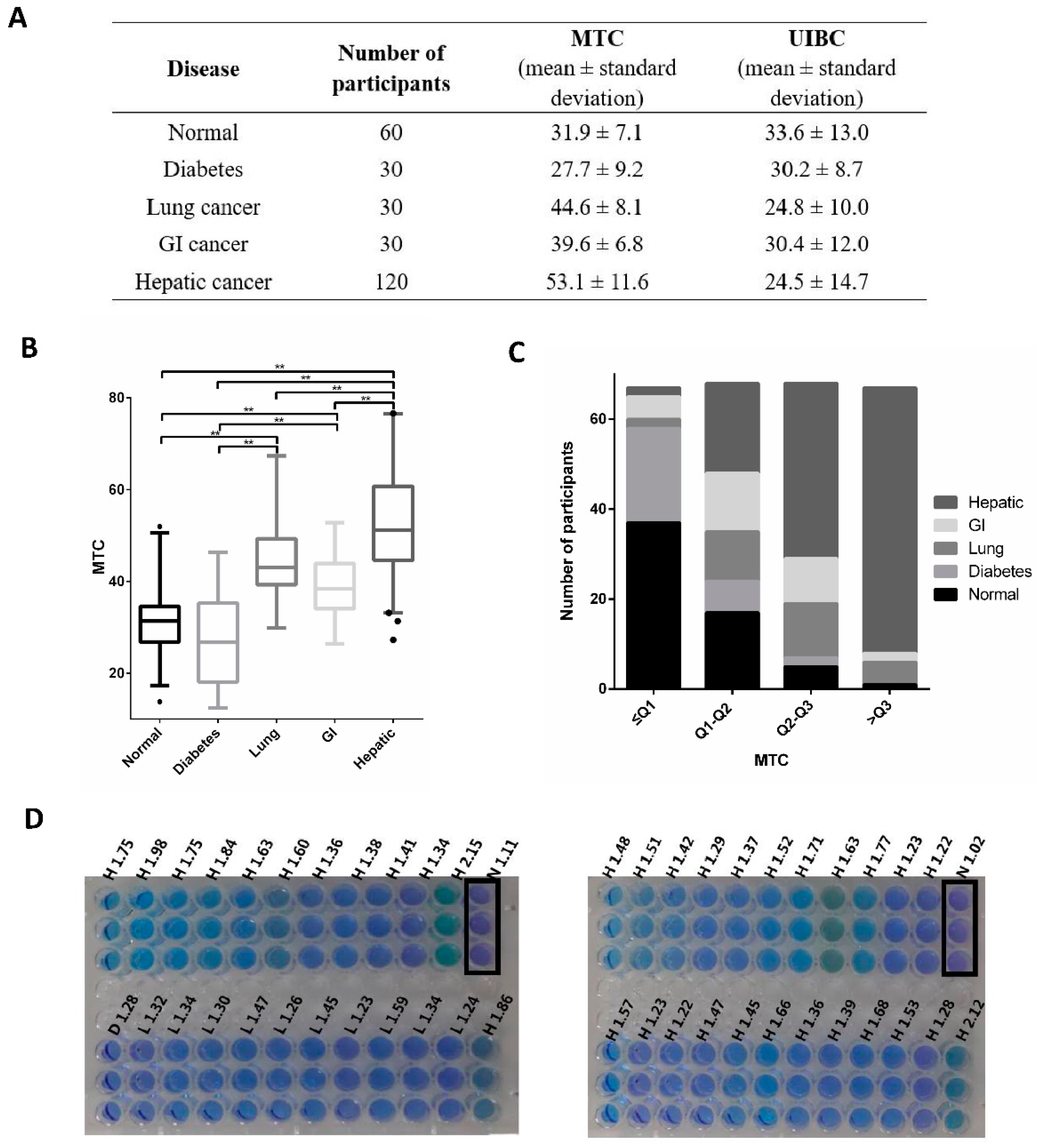
3.4. Systemic Comparison
3.5. Visible Indicator
4. Discussion and Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Winter, W.E.; Bazydlo, L.A.; Harris, N.S. The molecular biology of human iron metabolism. Lab. Med. 2014, 45, 92–102. [Google Scholar] [PubMed]
- Pantopoulos, K. Inherited Disorders of Iron Overload. Front. Nutr. 2018, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Trenor, C.C., 3rd; Campagna, D.R.; Sellers, V.M.; Andrews, N.C.; Fleming, M.D. The molecular defect in hypotransferrinemic mice. Blood 2000, 96, 1113–1118. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Bird, R.; Clague, A.; Carter, A. Clinical utility of serum soluble transferrin receptor levels and comparison with bone marrow iron stores as an index for iron-deficient erythropoiesis in a heterogeneous group of patients. Pathology 2007, 39, 349–353. [Google Scholar] [CrossRef]
- Wish, J.B. Assessing iron status: Beyond serum ferritin and transferrin saturation. Clin. J. Am. Soc. Nephrol. 2006, 1, S4–S8. [Google Scholar] [CrossRef]
- Gambino, R.; Desvarieux, E.; Orth, M.; Matan, H.; Ackattupathil, T.; Lijoi, E.; Wimmer, C.; Bower, J.; Gunter, E. The relation between chemically measured total iron-binding capacity concentrations and immunologically measured transferrin concentrations in human serum. Clin. Chem. 1997, 43, 2408–2412. [Google Scholar] [CrossRef]
- Persijn, J.P.; van der Slik, W.; Riethorst, A. Determination of serum iron and latent iron-binding capacity (LIBC). Clin. Chim. Acta 1971, 35, 91–98. [Google Scholar]
- Pfeiffer, C.M.; Looker, A.C. Laboratory methodologies for indicators of iron status: Strengths, limitations, and analytical challenges. Am. J. Clin. Nutr. 2017, 106, 1606S–1614S. [Google Scholar]
- Koseoglu, M.; Hur, A.; Atay, A.; Cuhadar, S. Effects of hemolysis interferences on routine biochemistry parameters. Biochem. Med. 2011, 21, 79–85. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, J.; Uh, Y.; Lee, S.I.; Seo, D.M.; Kim, K.S.; Jang, J.Y.; Lee, M.H.; Yoon, K.R.; Yoon, K.J. Development of an integrated reporting system for verifying hemolysis, icterus, and lipemia in clinical chemistry results. Ann. Lab. Med. 2014, 34, 307–312. [Google Scholar] [CrossRef]
- Blanck, H.M.; Pfeiffer, C.M.; Caudill, S.P.; Reyes, M.; Gunter, E.W.; Imperatore, G.; van Assendelft, O.W.; Strider, S.; Death, T. Serum iron and iron-binding capacity: A round-robin interlaboratory comparison study. Clin. Chem. 2003, 49, 1672–1675. [Google Scholar] [PubMed][Green Version]
- Gkamprela, E.; Deutsch, M.; Pectasides, D. Iron deficiency anemia in chronic liver disease: Etiopathogenesis, diagnosis and treatment. Ann. Gastroenterol. 2017, 30, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Shi, L.; Lan, L.; Zhao, Z.; Yang, D.; Fu, Y.; Tang, Y.; Zhang, X. Visualized detection of apo-transferrin based on cyanine dye supramolecular assembly. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 215, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Z.; Lan, L.; Yang, Q.; Zhang, P.; Shi, L.; Lang, Y.; Tabib-Salazar, A.; Wigneshweraraj, S.; Zhang, J.; et al. A Rapid Colorimetric Method to Visualize Protein Interactions. Chemistry 2018, 24, 6727–6731. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Montagnana, M.; Brocco, G.; Guidi, G.C. Influence of hemolysis on routine clinical chemistry testing. Clin. Chem. Lab. Med. 2006, 44, 311–316. [Google Scholar] [CrossRef]
- Rifai, N.; Horvath, A.R.; Wittwer, C. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Shindy, H.A. Fundamentals in the chemistry of cyanine dyes: A review. Dye. Pigment. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Im, J.; Lee, J.; Loffler, F.E. Interference of ferric ions with ferrous iron quantification using the ferrozine assay. J. Microbiol. Methods 2013, 95, 366–367. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Ronicke, F.; Schepers, U.; Wagenknecht, H.A. A new structure-activity relationship for cyanine dyes to improve photostability and fluorescence properties for live cell imaging. Chem. Sci. 2018, 9, 6557–6563. [Google Scholar] [CrossRef]
- Iqbal, A.; Wang, L.; Thompson, K.C.; Lilley, D.M.; Norman, D.G. The structure of cyanine 5 terminally attached to double-stranded DNA: Implications for FRET studies. Biochemistry 2008, 47, 7857–7862. [Google Scholar] [CrossRef] [PubMed]


| Disease Group | Male | Female | p 1 | ||
|---|---|---|---|---|---|
| Number of Participants | MTC (Mean ± Standard Deviation) | Number of Participants | MTC (Mean ± Standard Deviation) | ||
| Normal | 33 | 31.2 ± 6.0 | 27 | 32.9 ± 8.2 | 0.453 |
| Diabetes | 23 | 26.6 ± 8.5 | 7 | 31.2 ± 11.1 | 0.413 |
| Lung cancer | 23 | 45.7 ± 7.4 | 7 | 41.1 ± 9.9 | 0.288 |
| GI cancer | 21 | 39.1 ± 6.8 | 9 | 40.8 ± 7.1 | 0.397 |
| Hepatic cancer | 89 | 52.9 ± 10.8 | 31 | 53.6 ± 13.8 | 0.995 |
| Disease Group | Male | Female | p 1 | ||
|---|---|---|---|---|---|
| Number of Participants | UIBC (Mean ± Standard Deviation) | Number of Participants | UIBC (Mean ± Standard Deviation) | ||
| Normal | 33 | 30.2 ± 8.3 | 27 | 37.7 ± 16.4 | 0.115 |
| Diabetes | 23 | 28.2 ± 7.3 | 7 | 36.9 ± 9.9 | 0.033 |
| Lung cancer | 23 | 23.3 ± 10.1 | 7 | 29.7 ± 8.7 | 0.144 |
| GI cancer | 20 | 29.7 ± 10.0 | 9 | 32.0 ± 16.1 | 0.982 |
| Hepatic cancer | 84 | 23.1 ± 14.3 | 30 | 28.5 ± 15.4 | 0.059 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Gao, J.; Hui, L.; Li, Y.; Liu, J.; Fu, Y.; Shi, L.; Wang, Y.; Liu, B. An Improved Method for Quick Quantification of Unsaturated Transferrin. Biosensors 2022, 12, 708. https://doi.org/10.3390/bios12090708
Guo R, Gao J, Hui L, Li Y, Liu J, Fu Y, Shi L, Wang Y, Liu B. An Improved Method for Quick Quantification of Unsaturated Transferrin. Biosensors. 2022; 12(9):708. https://doi.org/10.3390/bios12090708
Chicago/Turabian StyleGuo, Ruirui, Juanjuan Gao, Lingyun Hui, Yanqing Li, Junhui Liu, Yao Fu, Lei Shi, Yawen Wang, and Bing Liu. 2022. "An Improved Method for Quick Quantification of Unsaturated Transferrin" Biosensors 12, no. 9: 708. https://doi.org/10.3390/bios12090708
APA StyleGuo, R., Gao, J., Hui, L., Li, Y., Liu, J., Fu, Y., Shi, L., Wang, Y., & Liu, B. (2022). An Improved Method for Quick Quantification of Unsaturated Transferrin. Biosensors, 12(9), 708. https://doi.org/10.3390/bios12090708





