Gluconobacter Oxydans-Based MFC with PEDOT:PSS/Graphene/Nafion Bioanode for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Anode Fabrication
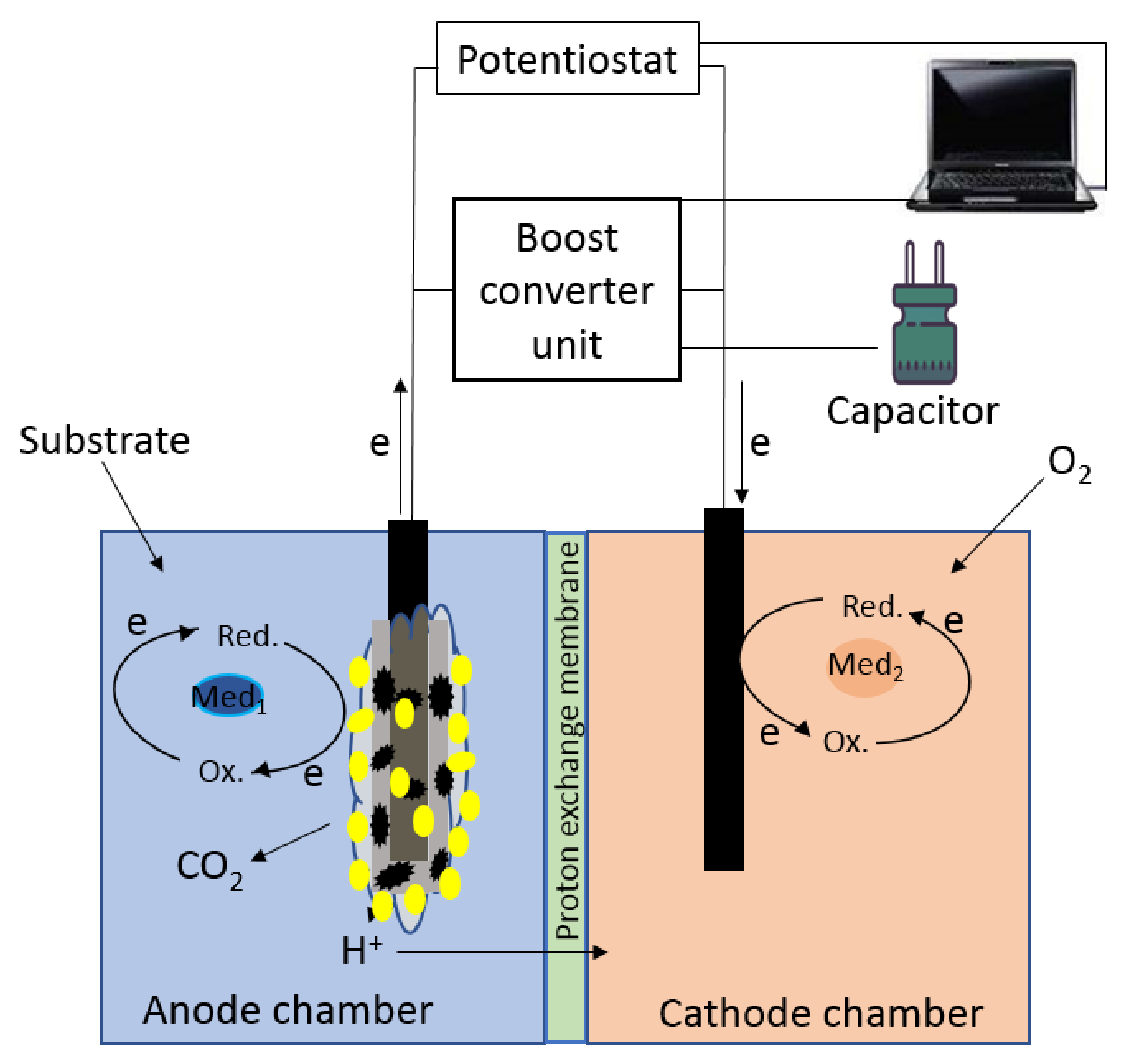
2.3. MFC Setup and Operation
2.4. Electrochemical Characterization
2.5. Raman Spectroscopy
3. Results
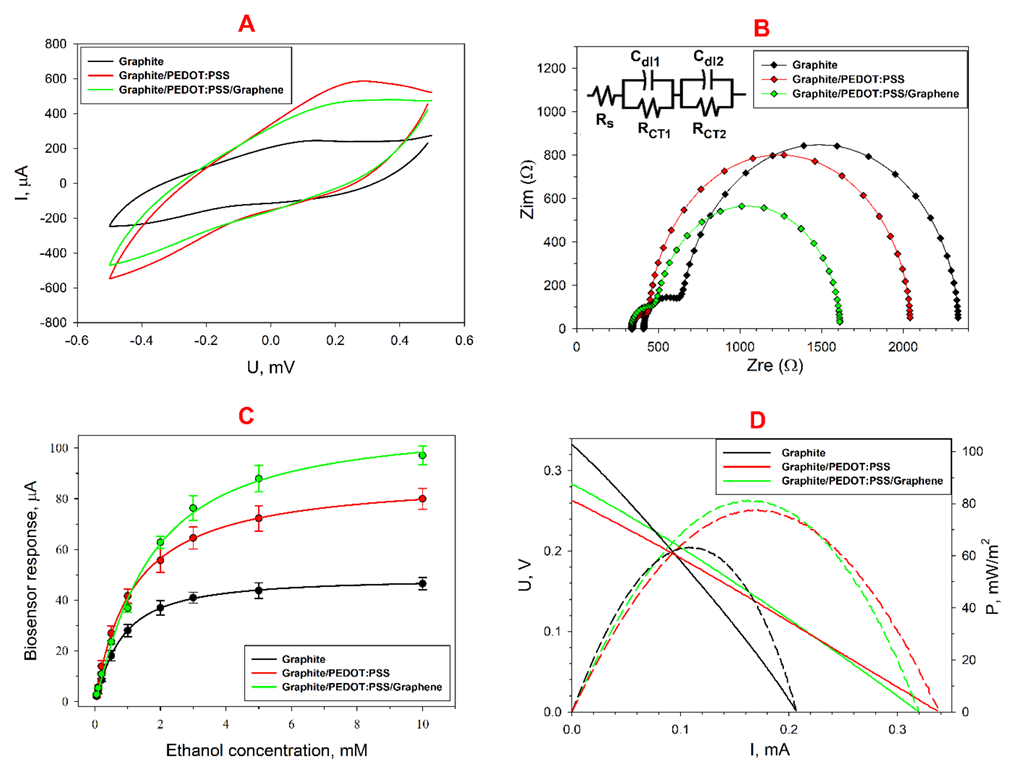
3.1. The Structure of the Composite
3.2. Electrochemical Characterization of the Biocomposite
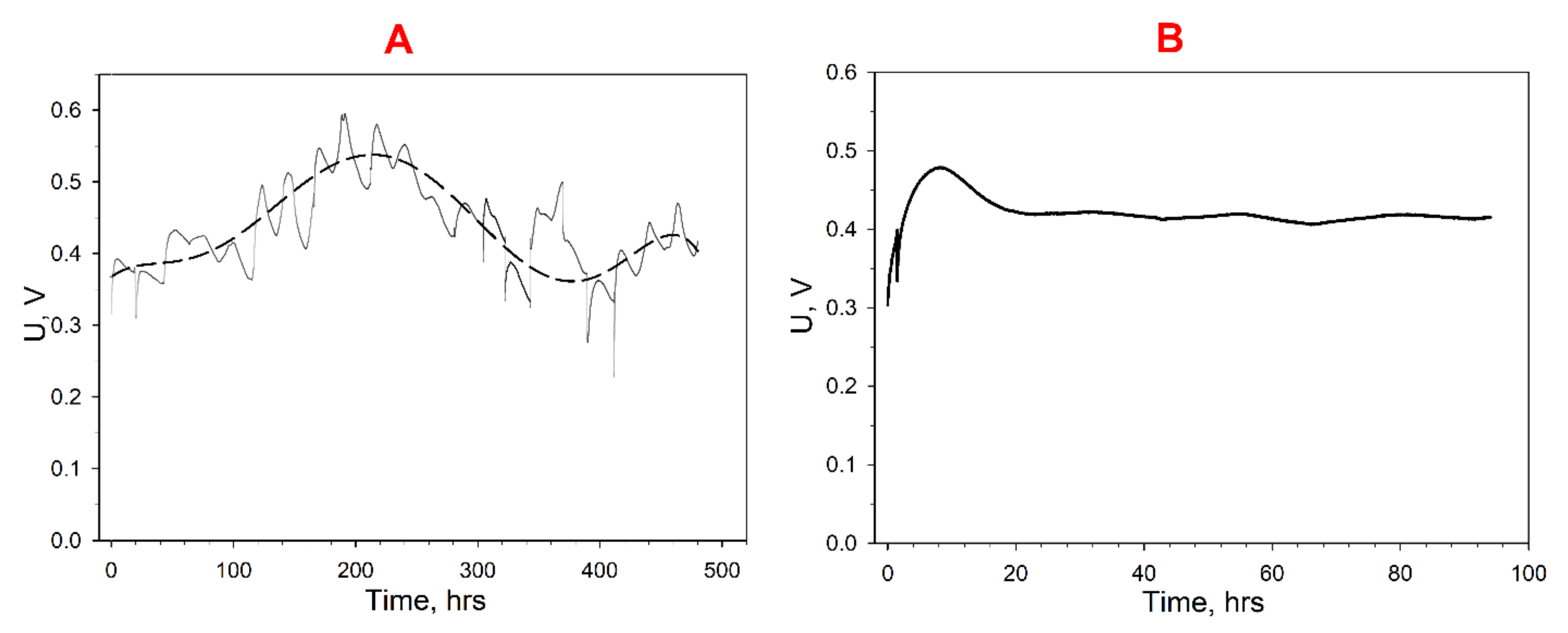
3.3. Wastewater Treatment
3.4. Accumulation of Electric Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hassani, A.; Malhotra, M.; Karim, A.V.; Krishnan, S.; Nidheesh, P.V. Recent progress on ultrasound-assisted electrochemical processes: A review on mechanism, reactor strategies, and applications for wastewater treatment. Environ. Res. 2022, 205, 112463. [Google Scholar] [CrossRef] [PubMed]
- Tuholske, C.; Halpern, B.S.; Blasco, G.; Villasenor, J.C.; Frazier, M.; Caylor, K. Mapping global inputs and impacts from of human sewage in coastal ecosystems. PLoS ONE 2021, 16, e0258898. [Google Scholar] [CrossRef] [PubMed]
- Barbusiński, K.; Filipek, K. Water and wastewater treatment: Selected topics. Clean Technol. 2022, 4, 91–96. [Google Scholar] [CrossRef]
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily wastewater treatment: Overview of conventional and modern methods, challenges, and future opportunities. Water 2021, 13, 980. [Google Scholar] [CrossRef]
- Yap, K.-L.; Ho, L.-N.; Ong, S.-A.; Guo, K.; Liew, Y.-M.; Oon, Y.-S.; Thor, S.-H.; Tan, S.-M.; Teoh, T.-P. Primary insights into the effects of organic pollutants and carbon-based cathode materials in a double chambered microbial fuel cell integrated electrocatalytic process. J. Water Process Eng. 2021, 44, 102358. [Google Scholar] [CrossRef]
- Oon, Y.-S.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Oon, Y.-L.; Lehl, H.K.; Thung, W.-E.; Nordin, N. Disclosing the synergistic mechanisms of azo dye degradation and bioelectricity generation in a microbial fuel cell. Chem. Eng. J. 2018, 344, 236–245. [Google Scholar] [CrossRef]
- Nawaz, A.; ul Haq, I.; Qaisar, K.; Gunes, B.; Raja, S.I.; Mohyuddin, K.; Amin, H. Microbial fuel cells: Insight into simultaneous wastewater treatment and bioelectricity generation. Process Saf. Environ. Prot. 2022, 161, 357–373. [Google Scholar] [CrossRef]
- Mohyudin, S.; Farooq, R.; Jubeen, F.; Rasheed, T.; Fatima, M.; Sher, F. Microbial fuel cells a state-of-the-art technology for wastewater treatment and bioelectricity generation. Environ. Res. 2022, 204, 112387. [Google Scholar] [CrossRef]
- Esfandyari, M.; Fanaei, M.A.; Gheshlaghi, R.; Akhavan Mahdavi, M. Mathematical modeling of two-chamber batch microbial fuel cell with pure culture of Shewanella. Chem. Eng. Res. Des. 2017, 117, 34–42. [Google Scholar] [CrossRef]
- Ren, H.; Tian, H.; Gardner, C.L.; Ren, T.-L.; Chae, J. A miniaturized microbial fuel cell with three-dimensional graphene macroporous scaffold anode demonstrating a record power density of over 10,000 W m−3. Nanoscale 2016, 8, 3539–3547. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, V. Recent trends in upgrading the performance of yeast as electrode biocatalyst in microbial fuel cells. Chemosphere 2021, 284, 131383. [Google Scholar] [CrossRef] [PubMed]
- Boas, J.V.; Peixoto, L.; Oliveira, V.B.; Simões, M.; Pinto, A.M.F.R. Cyclic voltammetry study of a yeast-based microbial fuel cell. Bioresour. Technol. Rep. 2022, 17, 100974. [Google Scholar] [CrossRef]
- Khilyas, I.V.; Sorokin, A.A.; Kiseleva, L.; Simpson, D.J.W.; Fedorovich, V.; Sharipova, M.R.; Kainuma, M.; Cohen, M.F.; Goryanin, I. Comparative metagenomic analysis of electrogenic microbial communities in differentially inoculated swine wastewater-fed microbial fuel cells. Scientifica 2017, 2017, 7616359. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; An, X.; Wu, D.; Xu, J.; Chen, Y.; Li, W.; Cao, Y.; Guo, X.; Lin, X.; Li, C.; et al. Engineering microbial consortia for high-performance cellulosic hydrolyzates-fed microbial fuel cells. Front. Microbiol. 2019, 10, 409. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zaveri, P.; Patel, R.; Shah, M.T.; Munshi, N.S. Optimization of microbial fuel cell process using a novel consortium for aromatic hydrocarbon bioremediation and bioelectricity generation. J. Environ. Manag. 2021, 298, 113546. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the anode of microbial fuel cells: Pure cultures versus mixed communities. Microb. Cell Factories 2019, 18, 39. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; AlMomani, F.; Al-Ghouti, M.; Hassan, M.K. From waste to watts: Updates on key applications of microbial fuel cells in wastewater treatment and energy production. Sustainability 2022, 14, 955. [Google Scholar] [CrossRef]
- Jothinathan, D.; Wilson, R.T. Comparative analysis of power production of pure, coculture, and mixed culture in a microbial fuel cell. Energy Source Part A 2017, 39, 520–527. [Google Scholar] [CrossRef]
- Ren, J.; Li, N.; Du, M.; Zhang, Y.; Hao, C.; Hu, R. Study on the effect of synergy effect between the mixed cultures on the power generation of microbial fuel cells. Bioengineered 2021, 12, 844–854. [Google Scholar] [CrossRef]
- Parihar, P.S.; Keshavkant, S.; Jadhav, S.K. Electrogenic potential of Enterococcus faecalis DWW1 isolated from the anodic biofilm of a dairy wastewater fed dual chambered microbial fuel cell. J. Water Process Eng. 2022, 45, 102503. [Google Scholar] [CrossRef]
- Friman, H.; Schechter, A.; Ioffe, Y.; Nitzan, Y.; Cahan, R. Current production in a microbial fuel cell using a pure culture of Cupriavidus basilensis growing in acetate or phenol as a carbon source. Microb. Biotechnol. 2013, 6, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, K.S. Synergistic effects in a microbial fuel cell between co-cultures and a photosynthetic alga Chlorella vulgaris improve performance. Heliyon 2021, 7, e05935. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, L.; Zularisam, A.W. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 2016, 56, 1322–1336. [Google Scholar] [CrossRef]
- Pankratova, G.; Gorton, L. Electrochemical communication between living cells and conductive surfaces. Curr. Opin. Electrochem. 2017, 5, 193–202. [Google Scholar] [CrossRef]
- Mahmoud, R.H.; Gomaa, O.M.; Hassan, R.Y.A. Bio-electrochemical frameworks governing microbial fuel cell performance: Technical bottlenecks and proposed solutions. RSC Adv. 2022, 12, 5749–5764. [Google Scholar] [CrossRef]
- Bertokova, A.; Bertok, T.; Filip, J.; Tkac, J. Gluconobacter sp. cells for manufacturing of effective electrochemical biosensors and biofuel cells. Chem. Pap. 2015, 69, 27–41. [Google Scholar] [CrossRef]
- Truong, V.K.; Bhadra, C.M.; Christofferson, A.J.; Yarovsky, I.; Al Kobaisi, M.; Garvey, C.J.; Ponamoreva, O.N.; Alferov, S.V.; Alferov, V.A.; Tharushi Perera, P.G.; et al. Three-Dimensional Organization of Self-Encapsulating Gluconobacter oxydans Bacterial Cells. ACS Omega 2017, 2, 8099–8107. [Google Scholar] [CrossRef]
- Plekhanova, Y.; Tarasov, S.; Reshetilov, A. Use of PEDOT:PSS/Graphene/Nafion Composite in Biosensors Based on Acetic Acid Bacteria. Biosensors 2021, 11, 332. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, V.K.; Qazi, G.N.; Kumar, A. Gluconobacter oxydans: Its biotechnological applications. J. Mol. Microbiol. Biotechnol. 2001, 3, 445–456. [Google Scholar]
- Pappenberger, G.; Hohmann, H.-P. Industrial Production of l-Ascorbic Acid (Vitamin C) and d-Isoascorbic Acid. In Biotechnology of Food and Feed Additives, Advances in Biochemical Engineering/Biotechnology; Zorn, H., Czermak, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 143–188. [Google Scholar] [CrossRef]
- Ke, X.; Wang, N.-N.; Yu, P.-H.; Lu, Y.-H.; Hu, Z.-C.; Zheng, Y.-G. Biosynthesis of miglitol intermediate 6-(N-hydroxyethyl)-amino-6-deoxy-α-l-sorbofuranose by an improved d-sorbitol dehydrogenase from Gluconobacter oxydans. 3 Biotech 2018, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.E.; Al-Barha, N.S.; Sharaf, A.-A.M.; Al-Maqtari, Q.A.; Mohedein, A.; Mohammed, H.H.H.; Chen, F. A novel strain of acetic acid bacteria Gluconobacter oxydans FBFS97 involved in riboflavin production. Sci. Rep. 2020, 10, 13527. [Google Scholar] [CrossRef] [PubMed]
- de la Morena, S.; Wojtusik, M.; Santos, V.E.; Garcia-Ochoa, F. Kinetic Modeling of Dihydroxyacetone Production from Glycerol by Gluconobacter oxydans ATCC 621 Resting Cells: Effect of Fluid Dynamics Conditions. Catalysts 2020, 10, 101. [Google Scholar] [CrossRef]
- Reshetilov, A.N.; Plekhanova, Y.V.; Tarasov, S.E.; Arlyapov, V.A.; Kolesov, V.V.; Gutorov, M.A.; Gotovtsev, P.M.; Vasilov, R.G. Effect of some carbon nanomaterials on ethanol oxidation by Gluconobacter oxydans bacterial cells. Appl. Biochem. Microbiol. 2017, 53, 123–129. [Google Scholar] [CrossRef]
- BQ25504 Ultra Low-Power Boost Converter with Battery Management for Energy Harvester Applications. Available online: https://www.ti.com/lit/ds/symlink/bq25504.pdf (accessed on 21 July 2022).
- State Standard GOST 31859-2012; Water. Method for Determination of Chemical Oxygen Demand. Standardinform: Moscow, Russia, 2014.
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Seekaew, Y.; Lokavee, S.; Phokharatkul, D.; Wisitsoraat, A.; Kerdcharoen, T.; Wongchoosuk, C. Low-cost and flexible printed graphene—PEDOT:PSS gas sensor for ammonia detection. Org. Electron. 2014, 15, 2971–2981. [Google Scholar] [CrossRef]
- Rutledge, S.A.; Helmy, A.S. Etch-free patterning of poly(3,4-ethylenedioxythiophene)—poly(styrenesulfonate) for optoelectronics. ACS Appl. Mater. Interfaces 2015, 7, 3940–3948. [Google Scholar] [CrossRef]
- Hossain, J.; Liu, Q.; Miura, T.; Kasahara, K.; Harada, D.; Ishikawa, R.; Ueno, K.; Shirai, H. Nafion-modified PEDOT:PSS as a transparent hole-transporting layer for high-performance crystalline-Si/organic heterojunction solar cells with improved light soaking stability. ACS Appl. Mater. Interfaces 2016, 8, 31926–31934. [Google Scholar] [CrossRef]
- Lee, J.T.; Choi, S.H.; Kim, J.Y. Electrical conductivity of poly(3,4-ethylenedioxythiophene):p-toluene sulfonate films hybridized with reduced graphene oxide. Nanoscale Res. Lett. 2014, 9, 644. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Yang, Y.; Li, S.; Yang, W.; Peng, T.; Mao, X.; Zhao, Y. PEDOT:PSS/graphene/PEDOT ternary film for high performance electrochemical electrode. J. Mater. Sci. Mater. Electron. 2015, 26, 8292–8300. [Google Scholar] [CrossRef]
- Kim, J.C.; Rahman, M.M.; Ju, M.J.; Lee, J.-J. Highly conductive and stable graphene/PEDOT:PSS composite as a metal free cathode for organic dye-sensitized solar cells. RSC Adv. 2018, 8, 19058–19066. [Google Scholar] [CrossRef] [PubMed]
- Badri, M.A.S.; Noor, N.F.M.; Zain, A.R.M.; MatSalleh, M.; Aziz, T.H.T.A. Exfoliated graphene-alkaline lignin-PEDOT: PSS composite as a transparent conductive electrode. Nanomater. Nanotechnol. 2021, 11, 18479804211015010. [Google Scholar] [CrossRef]
- Vacca, A.; Mascia, M.; Rizzardini, S.; Corgiolu, S.; Palmas, S.; Demelas, M.; Bonfiglio, A.; Ricci, P.C. Preparation and characterisation of transparent and flexible PEDOT:PSS/PANI electrodes by ink-jet printing and electropolymerisation. RSC Adv. 2015, 5, 79600–79606. [Google Scholar] [CrossRef]
- Salar-Garcia, M.J.; Montilla, F.; Quijada, C.; Morallon, E.; Ieropoulos, I. Improving the power performance of urine-fed microbial fuel cells using PEDOT-PSS modified anodes. Appl. Energy 2020, 278, 115528. [Google Scholar] [CrossRef]
- Rajendran, J.; Shetty, B.H.; Ganapathy, D.; Murugan, P.; Atchudan, R.; Umapathy, D.; Khosla, A.; Sundramoorthy, A.K. Thermally expanded graphite incorporated with PEDOT:PSS based anode for microbial fuel cells with high bioelectricity production. J. Electrochem. Soc. 2022, 169, 017515. [Google Scholar] [CrossRef]
- De Muynck, C.; Pereira, C.S.S.; Naessens, M.; Parmentier, S.; Soetaert, W.; Vandamme, E.J. The genus Gluconobacter oxydans: Comprehensive overview of biochemistry and biotechnological applications. Crit. Rev. Biotechnol. 2007, 27, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Reshetilov, A.N.; Trotsenko, J.A.; Morozova, N.O.; Iliasov, P.V.; Ashin, V.V. Characteristics of Gluconobacter oxydans B-1280 and Pichia methanolica MN4 cell based biosensors for detection of ethanol. Process Biochem. 2001, 36, 1015–1020. [Google Scholar] [CrossRef]
- Algar, C.K.; Howard, A.; Ward, C.; Wanger, G. Sediment microbial fuel cells as a barrier to sulfide accumulation and their potential for sediment remediation beneath aquaculture pens. Sci. Rep. 2020, 10, 13087. [Google Scholar] [CrossRef]
- Vejarano, F.; Benítez-Campo, N.; Bravo, E.; Loaiza, O.A.; Lizcano-Valbuena, W.H.; Vejarano, F.; Benítez-Campo, N.; Bravo, E.; Loaiza, O.A.; Lizcano-Valbuena, W.H. Electrochemical monitoring and microbial characterization of a domestic wastewater-fed microbial fuel cell inoculated with anaerobic sludge. Rev. Cienc. 2018, 22, 13–32. [Google Scholar] [CrossRef]
- Juang, D.-F. Organic removal efficiencies and power production capabilities of microbial fuel cells with pure cultures and mixed culture. APCBEE Procedia 2012, 1, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Vilas Boas, J.; Oliveira, V.B.; Marcon, L.R.C.; Simões, M.; Pinto, A.M.F.R. Optimization of a single chamber microbial fuel cell using Lactobacillus pentosus: Influence of design and operating parameters. Sci. Total Environ. 2019, 648, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Žalnėravičius, R.; Paškevičius, A.; Samukaitė-Bubnienė, U.; Ramanavičius, S.; Vilkienė, M.; Mockevičienė, I.; Ramanavičius, A. Microbial Fuel Cell Based on Nitrogen-Fixing Rhizobium anhuiense Bacteria. Biosensors 2022, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Mohd Azmi, N.; Mohd Sabri, M.N.I.; Tajarudin, H.A.; Shoparwe, N.F.; Makhtar, M.M.Z.; Shukor, H.; Alam, M.; Siddiqui, M.R.; Rafatullah, M. The Effect of Different Pretreatment of Chicken Manure for Electricity Generation in Membrane-Less Microbial Fuel Cell. Catalysts 2022, 12, 810. [Google Scholar] [CrossRef]
- Choudhury, P.; Bhunia, B.; Mahata, N.; Bandyopadhyay, T.K. Optimization for the improvement of power in equal volume of single chamber microbial fuel cell using dairy wastewater. J. Indian Chem. Soc. 2022, 99, 100489. [Google Scholar] [CrossRef]
- Das, S.; Calay, R.K. Experimental Study of Power Generation and COD Removal Efficiency by Air Cathode Microbial Fuel Cell Using Shewanella baltica 20. Energies 2022, 15, 4152. [Google Scholar] [CrossRef]
- Al-saned, A.J.O.; Kitafa, B.A.; Badday, A.S. Microbial fuel cells (MFC) in the treatment of dairy wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1067, 012073. [Google Scholar] [CrossRef]
- Ojima, Y.; Kawaguchi, T.; Fukui, S.; Kikuchi, R.; Terao, K.; Koma, D.; Ohmoto, T.; Azuma, M. Promoted performance of microbial fuel cells using Escherichia coli cells with multiple-knockout of central metabolism genes. Bioprocess Biosyst. Eng. 2020, 43, 323–332. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayashi, T.; Iwasaki, H.; Awatsu, M.; Yokoyama, H. Ultra-low-power energy harvester for microbial fuel cells and its application to environmental sensing and long-range wireless data transmission. J. Power Sources 2019, 430, 1–11. [Google Scholar] [CrossRef]



| Electrode Modification | Control | PEDOT:PSS | PEDOT:PSS/Graphene | |
|---|---|---|---|---|
| Parameter | ||||
| , µA | 48.8 | 88.6 | 109.9 | |
| Sensitivity, µA/mM | 34.6 | 50.6 | 40.3 | |
| KM | 0.77 | 1.14 | 1.58 | |
| h | 1.18 | 1.01 | 1.16 | |
| Linear detection limit, mM | 0.05–0.50 | 0.05–0.50 | 0.05–1.00 | |
| Minimal detection limit, mM | 0.05 | 0.05 | 0.05 | |
| Detection range, mM | 0.05–10 | 0.05–10 | 0.05–10 | |
| Anode Compartment | Carbon Source | Operation Time, h | Maximum Voltage, mV | Power Density, mW/m2 | Coulomb Efficiency, % | COD Removal Rate, % | Reference |
|---|---|---|---|---|---|---|---|
| Carbon felt/Rhizobium anhuiense | Glucose | 140 | 635 | 2.59 | - | - | [55] |
| Graphite felt/Bacillus subtilis | Chicken manure | 168 | 262 | 207.1 | - | 81 | [56] |
| Carbon cloth/Pseudomonas aeruginosa | Dairy wastewater | 360 | 1025 | 105 | 37.1 | 96 | [57] |
| Carbon felt/Shewanella. Baltica | Artificial wastewater | 1.5 | 190 | 12 | - | 57 | [58] |
| Graphite plate/Saccharomyces cerevisiae | Dairy wastewater | 264 | 850 | - | - | 92 | [59] |
| Carbon fiber/Escherichia coli | Glucose | 18 | - | 0.27 mW/cm3 | 21.3 | - | [60] |
| Graphite rod/Gluconobacter oxydans/PEDOT:PSS/Graphene/Nafion | Glucose (3 mM) | 96 | 550 | 82 | 16.5 | 85 | This work |
| Wastewater (279 mg O2/L) | 96 | 480 | 65 | 39.8 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasov, S.; Plekhanova, Y.; Kashin, V.; Gotovtsev, P.; Signore, M.A.; Francioso, L.; Kolesov, V.; Reshetilov, A. Gluconobacter Oxydans-Based MFC with PEDOT:PSS/Graphene/Nafion Bioanode for Wastewater Treatment. Biosensors 2022, 12, 699. https://doi.org/10.3390/bios12090699
Tarasov S, Plekhanova Y, Kashin V, Gotovtsev P, Signore MA, Francioso L, Kolesov V, Reshetilov A. Gluconobacter Oxydans-Based MFC with PEDOT:PSS/Graphene/Nafion Bioanode for Wastewater Treatment. Biosensors. 2022; 12(9):699. https://doi.org/10.3390/bios12090699
Chicago/Turabian StyleTarasov, Sergei, Yulia Plekhanova, Vadim Kashin, Pavel Gotovtsev, Maria Assunta Signore, Luca Francioso, Vladimir Kolesov, and Anatoly Reshetilov. 2022. "Gluconobacter Oxydans-Based MFC with PEDOT:PSS/Graphene/Nafion Bioanode for Wastewater Treatment" Biosensors 12, no. 9: 699. https://doi.org/10.3390/bios12090699
APA StyleTarasov, S., Plekhanova, Y., Kashin, V., Gotovtsev, P., Signore, M. A., Francioso, L., Kolesov, V., & Reshetilov, A. (2022). Gluconobacter Oxydans-Based MFC with PEDOT:PSS/Graphene/Nafion Bioanode for Wastewater Treatment. Biosensors, 12(9), 699. https://doi.org/10.3390/bios12090699







