Label-Free Microfluidic Impedance Cytometry for Acrosome Integrity Assessment of Boar Spermatozoa
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
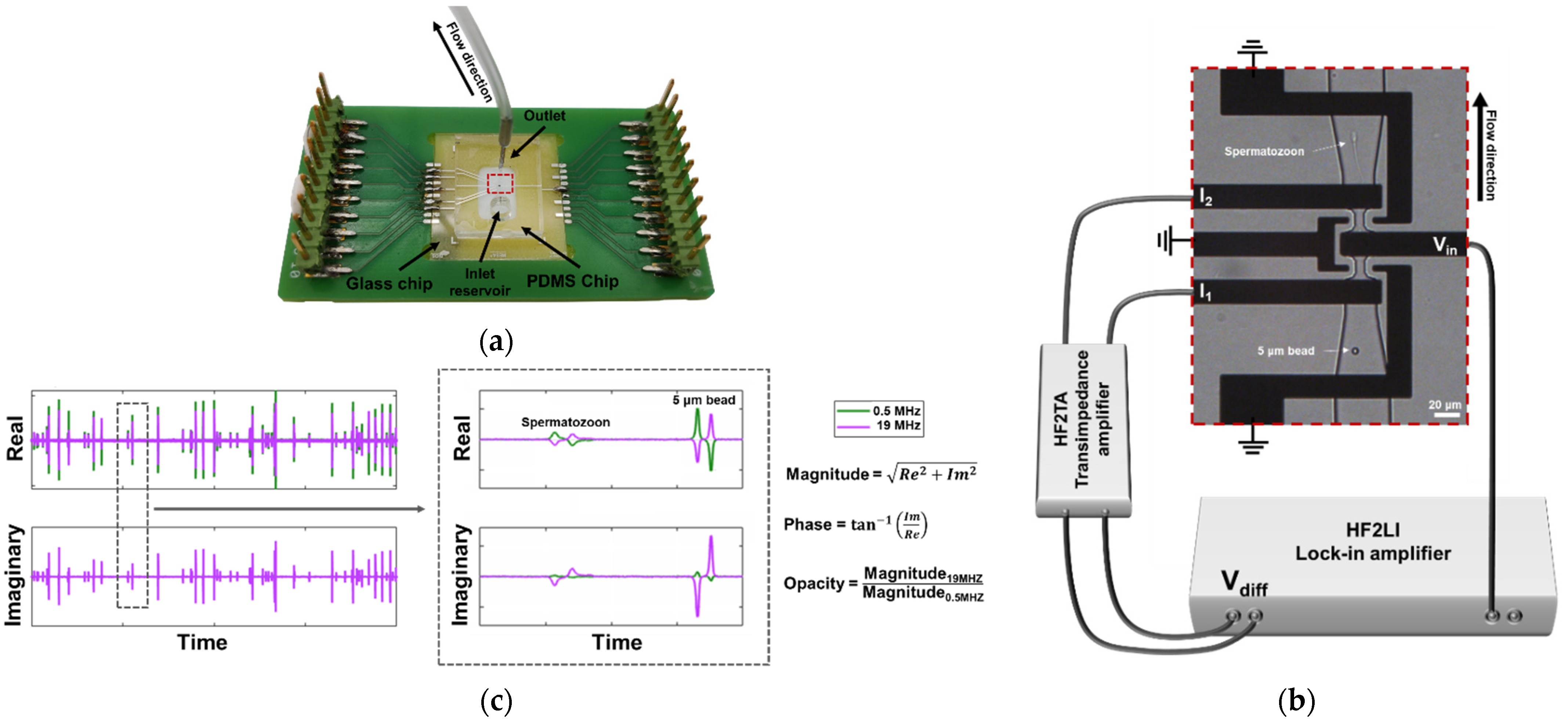
2.2. Chip Design & Fabrication
2.3. Chip Preparation & Operation
2.4. Data Analysis
3. Results & Discussion
3.1. Sample Treatment and Staining Results
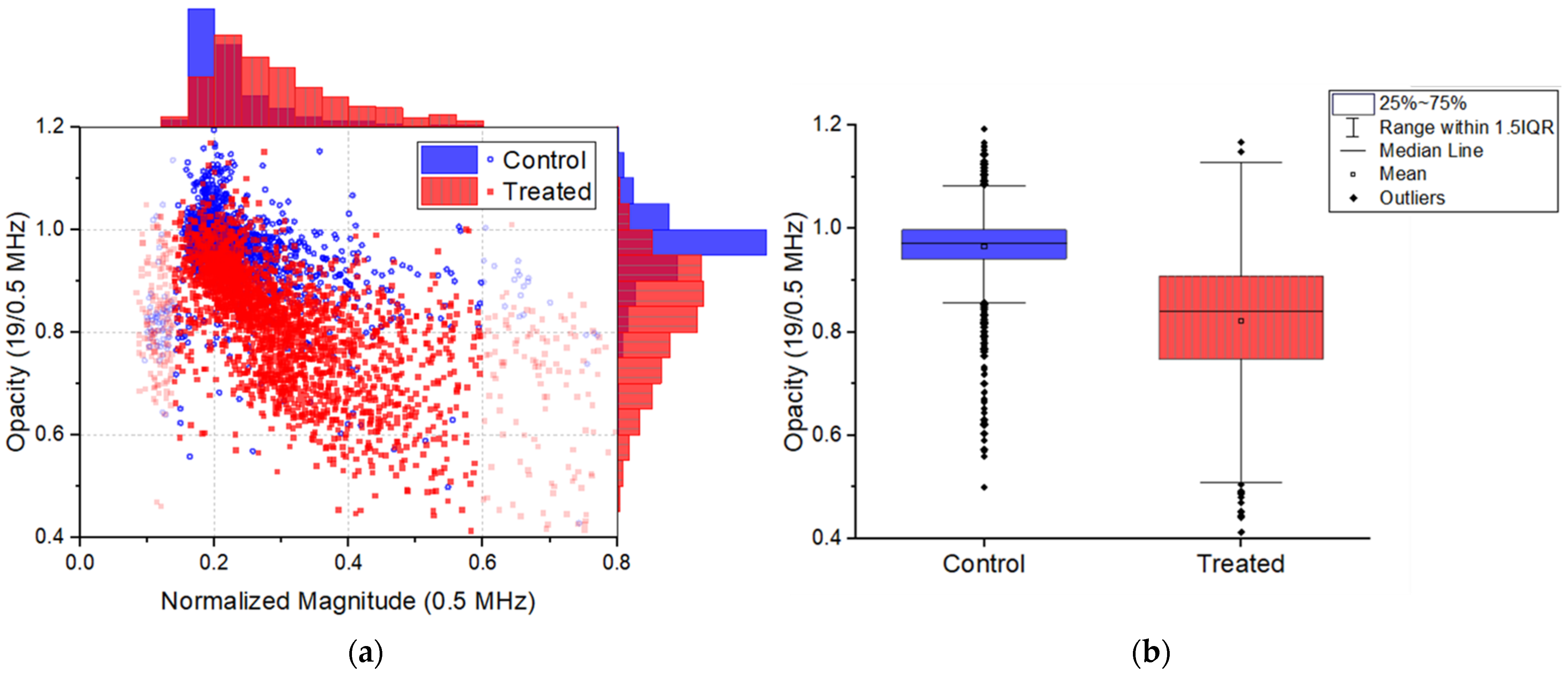
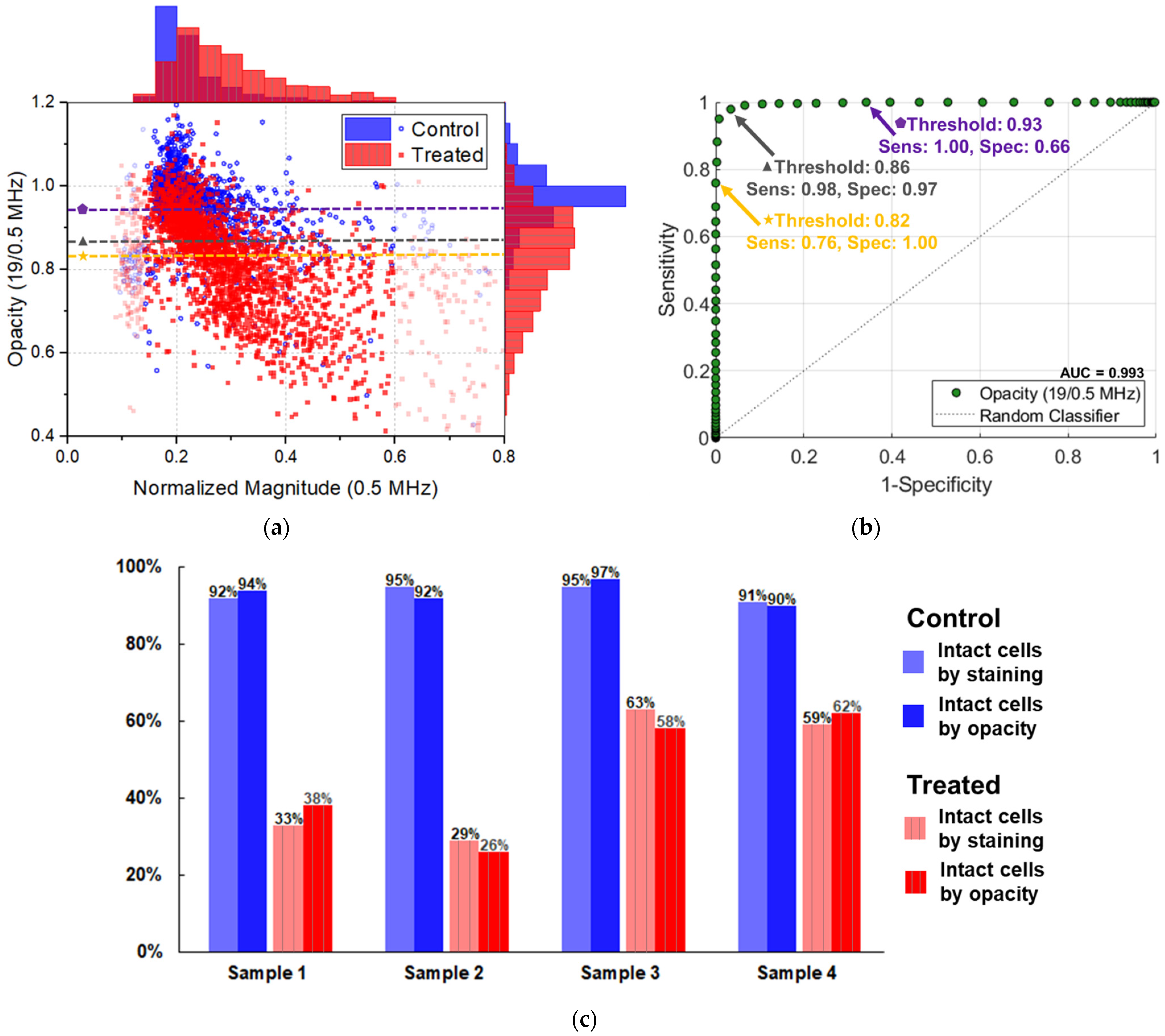
3.2. Impedance Cytometry Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morell, J.M. Artificial Insemination: Current and Future Trenfs. In Artificial Insemination in Farm Animals; Manafi, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 1–14. ISBN 978-953-307-312-5. [Google Scholar]
- Foote, R.H. Fertility estimation: A review of past experience and future prospects. Anim. Reprod. Sci. 2003, 75, 119–139. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; Cooper, T.G., Ed.; WHO Press: Geneva, Switzerland, 2010; ISBN 978-9-24-154778-9. [Google Scholar]
- Hirano, Y.; Shibahara, H.; Obara, H.; Suzuki, T.; Takamizawa, S.; Yamaguchi, C.; Tsunoda, H.; Sato, I. Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J. Assist. Reprod. Genet. 2001, 18, 213–218. [Google Scholar] [CrossRef]
- Amann, R.P.; Waberski, D. Computer-assisted sperm analysis (CASA): Capabilities and potential developments. Theriogenology 2014, 81, 5–17. [Google Scholar] [CrossRef]
- Holman, L. Sperm viability staining in ecology and evolution: Potential pitfalls. Behav. Ecol. Sociobiol. 2009, 63, 1679–1688. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R. Acrosome Reaction Measurement. In Andrological Evaluation of Male Infertility; Springer: Cham, Switzerland, 2016; pp. 143–146. ISBN 978-3-319-26797-5. [Google Scholar]
- Ribas-Maynou, J.; García-Peiró, A.; Fernández-Encinas, A.; Abad, C.; Amengual, M.J.; Prada, E.; Navarro, J.; Benet, J. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assay. Andrology 2013, 1, 715–722. [Google Scholar] [CrossRef]
- Parrish, J.J.; KROGENAES, A.; Susko-Parrish, J. Effect of Bovine sperm separation by either swim-up of percoll method on success of in vitro fertilization and early embryonic development. Theriogenology 1995, 44, 859–869. [Google Scholar] [CrossRef]
- Khatun, A.; Rahman, M.S.; Pang, M.G. Clinical assessment of the male fertility. Obstet. Gynecol. Sci. 2018, 61, 179–191. [Google Scholar] [CrossRef]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef]
- Nosrati, R.; Graham, P.J.; Zhang, B.; Riordon, J.; Lagunov, A.; Hannam, T.G.; Escobedo, C.; Jarvi, K.; Sinton, D. Microfluidics for sperm analysis and selection. Nat. Rev. Urol. 2017, 14, 707–730. [Google Scholar] [CrossRef]
- Cho, B.S.; Schuster, T.G.; Zhu, X.; Chang, D.; Smith, G.D.; Takayama, S. Passively driven integrated microfluidic system for separation of motile sperm. Anal. Chem. 2003, 75, 1671–1675. [Google Scholar] [CrossRef]
- Tasoglu, S.; Safaee, H.; Zhang, X.; Kingsley, J.L.; Catalano, P.N.; Gurkan, U.A.; Nureddin, A.; Kayaalp, E.; Anchan, R.M.; Maas, R.L.; et al. Exhaustion of racing sperm in nature-mimicking microfluidic channels during sorting. Small 2013, 9, 3374–3384. [Google Scholar] [CrossRef]
- Lopez-Garcia, M.D.C.; Monson, R.L.; Haubert, K.; Wheeler, M.B.; Beebe, D.J. Sperm motion in a microfluidic fertilization device. Biomed. Microdevices 2008, 10, 709–718. [Google Scholar] [CrossRef]
- Kantsler, V.; Dunkel, J.; Blayney, M.; Goldstein, R.E. Rheotaxis facilitates upstream navigation of mammalian sperm cells. Elife 2014, 3, e02403. [Google Scholar] [CrossRef]
- Xie, L.; Ma, R.; Han, C.; Su, K.; Zhang, Q.; Qiu, T.; Wang, L.; Huang, G.; Qiao, J.; Wang, J.; et al. Integration of sperm motility and chemotaxis screening with a microchannel-based device. Clin. Chem. 2010, 56, 1270–1278. [Google Scholar] [CrossRef]
- Koyama, S.; Amarie, D.; Soini, H.A.; Novotny, M.V.; Jacobson, S.C. Chemotaxis assays of mouse sperm on microfluidic devices. Anal. Chem. 2006, 78, 3354–3359. [Google Scholar] [CrossRef]
- Berendsen, J.T.W.; Kruit, S.A.; Atak, N.; Willink, E.; Segerink, L.I. Flow-Free Microfluidic Device for Quantifying Chemotaxis in Spermatozoa. Anal. Chem. 2020, 92, 3302–3306. [Google Scholar] [CrossRef]
- Segerink, L.I.; Sprenkels, A.J.; Ter Braak, P.M.; Vermes, I.; Van Den Berg, A. On-chip determination of spermatozoa concentration using electrical impedance measurements. Lab Chip 2010, 10, 1018–1024. [Google Scholar] [CrossRef]
- Kanakasabapathy, M.K.; Sadasivam, M.; Singh, A.; Preston, C.; Thirumlaraju, P.; Venkataraman, M.; Bormann, C.L.; Draz, M.S.; Petrozza, J.C.; Shafiee, H. An automated smartphone-based diagnostic assay for point-of-care semen analysis. Sci. Transl. Med. 2017, 9, eaai7863. [Google Scholar] [CrossRef]
- De Wagenaar, B.; Dekker, S.; De Boer, H.L.; Bomer, J.G.; Olthuis, W.; Van Den Berg, A.; Segerink, L.I. Towards microfluidic sperm refinement: Impedance-based analysis and sorting of sperm cells. Lab Chip 2016, 16, 1514–1522. [Google Scholar] [CrossRef]
- De Wagenaar, B.; Berendsen, J.T.W.; Bomer, J.G.; Olthuis, W.; Van Den Berg, A.; Segerink, L.I. Microfluidic single sperm entrapment and analysis. Lab Chip 2015, 15, 1294–1301. [Google Scholar] [CrossRef]
- Wongtawan, T.; Dararatana, N.; Thongkittidilok, C.; Kornmatitsuk, S.; Oonkhanond, B. Enrichment of bovine X-sperm using microfluidic dielectrophoretic chip: A proof-of- concept study. Heliyon 2020, 6, e05483. [Google Scholar] [CrossRef]
- Phiphatanaphiphop, C.; Leksakul, K.; Phatthanakun, R.; Busayaporn, W. Multiwalled Carbon Nanotubes in Microfluidic Chip for the Separation of X- and Y-Sperm Based on a Photolithography Technique. J. Microelectromech. Syst. 2020, 29, 1264–1277. [Google Scholar] [CrossRef]
- Johnson, L.A.; Clarke, R.N. Flow sorting of X and Y chromosome-bearing mammalian sperm: Activation and pronuclear development of sorted bull, boar, and ram sperm microinjected into hamster oocytes. Gamete Res. 1988, 21, 335–343. [Google Scholar] [CrossRef]
- Engelmann, U.; Krassnigg, F.; Schatz, H.; Schill, W. Separation of Human X and Y Spermatozoa by Free-Flow Electrophoresis. Gamete Res. 1988, 159, 151–159. [Google Scholar] [CrossRef]
- Spencer, D.; Morgan, H. High-Speed Single-Cell Dielectric Spectroscopy. ACS Sens. 2020, 5, 423–430. [Google Scholar] [CrossRef]
- Honrado, C.; Ciuffreda, L.; Spencer, D.; Ranford-Cartwright, L.; Morgan, H. Dielectric characterization of Plasmodium falciparum-infected red blood cells using microfluidic impedance cytometry. J. R. Soc. Interface 2018, 15, 20180416. [Google Scholar] [CrossRef]
- Spencer, D.; Hollis, V.; Morgan, H. Microfluidic impedance cytometry of tumour cells in blood. Biomicrofluidics 2014, 8, 064124. [Google Scholar] [CrossRef]
- McGrath, J.S.; Honrado, C.; Moore, J.H.; Adair, S.J.; Varhue, W.B.; Salahi, A.; Farmehini, V.; Goudreau, B.J.; Nagdas, S.; Blais, E.M.; et al. Electrophysiology-based stratification of pancreatic tumorigenicity by label-free single-cell impedance cytometry. Anal. Chim. Acta 2020, 1101, 90–98. [Google Scholar] [CrossRef]
- Shaker, M.; Colella, L.; Caselli, F.; Bisegna, P.; Renaud, P. An impedance-based flow microcytometer for single cell morphology discrimination. Lab Chip 2014, 14, 2548–2555. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Z.; Ge, X.; Zhao, X.; Hao, L.; Cheng, Z.; Zhou, W.; Du, Y.; Wang, L.; Tian, F.; et al. Particle Self-Aligning, Focusing, and Electric Impedance Microcytometer Device for Label-Free Single Cell Morphology Discrimination and Yeast Budding Analysis. Anal. Chem. 2019, 91, 13398–13406. [Google Scholar] [CrossRef]
- Honrado, C.; Bisegna, P.; Swami, N.S.; Caselli, F. Single-cell microfluidic impedance cytometry: From raw signals to cell phenotypes using data analytics. Lab Chip 2021, 21, 22–54. [Google Scholar] [CrossRef]
- Sun, T.; Morgan, H. Single-cell microfluidic impedance cytometry: A review. Microfluid. Nanofluidics 2010, 8, 423–443. [Google Scholar] [CrossRef]
- Ostermann, M.; Sauter, A.; Xue, Y.; Birkeland, E.; Schoelermann, J.; Holst, B.; Cimpan, M.R. Label-free impedance flow cytometry for nanotoxicity screening. Sci. Rep. 2020, 10, 142. [Google Scholar] [CrossRef]
- Crocetti, S.; Beyer, C.; Schade, G.; Egli, M.; Fröhlich, J.; Franco-Obregón, A. Low intensity and frequency pulsed electromagnetic fields selectively impair breast cancer cell viability. PLoS ONE 2013, 8, e72944. [Google Scholar] [CrossRef]
- De Ninno, A.; Reale, R.; Giovinazzo, A.; Bertani, F.R.; Businaro, L.; Bisegna, P.; Matteucci, C.; Caselli, F. High-throughput label-free characterization of viable, necrotic and apoptotic human lymphoma cells in a coplanar-electrode microfluidic impedance chip. Biosens. Bioelectron. 2020, 150, 111887. [Google Scholar] [CrossRef]
- Opitz, C.; Schade, G.; Kaufmann, S.; Di Berardino, M.; Ottiger, M.; Grzesiek, S. Rapid determination of general cell status, cell viability, and optimal harvest time in eukaryotic cell cultures by impedance flow cytometry. Appl. Microbiol. Biotechnol. 2019, 103, 8619–8629. [Google Scholar] [CrossRef]
- Pierzchalski, A.; Hebeisen, M.; Mittag, A.; Bocsi, J.; Di Berardino, M.; Tarnok, A. Label-free hybridoma cell culture quality control by a chip-based impedance flow cytometer. Lab Chip 2012, 12, 4533–4543. [Google Scholar] [CrossRef]
- Tang, T.; Liu, X.; Kiya, R.; Shen, Y.; Yuan, Y.; Zhang, T.; Suzuki, K.; Tanaka, Y.; Li, M.; Hosokawa, Y.; et al. Microscopic impedance cytometry for quantifying single cell shape. Biosens. Bioelectron. 2021, 193, 113521. [Google Scholar] [CrossRef]
- Tang, T.; Liu, X.; Yuan, Y.; Kiya, R.; Shen, Y.; Zhang, T.; Suzuki, K.; Tanaka, Y.; Li, M.; Hosokawa, Y.; et al. Dual-frequency impedance assays for intracellular components in microalgal cells. Lab Chip 2022, 22, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Cafe, S.L.; Bromfield, E.G.; De Iuliis, G.N.; Dun, M.D. Capacitation and Acrosome Reaction: Histochemical Techniques to Determine Acrosome Reaction. In Manual of Sperm Function Testing in Human Assisted Reproduction; Agarwal, A., Henkel, R., Majzoub, A., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 81–92. [Google Scholar]
- Glazar, A.I. Assessment of Sperm Acrosomal Status: FITC PNA. In Equine Reproductive Procedures; Dascanio, J., McCue, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 483–485. [Google Scholar]
- Hoffman, R.A.; Johnson, T.S.; Britt, W.B. Flow cytometric electronic direct current volume and radiofrequency impedance measurements of single cells and particles. Cytometry 1981, 1, 377–384. [Google Scholar] [CrossRef]
- El-Ghobashy, A.A.; West, C.R. The human sperm head: A key for successful fertilization. J. Androl. 2003, 24, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Daguerre, H.; Solsona, M.; Cottet, J.; Gauthier, M.; Renaud, P.; Bolopion, A. Positional dependence of particles and cells in microfluidic electrical impedance flow cytometry: Origin, challenges and opportunities. Lab Chip 2020, 20, 3665–3689. [Google Scholar] [CrossRef]
- Sun, T.; Green, N.G.; Gawad, S.; Morgan, H. Analytical electric field and sensitivity analysis for two microfluidic impedance cytometer designs. IET Nanobiotechnol. 2007, 1, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Cottet, J.; Kehren, A.; van Lintel, H.; Buret, F.; Frénéa-Robin, M.; Renaud, P. How to improve the sensitivity of coplanar electrodes and micro channel design in electrical impedance flow cytometry: A study. Microfluid. Nanofluidics 2019, 23, 11. [Google Scholar] [CrossRef]
- Clausen, C.H.; Skands, G.E.; Bertelsen, C.V.; Svendsen, W.E. Coplanar electrode layout optimized for increased sensitivity for electrical impedance spectroscopy. Micromachines 2015, 6, 110–120. [Google Scholar] [CrossRef]
- De Bruijn, D.S.; Braak, P.M.; Van De Waal, D.B.; Bomer, J.G.; Berg, A. Van Den Calcification State of Algae studied with Impedance Flow Cytometry. In Proceedings of the 25th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2021, Palm Springs, CA, USA, 10–14 October 2021; pp. 559–560. [Google Scholar]
- González-Murillo, J.J.; Monge-Azemar, M.; Bartoli, J.; Flores, A.; Moreno, M.; García-Celma, M.; Romano-Rodríguez, A.; Svendsen, W.; Samitier, J.; Rodríguez-Trujillo, R. Electrical Impedance Spectroscopy Microflow Cytometer for Cell Viability Tests. In Proceedings of the 2018 Spanish Conference on Electron Devices (CDE), Salamanca, Spain, 14–16 November 2018; pp. 1–4. [Google Scholar] [CrossRef]
- de Wagenaar, B.; Geijs, D.J.; de Boer, H.; Bomer, J.G.; Olthuis, W.; van den Berg, A.; Segerink, L.I. Spermometer: Electrical characterization of single boar sperm motility. Fertil. Steril. 2016, 106, 773–780.e6. [Google Scholar] [CrossRef]
- Dolors Briz, M.; Fàbrega, A. The Boar Spermatozoon. In Boar Reproduction: Fundamentals and New Biotechnological Trends; Bonet, S., Casas, I., Holt, W.V., Yeste, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–47. ISBN 978-3-642-35048-1. [Google Scholar]
- Cummins, J.M.; Pember, S.M.; Jequier, A.M.; Yovich, J.L.; Hartmann, P.E. A test of the human sperm acrosome reaction following ionophore challenge: Relationship to fertility and other seminal parameters. J. Androl. 1991, 12, 98–103. [Google Scholar]
- Samplaski, M.K.; Dimitromanolakis, A.; Lo, K.C.; Grober, E.D.; Mullen, B.; Garbens, A.; Jarvi, K.A. The relationship between sperm viability and DNA fragmentation rates. Reprod. Biol. Endocrinol. 2015, 13, 4–9. [Google Scholar] [CrossRef]
- Makkar, G.; Ng, E.H.Y.; Yeung, W.S.B.; Ho, P.C. The significance of the ionophore-challenged acrosome reaction in the prediction of successful outcome of controlled ovarian stimulation and intrauterine insemination. Hum. Reprod. 2003, 18, 534–539. [Google Scholar] [CrossRef][Green Version]
- Sigman, M.; Zini, A. Semen analysis and sperm function assays: What do they mean? Semin. Reprod. Med. 2009, 27, 115–123. [Google Scholar] [CrossRef]

| Control | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Live intact % | 76.0 | 65.7 | 52.1 | 52.6 |
| Live reacted % | 0.8 | 0.4 | 0.7 | 0.0 |
| Dead intact % | 16.0 | 29.4 | 42.7 | 38.3 |
| Dead reacted % | 7.2 | 4.5 | 5.5 | 9.1 |
| Treated | ||||
| 1 | 2 | 3 | 4 | |
| Live intact % | 1.2 | 7.8 | 7.0 | 1.9 |
| Live reacted % | 1.6 | 0.4 | 0.0 | 1.2 |
| Dead intact % | 32.1 | 21.2 | 55.6 | 57.0 |
| Dead reacted % | 65.0 | 70.6 | 37.4 | 39.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruit, S.A.; de Bruijn, D.S.; Broekhuijse, M.L.W.J.; Olthuis, W.; Segerink, L.I. Label-Free Microfluidic Impedance Cytometry for Acrosome Integrity Assessment of Boar Spermatozoa. Biosensors 2022, 12, 679. https://doi.org/10.3390/bios12090679
Kruit SA, de Bruijn DS, Broekhuijse MLWJ, Olthuis W, Segerink LI. Label-Free Microfluidic Impedance Cytometry for Acrosome Integrity Assessment of Boar Spermatozoa. Biosensors. 2022; 12(9):679. https://doi.org/10.3390/bios12090679
Chicago/Turabian StyleKruit, Stella A., Douwe S. de Bruijn, Marleen L. W. J. Broekhuijse, Wouter Olthuis, and Loes I. Segerink. 2022. "Label-Free Microfluidic Impedance Cytometry for Acrosome Integrity Assessment of Boar Spermatozoa" Biosensors 12, no. 9: 679. https://doi.org/10.3390/bios12090679
APA StyleKruit, S. A., de Bruijn, D. S., Broekhuijse, M. L. W. J., Olthuis, W., & Segerink, L. I. (2022). Label-Free Microfluidic Impedance Cytometry for Acrosome Integrity Assessment of Boar Spermatozoa. Biosensors, 12(9), 679. https://doi.org/10.3390/bios12090679





