A Flexible and Attachable Colorimetric Film Sensor for the Detection of Gaseous Ammonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
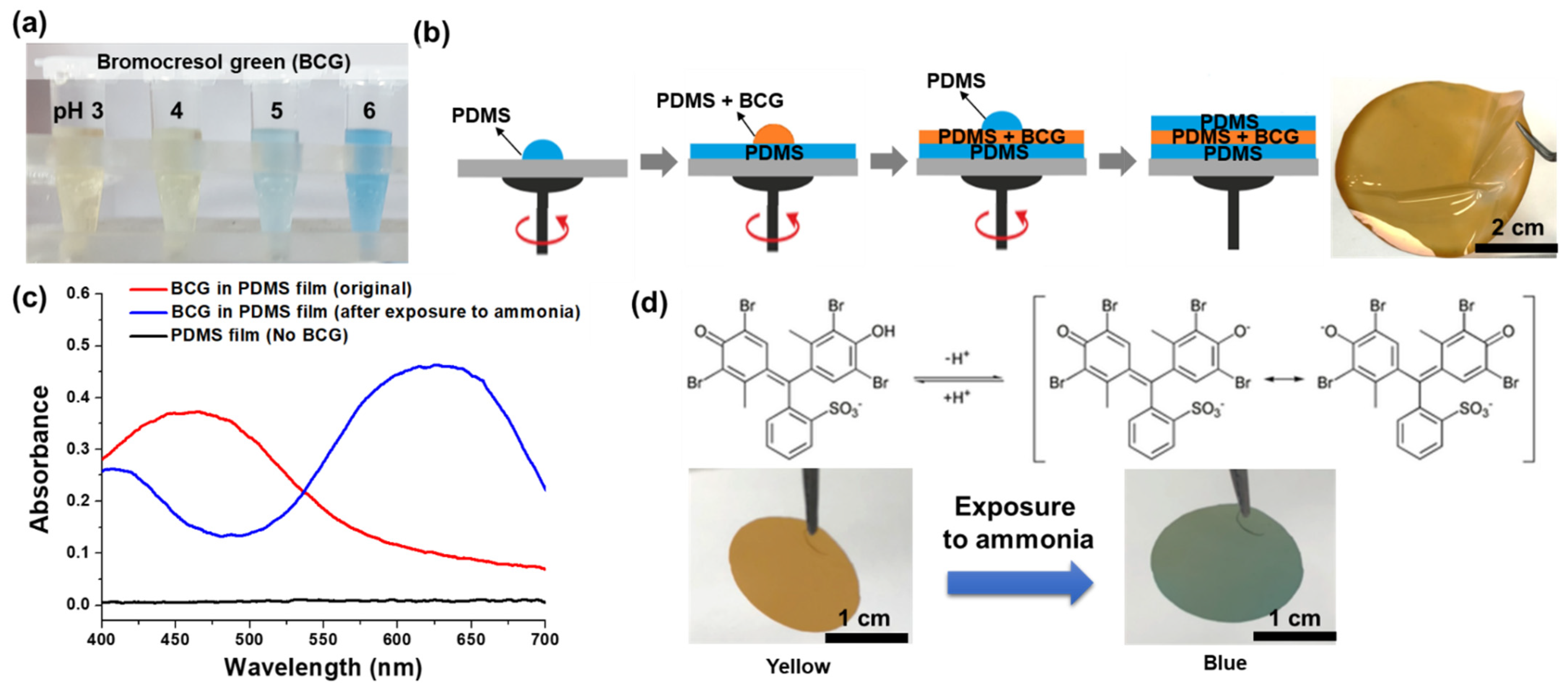
2.2. Fabrication of the PDMS-Based Colorimetric Film Sensor for Gaseous Ammonia Detection
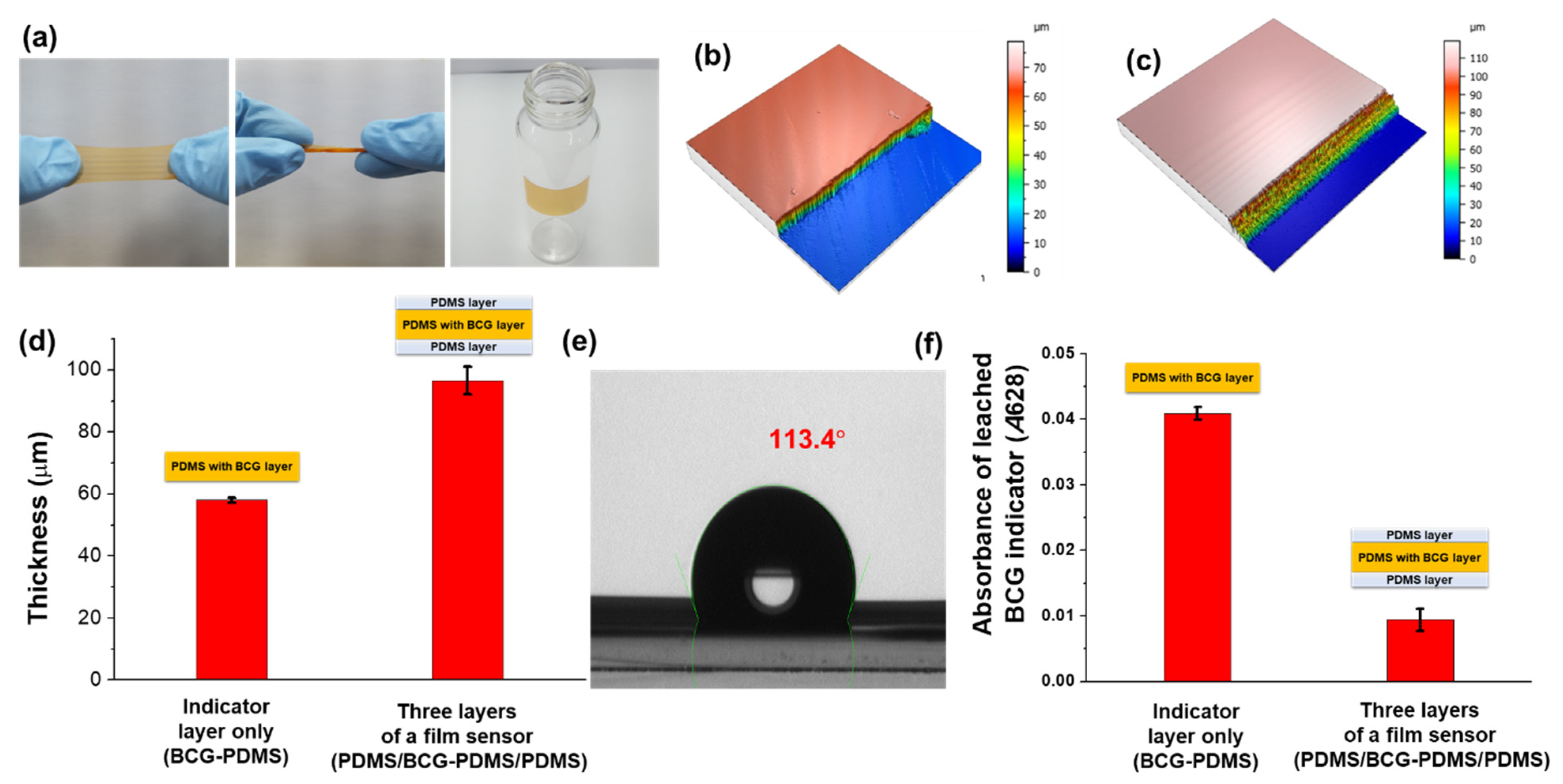
2.3. Sensor Thickness Measurements
2.4. Sensor Contact Angle Measurements
2.5. Leaching of Bromocresol Green (BCG) Indicator from the Sensor
2.6. Optimization of Bromocresol Green (BCG) Concentration in the Sensor
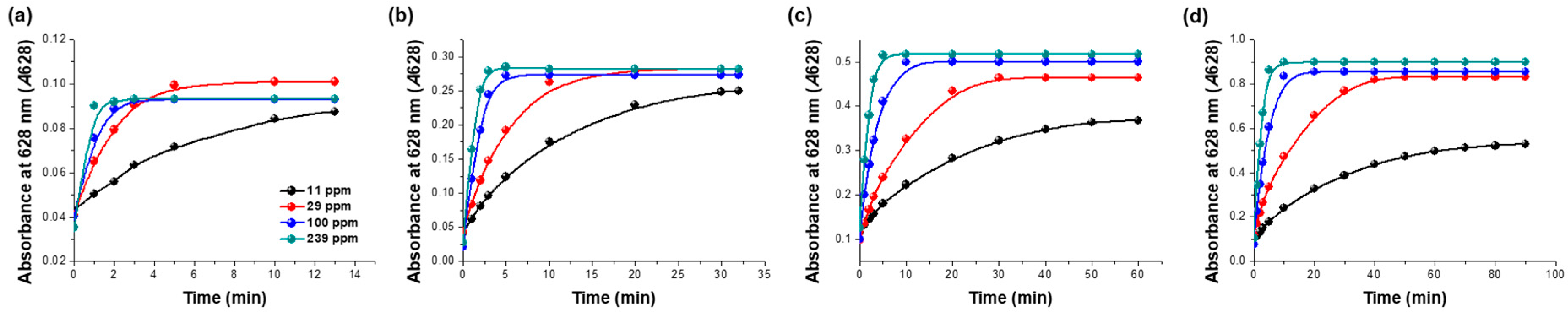
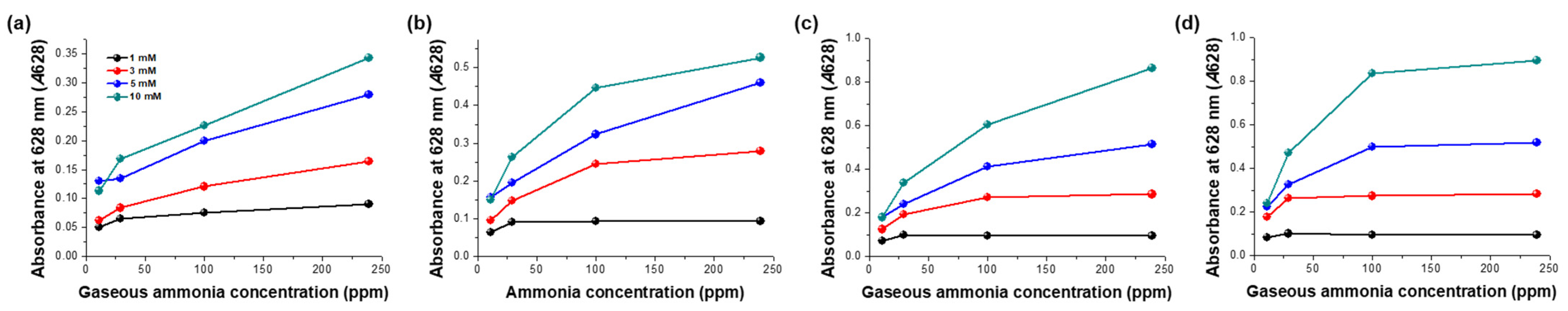
2.7. Sensitivity of the Sensor for the Detection of Gaseous Ammonia
2.8. Selectivity of the Sensor
2.9. Application of the Colorimetric Film Sensor to the Detection of Released Ammonia Gas
3. Results and Discussion
3.1. Properties of the Colorimetric Film Sensor
3.2. Optimization of the Concentration of Bromocresol Green (BCG) Indicator in the Sensor
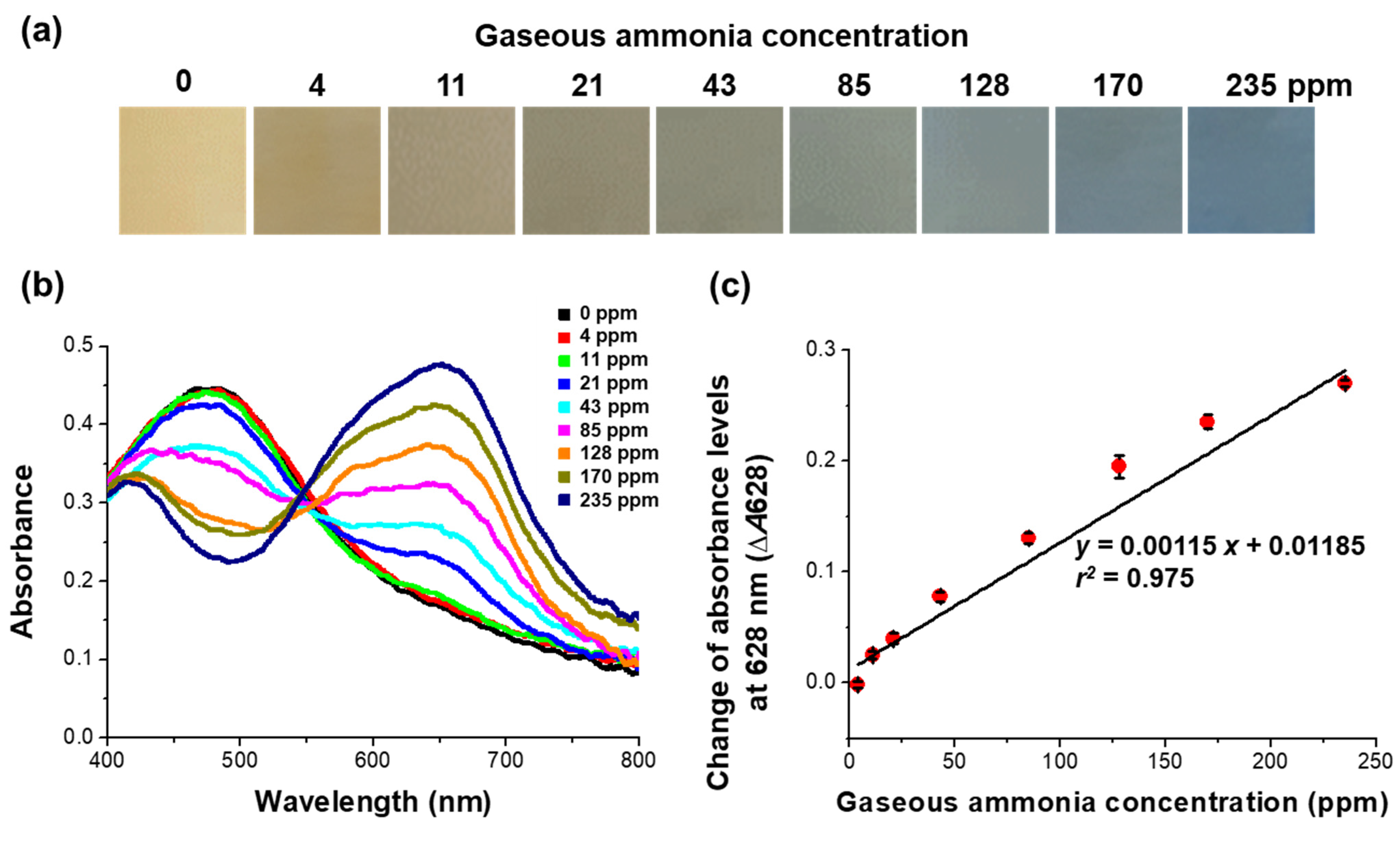
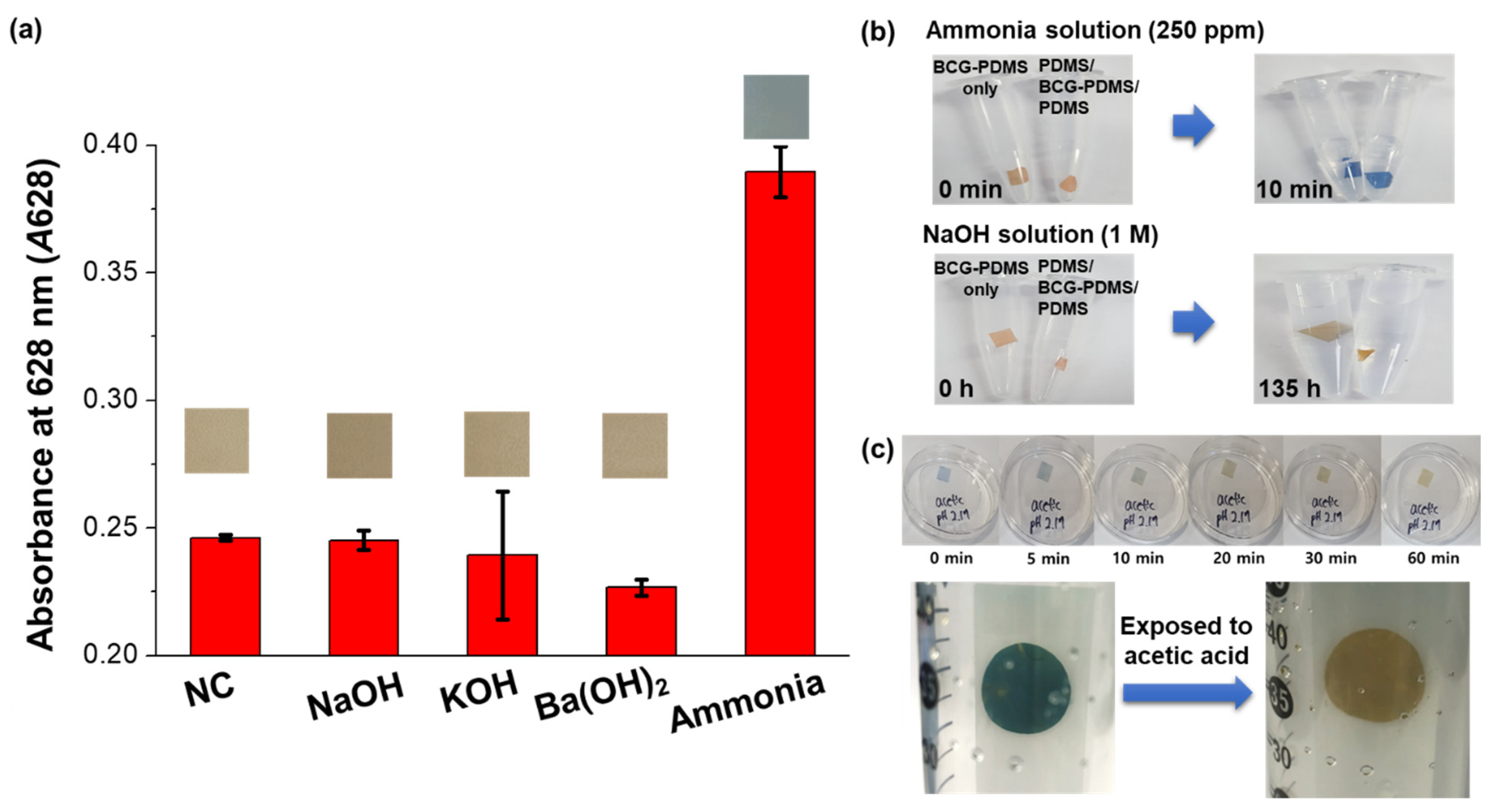
3.3. Validation of the Sensitivity and Selectivity of the Sensor Specific for Gaseous Ammonia
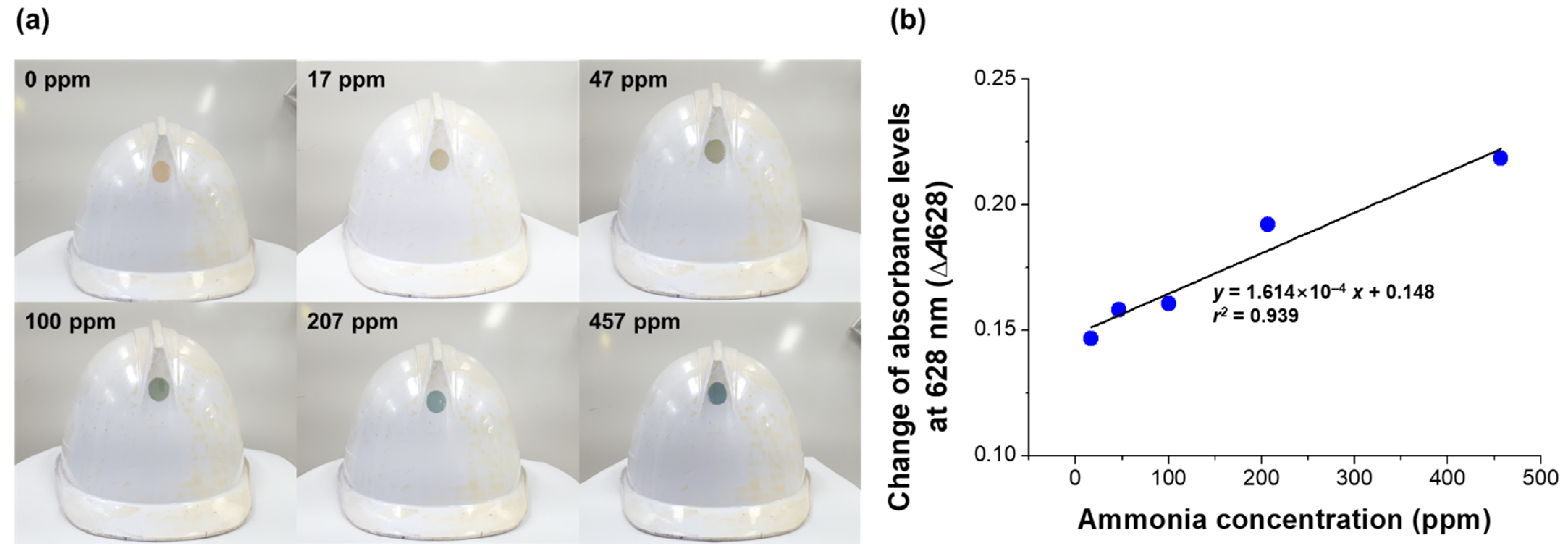
3.4. Application of the Sensor for the Detection of Released Ammonia Gas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ward, B.K.; Dufault, R.J.; Hassell, R.; Cutulle, M.A. Affinity of hyperammonia-producing bacteria to produce bioammonium/ammonia utilizing five organic nitrogen substrates for potential use as an organic liquid fertilizer. ACS Omega 2018, 3, 11817–11822. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L. Global production capacity of ammonia 2018–2030. Statista 2022. Available online: https://www.statista.com/statistics/1065865/ammonia-production-capacity-globally/ (accessed on 20 July 2022).
- Giddey, S.; Badwal, S.P.S.; Munnings, C.; Dolan, M. Ammonia as a renewable energy transportation media. ACS Sustain. Chem. Eng. 2017, 5, 10231–10239. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A roadmap to the ammonia economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Rafiqul, I.; Weber, C.; Lehmann, B.; Voss, A. Energy efficiency improvements in ammonia production—Perspectives and uncertainties. Energy 2005, 30, 2487–2504. [Google Scholar] [CrossRef]
- Joshi, A.; Gangal, S.A.; Gupta, S.K. Ammonia sensing properties of polypyrrole thin films at room temperature. Sens. Actuators B Chem. 2011, 156, 938–942. [Google Scholar] [CrossRef]
- Van Damme, M.; Clarisse, L.; Whitburn, S.; Hadji-Lazaro, J.; Hurtmans, D.; Clerbaux, C.; Coheur, P.F. Industrial and agricultural ammonia point sources exposed. Nature 2018, 564, 99–103. [Google Scholar] [CrossRef]
- Sutton, M.A.; Reis, S.; Riddick, S.N.; Dragosits, U.; Nemitz, E.; Theobald, M.R.; Tang, Y.S.; Braban, C.F.; Vieno, M.; Dore, A.J.; et al. Towards a climate-dependent paradigm of ammonia emission and deposition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20130166. [Google Scholar] [CrossRef]
- BQ Prime Powered by Bloomberg. Ammonia Gas Leak at IFFCO Fertiliser Plant in Uttar Pradesh Leaves 2 Dead, 16 Injured. 2020. Available online: https://www.bqprime.com/business/two-iffco-officials-dead-16-employees-injured-in-ammonia-gas-leak-at-fertiliser-plant-in-up (accessed on 20 July 2021).
- National Research Council; Committee on Acute Exposure Guideline Levels. Ammonia Acute Exposure Guideline Levels; National Academies Press (US): Washington, DC, USA, 2008. [Google Scholar]
- The National Institute for Occupational Safety and Health (NIOSH). Ammonia. Available online: https://www.cdc.gov/niosh/topics/ammonia/ (accessed on 20 July 2021).
- Belson, M. Ammonia and nitrogen oxides. In Haddad and Winchester’s Clinical Management of Poisoning and Drug Overdose, 4th ed.; Shannon, M.W., Borron, S.W., Burns, M.J., Eds.; W. B. Saunders: Philadelphia, PA, USA, 2007; Chapter 97; pp. 1399–1406. [Google Scholar]
- Devos, M.P.F.; Rouault, J.; Laffort, P.; Van Gemert, L.J. Standardized Human Olfactory Thresholds; Oxford University Press: New York, NY, USA, 1990; Volume 1. [Google Scholar]
- Timmer, B.; Olthuis, W.; Berg, A.v.d. Ammonia sensors and their applications—a review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Mackin, C.; Schroeder, V.; Zurutuza, A.; Su, C.; Kong, J.; Swager, T.M.; Palacios, T. Chemiresistive graphene sensors for ammonia detection. ACS Appl. Mater. Interfaces 2018, 10, 16169–16176. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Nanda, K.K. Au nanocomposite based chemiresistive ammonia sensor for health monitoring. ACS Sens. 2015, 1, 55–62. [Google Scholar] [CrossRef]
- Veeralingam, S.; Sahatiya, P.; Badhulika, S. Low cost, flexible and disposable SnSe2 based photoresponsive ammonia sensor for detection of ammonia in urine samples. Sens. Actuators B Chem. 2019, 297, 126725. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.; Duan, Y. Development of a polyaniline-based optical ammonia sensor. Sens. Actuators B Chem. 2001, 72, 75–79. [Google Scholar]
- Dong, J.X.; Gao, Z.F.; Zhang, Y.; Li, B.L.; Li, N.B.; Luo, H.Q. A selective and sensitive optical sensor for dissolved ammonia detection via agglomeration of fluorescent Ag nanoclusters and temperature gradient headspace single drop microextraction. Biosens. Bioelectron. 2017, 91, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yang, X.; Shu, Y.; Chen, X.; Wang, J. Ionic liquid-based slab optical waveguide sensor for the detection of ammonia in human breath. J. Colloid. Interface Sci. 2018, 512, 819–825. [Google Scholar] [CrossRef]
- Ly, T.N.; Park, S. Highly sensitive ammonia sensor for diagnostic purpose using reduced graphene oxide and conductive polymer. Sci. Rep. 2018, 8, 18030. [Google Scholar] [CrossRef]
- Zhang, T.; Nix, M.B.; Yoo, B.-Y.; Deshusses, M.A.; Myung, N.V. Electrochemically functionalized single-walled carbon nanotube gas sensor. Electroanalysis 2006, 18, 1153–1158. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Firoozbakht, F. Determination of the acidity constants of neutral red and bromocresol green by solution scanometric method and comparison with spectrophotometric results. Beni-Suef. Univ. J. Basic Appl. Sci. 2016, 5, 13–20. [Google Scholar] [CrossRef][Green Version]
- Jo, B.; Lerberghe, L.M.V.; Motsegood, K.M.; Beebe, D.J. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J. Microelectromech. Syst. 2000, 9, 76–81. [Google Scholar] [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations-a mini review. Anal. Methods 2016, 8, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef]
- Cho, Y.B.; Jeong, S.H.; Chun, H.; Kim, Y.S. Selective colorimetric detection of dissolved ammonia in water via modified Berthelot’s reaction on porous paper. Sens. Actuators B Chem. 2018, 256, 167–175. [Google Scholar] [CrossRef]
- Khachornsakkul, K.; Hung, K.H.; Chang, J.J.; Dungchai, W.; Chen, C.H. A rapid and highly sensitive paper-based colorimetric device for the on-site screening of ammonia gas. Analyst 2021, 146, 2919–2927. [Google Scholar] [CrossRef]
- Chang, Y.C.; Bai, H.; Li, S.N.; Kuo, C.N. Bromocresol green/mesoporous silica adsorbent for ammonia gas sensing via an optical sensing instrument. Sensors 2011, 11, 4060–4072. [Google Scholar] [CrossRef]
- Markovics, Á.; Nagy, G.; Kovács, B. Reflection-based sensor for gaseous ammonia. Sens. Actuators B Chem. 2009, 139, 252–257. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Kysar, J.S. An electrochemical ammonia sensor on paper substrate. J. Electrochem. Soc. 2017, 164, B113–B117. [Google Scholar] [CrossRef]
- Seekaew, Y.; Pon-On, W.; Wongchoosuk, C. Ultrahigh selective room-temperature ammonia gas sensor based on tin-titanium dioxide/reduced graphene/carbon nanotube nanocomposites by the solvothermal method. ACS Omega 2019, 4, 16916–16924. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, E.-H.; Lee, S.-W. A Flexible and Attachable Colorimetric Film Sensor for the Detection of Gaseous Ammonia. Biosensors 2022, 12, 664. https://doi.org/10.3390/bios12080664
Lee S, Lee E-H, Lee S-W. A Flexible and Attachable Colorimetric Film Sensor for the Detection of Gaseous Ammonia. Biosensors. 2022; 12(8):664. https://doi.org/10.3390/bios12080664
Chicago/Turabian StyleLee, Sangwon, Eun-Hee Lee, and Seung-Woo Lee. 2022. "A Flexible and Attachable Colorimetric Film Sensor for the Detection of Gaseous Ammonia" Biosensors 12, no. 8: 664. https://doi.org/10.3390/bios12080664
APA StyleLee, S., Lee, E.-H., & Lee, S.-W. (2022). A Flexible and Attachable Colorimetric Film Sensor for the Detection of Gaseous Ammonia. Biosensors, 12(8), 664. https://doi.org/10.3390/bios12080664




