Application of Heat-Enhancement for Improving the Sensitivity of Quartz Crystal Microbalance
Abstract
:1. Introduction
2. Methods
2.1. Atomic Force Microscopy (AFM)
2.2. The Determination of Ion Concentration of Sodium Chloride and Phosphate-Buffered Saline (PBS)
2.3. The Monitoring of Self-Assembly of 11-Mercaptoundecanoic Acid (MUA)
2.4. Single Nucleotide Polymorphism (SNP) Detection
3. Results and Discussion
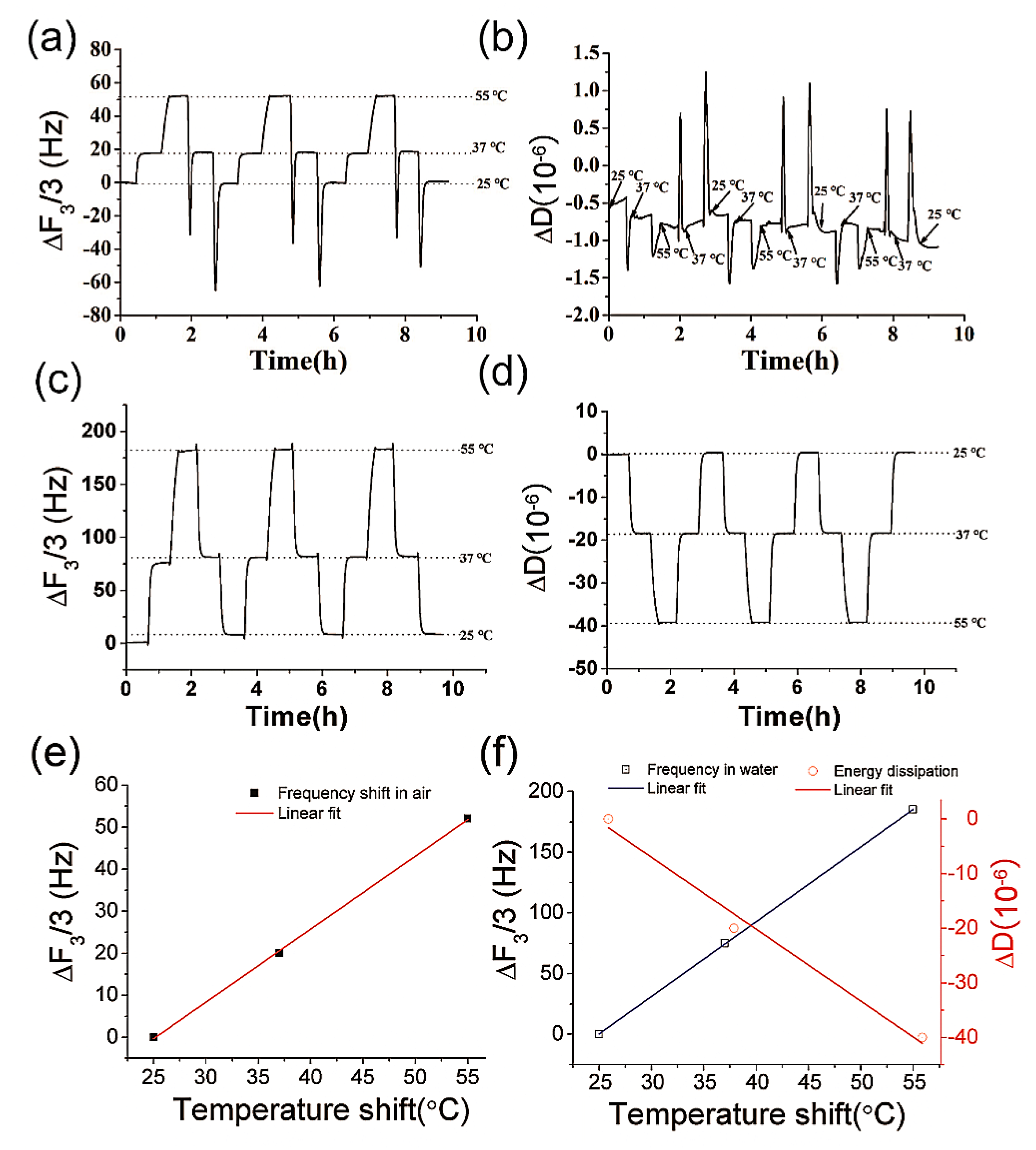
3.1. Temperature Dependency of the Resonant Frequency and Energy Dissipation of QCM
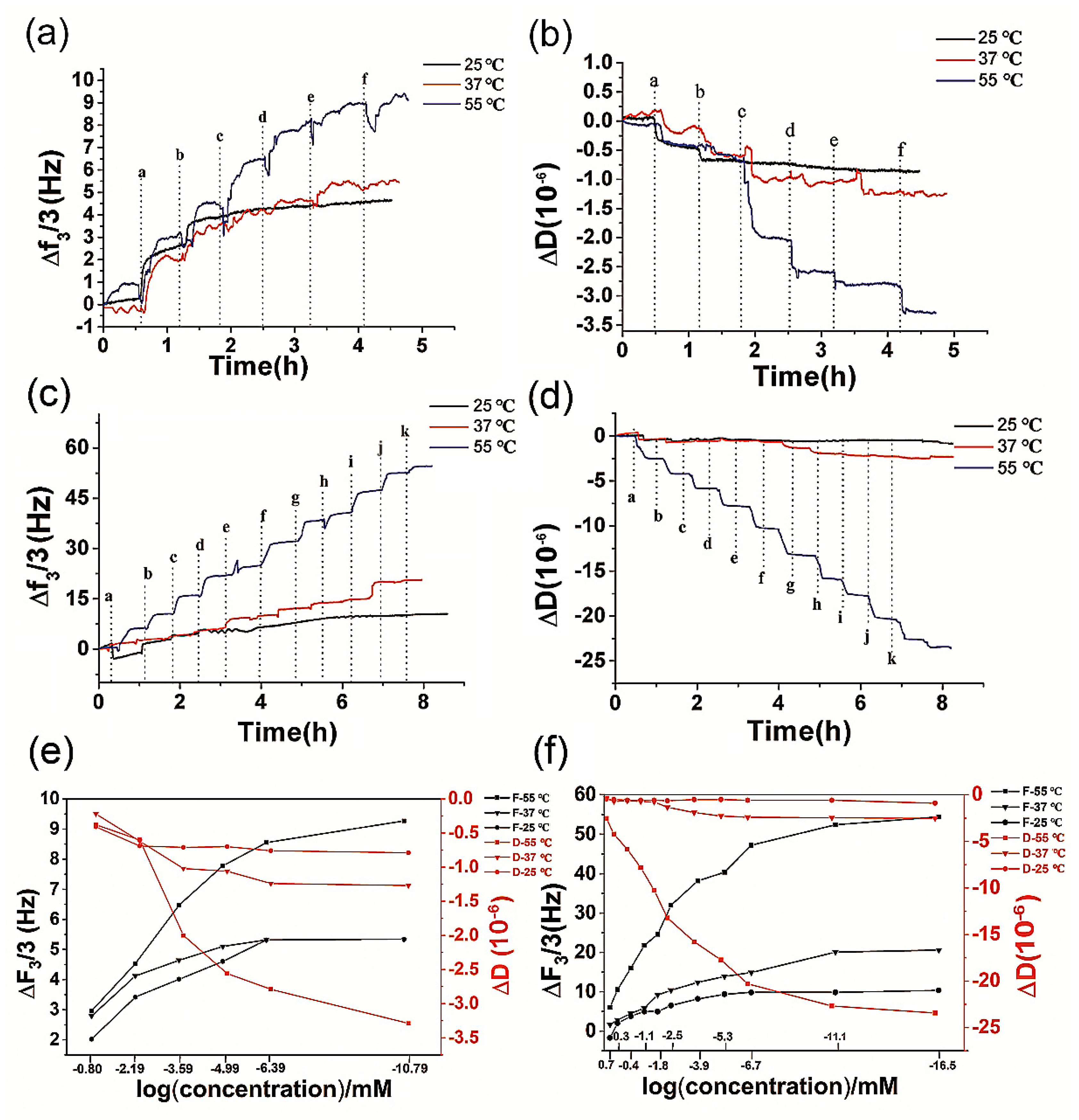
3.2. The Temperature Dependency of QCM Applied for the Ion Detection
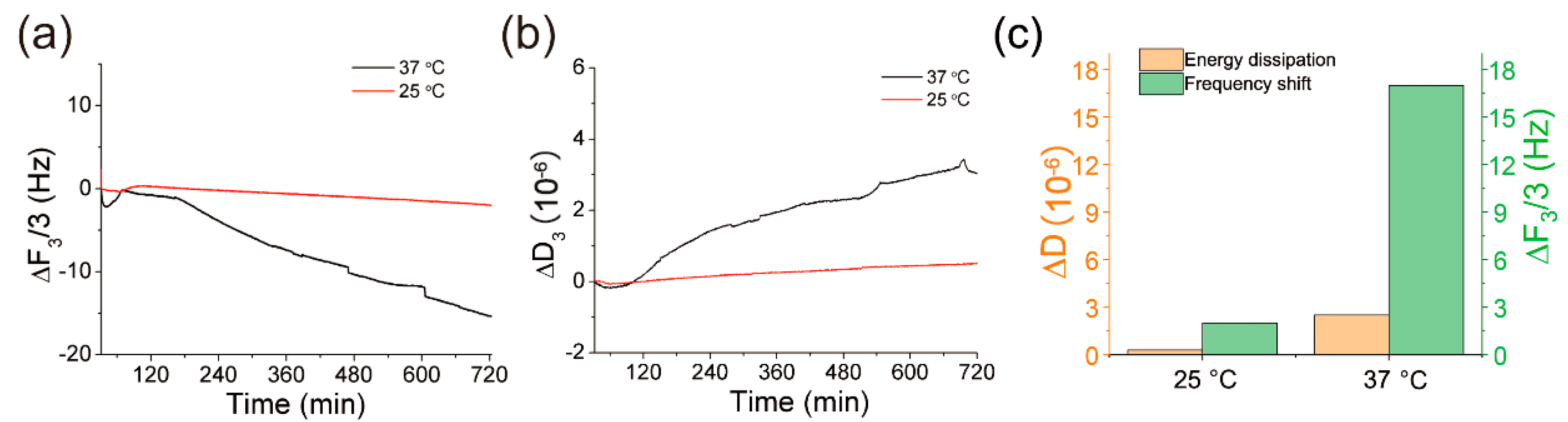
3.3. The Temperature Enhancement of QCM Applied for MUA SAMs Monitoring
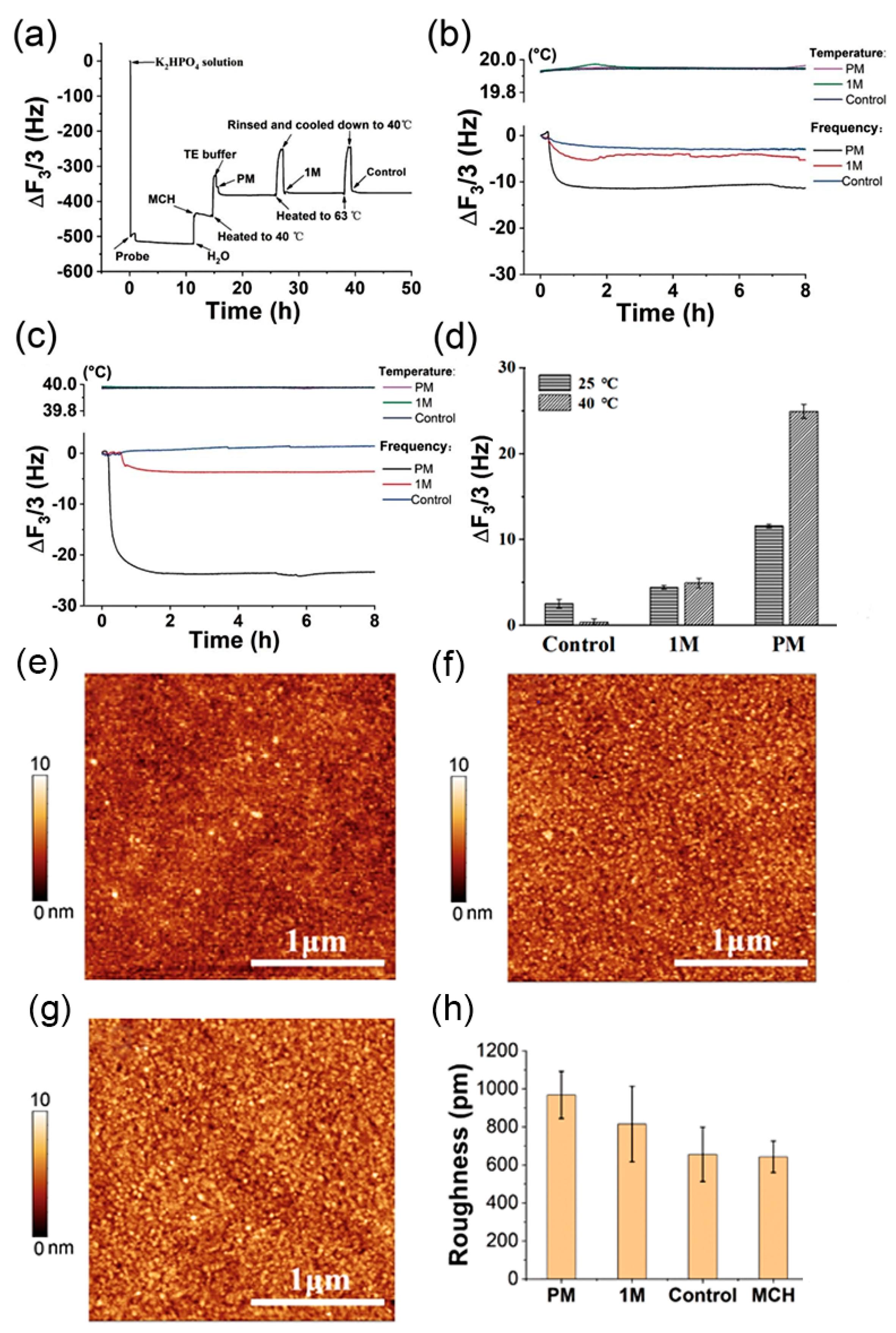
3.4. The Temperature Enhancement of QCM for the Single Nucleotide Polymorphism (SNP) Detection
3.5. The Mechanism of Temperature Enhancement of QCM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuo, B.; Li, W.; Zhang, T.; Zhang, H.X.; Liu, G.H. Detection of sarin with a fluorinated polymer-coated quartz crystal microbalance sensor. S. Afr. J. Chem. 2007, 60, 118–124. [Google Scholar]
- Hook, F.; Voros, J.; Rodahl, M.; Kurrat, R.; Boni, P.; Ramsden, J.J.; Textor, M.; Spencer, N.D.; Tengvall, P.; Gold, J.; et al. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloid Surf. B 2002, 24, 155–170. [Google Scholar] [CrossRef]
- Jordan, J.L.; Fernandez, E.J. QCM-D Sensitivity to Protein Adsorption Reversibility. Biotechnol. Bioeng. 2008, 101, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Hemmersam, A.G.; Rechendorff, K.; Foss, M.; Sutherland, D.S.; Besenbacher, F. Fibronectin adsorption on gold, Ti-, and Ta-oxide investigated by QCM-D and RSA modelling. J. Colloid Interface Sci. 2008, 320, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gao, Y.; Shen, H.; Yang, Y.; Yuan, L. A Quartz Crystal Microbalance (QCM) Study of Single-Strand DNA Hybridization and Hydrolytic Cleavage. J. Anal. Chem. 2005, 60, 780–783. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, J.; Yan, H.H.; Fu, W.L. Specific binding DNA-based piezoelectric quartz crystal microbalance biosensor array for the study of NF-kappa B. Sens. Actuators B Chem. 2010, 149, 259–263. [Google Scholar] [CrossRef]
- Hianik, T.; Ostatna, V.; Sonlajtnerova, M.; Grman, I. Influence of ionic strength, pH and aptamer configuration for binding affinity to thrombin. Bioelectrochemistry 2007, 70, 127–133. [Google Scholar] [CrossRef]
- Tombelli, S.; Minunni, M.; Luzi, E.; Mascini, M. Aptamer-based biosensors for the detection of HIV-1 Tat protein. Bioelectrochemistry 2005, 67, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Liss, M.; Petersen, B.; Wolf, H.; Prohaska, E. An aptamer-based quartz crystal protein biosensor. Anal. Chem. 2002, 74, 4488–4495. [Google Scholar] [CrossRef]
- Ogi, H.; Motohisa, K.; Hatanaka, K.; Ohmori, T.; Hirao, M.; Nishiyama, M. Concentration dependence of IgG-protein A affinity studied by wireless-electrodeless QCM. Biosens. Bioelectron. 2007, 22, 3238–3242. [Google Scholar] [CrossRef]
- He, J.A.; Lu, Y.D.; Fang, J.; Ma, H.W. The change in thickness of the solidified liquid layer rather than the immobilized mass determines the frequency response of a quartz crystal microbalance. Sci. China Chem. 2012, 55, 175–181. [Google Scholar] [CrossRef]
- Rodriguez-Pardo, L.; Rodriguez, J.F.; Gabrielli, C.; Brendel, R. Sensitivity, noise, and resolution in QCM sensors in liquid media. IEEE Sens. J. 2005, 5, 1251–1257. [Google Scholar] [CrossRef]
- Rocklein, M.N.; George, S.M. Temperature-Induced Apparent Mass Changes Observed during Quartz Crystal Microbalance Measurements of Atomic Layer Deposition. Anal. Chem. 2003, 75, 4975–4982. [Google Scholar] [CrossRef]
- Wang, D.; Mousavi, P.; Hauser, P.J.; Oxenham, W.; Grant, C.S. Quartz crystal microbalance in elevated temperature viscous liquids: Temperature effect compensation and lubricant degradation monitoring. Colloids Surf. A Physicochem. Eng. Asp. 2005, 268, 30–39. [Google Scholar] [CrossRef]
- Li, Q.; Gu, Y.; Xie, B. Hybrid temperature effect on a quartz crystal microbalance resonator in aqueous solutions. Chin. Phys. B 2017, 26, 67704. [Google Scholar] [CrossRef]
- Cao-Paz, A.; Rodriguez-Pardo, L.; Fariña, J. Temperature compensation of QCM sensors in liquid media. Sens. Actuators B Chem. 2014, 193, 78–81. [Google Scholar] [CrossRef]
- Werner, J.F. Crystal Oscillator Design and Temperature Compensation. Electron. Power 1979, 25, 219. [Google Scholar] [CrossRef]
- Sang-Wook, L.; Byung Il, C.; Jong, C.K.; Sang-Bong, W.; Yong-Gyoo, K.; Jeseung, Y.; Young-Soo, S. Reduction and compensation of humidity measurement errors at cold temperatures using dual QCM humidity sensors based on graphene oxides. Sens. Actuators B Chem. 2019, 284, 386–394. [Google Scholar]
- Peterson, A.W.; Wolf, L.K.; Georgiadis, R.M. Hybridization of Mismatched or Partially Matched DNA at Surfaces. J. Am. Chem. Soc. 2002, 124, 14601–14607. [Google Scholar] [CrossRef]
- Lu, C.; Czanderna, A.W. Application of Piezoelectric Quartz Crystal Microbalance; Elsevier: New York, NY, USA, 1984; Volume 7, pp. 1–393. [Google Scholar]
- Sinha, B.K.; Tiersten, H.F. First temperature derivatives of the fundamental elastic constants of quartz. J. Appl. Phys. 1979, 50, 2732–2739. [Google Scholar] [CrossRef]
- Bechmann, R. Frequency–temperature–angle characteristics of at-type resonators made of natural and synthetic quartz. Proc. IRE 1956, 44, 1600–1607. [Google Scholar] [CrossRef]
- Haines, J.; Cambon, O.; Keen, D.A.; Tucker, M.G.; Dove, M.T. Structural disorder and loss of piezoelectric properties in α-quartz at high temperature. Appl. Phys. Lett. 2002, 81, 2968–2970. [Google Scholar] [CrossRef]
| Samples | Sequence |
|---|---|
| probe | AGATCAGTGCGTCTGTACTAGCACA |
| PM | TGTGCTAGTACAGACGCACTGATCT |
| 1 M | TGTGCTAGTACAGACACACTGATCT |
| Control | AGATCAGTGCGTCTGTACTAGCACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Ma, Z.; Li, C.; Zhang, H.; Zhu, Z.; Wang, J. Application of Heat-Enhancement for Improving the Sensitivity of Quartz Crystal Microbalance. Biosensors 2022, 12, 643. https://doi.org/10.3390/bios12080643
Song C, Ma Z, Li C, Zhang H, Zhu Z, Wang J. Application of Heat-Enhancement for Improving the Sensitivity of Quartz Crystal Microbalance. Biosensors. 2022; 12(8):643. https://doi.org/10.3390/bios12080643
Chicago/Turabian StyleSong, Chenglong, Zhihao Ma, Chenglong Li, Hongxing Zhang, Zhiqiang Zhu, and Jie Wang. 2022. "Application of Heat-Enhancement for Improving the Sensitivity of Quartz Crystal Microbalance" Biosensors 12, no. 8: 643. https://doi.org/10.3390/bios12080643
APA StyleSong, C., Ma, Z., Li, C., Zhang, H., Zhu, Z., & Wang, J. (2022). Application of Heat-Enhancement for Improving the Sensitivity of Quartz Crystal Microbalance. Biosensors, 12(8), 643. https://doi.org/10.3390/bios12080643





