Production and Selection of Antibody–Antigen Pairs for the Development of Immunoenzyme Assay and Lateral Flow Immunoassay Methods for Carbofuran and Its Analogues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Syntheses of Immunogens and Coating Antigens
2.3. Production of mAb
2.4. Development of ELISA Method
2.5. Development of Colloidal Gold-Based LFIA Method
2.6. Development of Quantum Dot-Based LFIA Method
2.7. Standard Curves and IC50 for ELISA and QD-LFIA Methods
2.8. Cross-Reactivity
2.9. Analysis of Vegetable Samples by QD-LFIA Method
2.10. Comparison with HPLC-MS Methods
3. Results and Discussion
3.1. Production of mAb
3.2. Screening of Antibody–Antigen Pairs
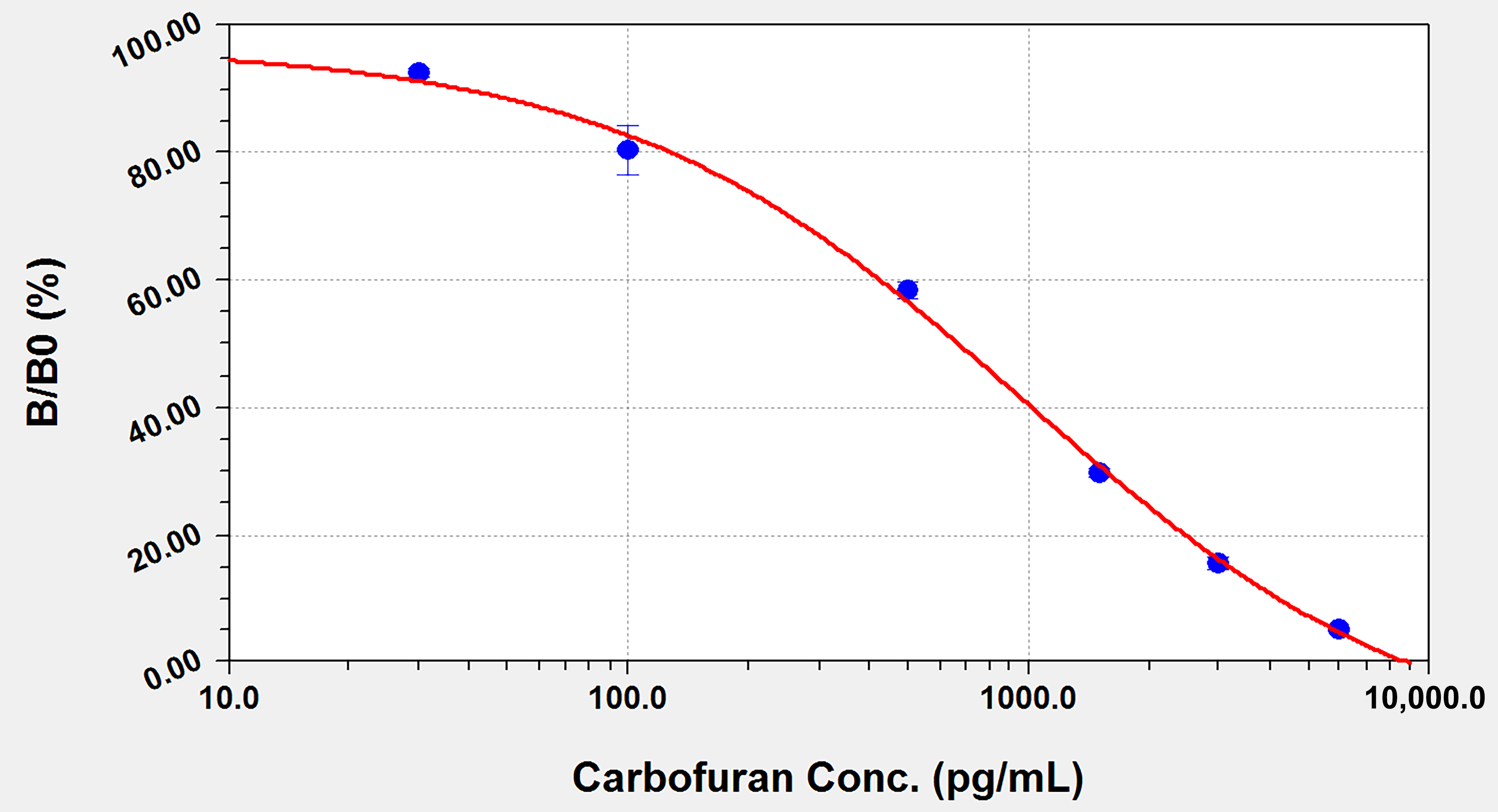
3.3. Detection of Carbofuran by ELISA
3.4. Detection of Carbofuran by QD-LFIA
3.5. Recovery of Carbofuran in Vegetable Samples by QD-LFIA Method
3.6. Comparison with HPLC-MS Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iesce, M.R.; Greca, M.D.; Cermola, F.; Rubino, M.; Isidori, M.; Pascarella, L. Transformation and Ecotoxicity of Carbamic Pesticides in Water (5 pp). Environ. Sci. Pollut. Res. 2006, 13, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Soler, C.; Hamilton, B.; Furey, A.; James, K.J.; Mañes, J.; Picó, Y. Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry Analysis of Carbosulfan, Carbofuran, 3-Hydroxycarbofuran, and Other Metabolites in Food. Anal. Chem. 2007, 79, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Abass, K.; Reponen, P.; Mattila, S.; Rautio, A.; Pelkonen, O. Comparative metabolism of benfuracarb in in vitro mammalian hepatic microsomes model and its implications for chemical risk assessment. Toxicol. Lett. 2014, 224, 290–299. [Google Scholar] [CrossRef]
- Mishra, S.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Bhatt, P.; Chen, S. Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere 2020, 259, 127419. [Google Scholar] [CrossRef] [PubMed]
- Soloneski, S.; Reigosa, M.A.; Molinari, G.; González, N.V.; Larramendy, M.L. Genotoxic and cytotoxic effects of carbofuran and furadan® on Chinese hamster ovary (CHOK1) cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 656, 68–73. [Google Scholar] [CrossRef]
- Gupta, R.C. Carbofuran toxicity. J. Toxicol. Environ. Health 1994, 43, 383–418. [Google Scholar] [CrossRef]
- Pessoa, P.C.; Luchmann, K.H.; Ribeiro, A.B.; Veras, M.M.; Correa, J.R.; Nogueira, A.J.; Bainy, A.C.; Carvalho, P.S. Cholinesterase inhibition and behavioral toxicity of carbofuran on Oreochromis niloticus early life stages. Aquat. Toxicol. 2011, 105, 312–320. [Google Scholar] [CrossRef]
- Rocha, O.; Neto, A.J.G.; Dos Santos Lima, J.C.; Freitas, E.C.; Miguel, M.; da Silva Mansano, A.; Moreira, R.A.; Daam, M.A. Sensitivities of three tropical indigenous freshwater invertebrates to single and mixture exposures of diuron and carbofuran and their commercial formulations. Ecotoxicology 2018, 27, 834–844. [Google Scholar] [CrossRef]
- Sills, J.; Kitowski, I.; Łopucki, R.; Stachniuk, A.; Fornal, E. Banned pesticide still poisoning EU raptors. Science 2021, 371, 1319–1320. [Google Scholar] [CrossRef]
- The Ministry of Agriculture and Rural Affairs. Announcement No. 199 of the Ministry of Agriculture, People’s Republic of China. Available online: http://www.moa.gov.cn/ztzl/ncpzxzz/flfg/200709/t20070919_893058.htm (accessed on 28 May 2022).
- European Commission. Commission Decision of 13 June 2007 Concerning the Non-Inclusion of Carbosulfan in Annex I to Council Directive 91/414/EEC and the Withdrawal of Authorisations for Plant Protection Products Containing That Substance. Available online: http://data.europa.eu/eli/dec/2007/415/oj (accessed on 28 May 2022).
- European Commission. Commission Decision of 13 June 2007 Concerning the Non-Inclusion of Carbofuran in Annex I to Council Directive 91/414/EEC and the Withdrawal of Authorisations for Plant Protection Products Containing That Substance. Available online: http://data.europa.eu/eli/dec/2007/416/oj (accessed on 28 May 2022).
- European Commission. Commission Decision of 20 September 2007 Concerning the Non-Inclusion of Benfuracarb in Annex I to Council Directive 91/414/EEC and the Withdrawal of Authorisations for Plant Protection Products Containing That Substance. Available online: http://data.europa.eu/eli/dec/2007/615/oj (accessed on 28 May 2022).
- United States Environmental Protection Agency. Interim Reregistration Eligibility Decision (IRED) for Carbofuran. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=901A0300.txt (accessed on 28 May 2022).
- GB 2763-2021; National Food Safety Standard—Maximum Residue Limits for Pesticides in Food. Nation Health Commission of the People’s Republic of China: Beijing, China, 2021.
- National Archives and Records Administration. 40 CFR 180—Tolerances and Exemptions for Pesticide Chemical Residues in Food. Available online: https://www.govinfo.gov/app/details/CFR-2021-title40-vol26/CFR-2021-title40-vol26-part180 (accessed on 15 July 2022).
- Li, H.; Chang, Q.; Bai, R.; Lv, X.; Cao, T.; Shen, S.; Liang, S.; Pang, G. Simultaneous determination and risk assessment of highly toxic pesticides in the market-sold vegetables and fruits in China: A 4-year investigational study. Ecotoxicol. Environ. Saf. 2021, 221, 112428. [Google Scholar] [CrossRef]
- Jiang, M.; Gao, H.; Liu, X.; Wang, Y.U.; Lan, J.; Li, Y.; Lv, S.; Zhu, K.; Gong, P. Detection of Pesticide Residues in Vegetables Sold in Changchun City, China. J. Food Prot. 2021, 84, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Casado, J.; Brigden, K.; Santillo, D.; Johnston, P. Screening of pesticides and veterinary drugs in small streams in the European Union by liquid chromatography high resolution mass spectrometry. Sci. Total Environ. 2019, 670, 1204–1225. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tan, G.; Wang, M.; Lin, H.; He, L.; Li, L.; Wang, B. Application of Immunoassays for Rapid Monitor of Carbofuran Residue in Vegetables. J. Food Sci. 2019, 84, 3296–3302. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, H.; Xiao, Z.; Fu, H.; Shen, Y.; Luo, L.; Wang, H.; Lei, H.; Hongsibsong, S.; Xu, Z. Rational hapten design to produce high-quality antibodies against carbamate pesticides and development of immunochromatographic assays for simultaneous pesticide screening. J. Hazard. Mater. 2021, 412, 125241. [Google Scholar] [CrossRef]
- Yao, L.; Liu, L.; Song, S.; Kuang, H.; Xu, C. Development of indirect competitive enzyme-linked immunosorbent and immunochromatographic strip assays for carbofuran detection in fruits and vegetables. Food Agric. Immunol. 2017, 28, 639–651. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Qu, Q.; Chen, S.; Liu, X.; Li, P. A double-label time-resolved fluorescent strip for rapidly quantitative detection of carbofuran residues in agro-products. Food Chem. 2017, 231, 295–300. [Google Scholar] [CrossRef]
- Gui, W.; Jin, M.; Sun, L.; Guo, Y.; Zhu, G. Residues determination of carbofuran in vegetables based on sensitive time-resolved fluorescence immunoassay. Food Agric. Immunol. 2009, 20, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Abad, A.; Moreno, M.J.; Montoya, A. Development of Monoclonal Antibody-Based Immunoassays to the N-Methylcarbamate Pesticide Carbofuran. J. Agric. Food Chem. 1999, 47, 2475–2485. [Google Scholar] [CrossRef]
- Abad, A.; Moreno, M.J.; Montoya, A. A monoclonal immunoassay for carbofuran and its application to the analysis of fruit juices. Anal. Chim. Acta 1997, 347, 103–110. [Google Scholar] [CrossRef]
- Lan, J.; Wang, M.; Ding, S.; Fan, Y.; Diao, X.; Li, Q.X.; Zhao, H. Simultaneous detection of carbofuran and 3-hydroxy-carbofuran in vegetables and fruits by broad-specific monoclonal antibody-based ELISA. Food Agric. Immunol. 2019, 30, 1085–1096. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Jiang, Y.; Sun, Y.; Pan, K.; Lei, H.; Wu, Q.; Shen, Y.; Xiao, Z.; Xu, Z. Development of an Enzyme-linked Immuno-Sorbent Assay (ELISA) Method for Carbofuran Residues. Molecules 2008, 13, 871–881. [Google Scholar] [CrossRef]
- Sun, W.; Liu, L.; Memon, A.G.; Zhou, X.; Zhao, H. Waveguide-Based Fluorescent Immunosensor for the Simultaneous Detection of Carbofuran and 3-Hydroxy-Carbofuran. Biosensors 2020, 10, 191. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Y.; Wang, X. Amperometric immunosensor based on deposited gold nanocrystals/4,4′-thiobisbenzenethiol for determination of carbofuran. Food Control 2012, 28, 184–191. [Google Scholar] [CrossRef]
- Liu, L.; Xu, D.; Hu, Y.; Liu, S.; Wei, H.; Zheng, J.; Wang, G.; Hu, X.; Wang, C. Construction of an impedimetric immunosensor for label-free detecting carbofuran residual in agricultural and environmental samples. Food Control 2015, 53, 72–80. [Google Scholar] [CrossRef]
- Chen, H.; Hu, O.; Fan, Y.; Xu, L.; Zhang, L.; Lan, W.; Hu, Y.; Xie, X.; Ma, L.; She, Y.; et al. Fluorescence paper-based sensor for visual detection of carbamate pesticides in food based on CdTe quantum dot and nano ZnTPyP. Food Chem. 2020, 327, 127075. [Google Scholar] [CrossRef]
- Sun, X.; Du, S.; Wang, X.; Zhao, W.; Li, Q. A Label-Free Electrochemical Immunosensor for Carbofuran Detection Based on a Sol-Gel Entrapped Antibody. Sensors 2011, 11, 9520–9531. [Google Scholar] [CrossRef]
- Zou, R.; Guo, Y.; Chen, Y.; Zhao, Y.; Zhao, L.; Zhu, G.; Liu, Y.; Peters, J.; Guo, Y. Computer-aided profiling of a unique broad-specific antibody and its application to an ultrasensitive fluoroimmunoassay for five N-methyl carbamate pesticides. J. Hazard. Mater. 2022, 426, 127845. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, B.; Cui, P.; Yu, T.; Shi, G.; Shen, Z. Development of a quantum dot–based lateral flow immunoassay with high reaction consistency to total aflatoxins in botanical materials. Anal. Bioanal. Chem. 2021, 413, 1629–1637. [Google Scholar] [CrossRef]
- Woychik, N.A.; Hinsdill, R.D.; Chu, F.S. Production and characterization of monoclonal antibodies against aflatoxin M1. Appl. Environ. Microbiol. 1984, 48, 1096–1099. [Google Scholar] [CrossRef] [Green Version]
- The Ministry of Agriculture and Rural Affairs. Determination of Carbonfuran, Chlordimeform and Amitraz in Feeds—Liquid Chromatography-Tandem Mass Spectrometry; The Ministry of Agriculture and Rural Affairs: Beijing, China, 2020. [Google Scholar]
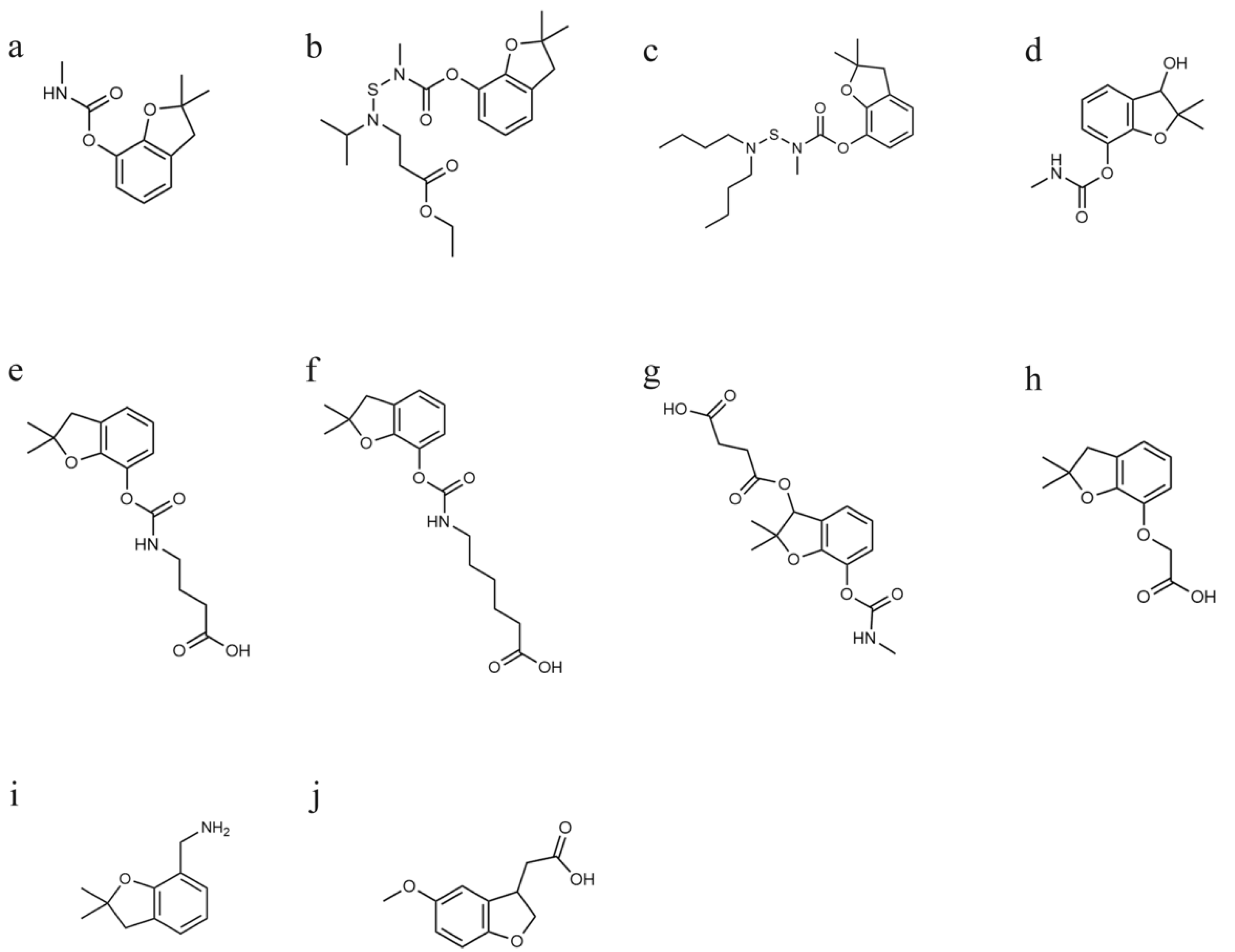



| Cell Lines | Carbofuran | Benfuracarb | Carbosulfan |
|---|---|---|---|
| 13C8 | 95% | 91% | 90% |
| 6D6 | 86% | 53% | 61% |
| 12D6 | 84% | 55% | 46% |
| 18G3 | 86% | 76% | 71% |
| 12H5 | 78% | 70% | 67% |
| 7F9 | 76% | 68% | 65% |
| Antibody | Antigen | IC50 (ELISA)ng/mL | IC50 (QD-LFIA) ng/mL |
|---|---|---|---|
| 13C8 | DDB-OVA | 0.22 ± 0.02 | >1000 |
| MDA-OVA | 0.17 ± 0.01 | 0.66 ± 0.03 | |
| DDB-BSA | 0.19 ± 0.01 | >1000 | |
| MDA-BSA | 0.20 ± 0.02 | 0.68 ± 0.03 |
| Chemicals | IC50 | CR (%) |
|---|---|---|
| carbofuran | 0.18 ± 0.03 | 100.0 |
| benfuracarb | 0.23 ± 0.03 | 78.3 |
| carbosulfan | 0.24 ± 0.04 | 75.0 |
| 3-hydroxy-carbofuran | 0.25 ± 0.05 | 72.0 |
| metolcarb | >20 | <1% |
| carbaryl | >20 | <1% |
| isoprocarb | >20 | <1% |
| aldicarb | >20 | <1% |
| Works | Chemicals | IC50 | CR(%) | Reference |
|---|---|---|---|---|
| 1 | carbofuran | 0.3 | / | [23] |
| 2 | carbofuran | 0.66 | / | [26] |
| 3 | carbofuran | 0.76 ± 0.07 | 100.0 | [28] |
| 3-hydroxy-carbofuran | 0.69 ± 0.08 | 110.1 | ||
| 4 | carbofuran | 8.97 | 100.0 | [35] |
| isoprocarb | 17.68 | 50.7 | ||
| propoxur | 34.75 | 25.8 | ||
| carbosulfan | 115.80 | 7.7 | ||
| carbaryl | 105.59 | 8.5 | ||
| 5 | carbofuran | 0.18 ± 0.03 | 100.0 | this work |
| benfuracarb | 0.23 ± 0.03 | 78.3 | ||
| carbosulfan | 0.24 ± 0.04 | 75.0 | ||
| 3-hydroxy-carbofuran | 0.25 ± 0.05 | 72.0 |
| Chemicals | IC50 | CR (%) |
|---|---|---|
| carbofuran | 0.67 ± 0.08 | 100.0 |
| benfuracarb | 0.81 ± 0.07 | 83.7 |
| carbosulfan | 0.84 ± 0.09 | 79.8 |
| 3-hydroxy-carbofuran | 0.87 ± 0.06 | 77.0 |
| metolcarb | >100 | <1% |
| carbaryl | >100 | <1% |
| isoprocarb | >100 | <1% |
| aldicarb | >100 | <1% |
| Spiked Concentration (ng/g) | Measured Concentration (ng/g) | Recovery (%) | CV (%) |
|---|---|---|---|
| 10 | 8.3 ± 0.6 | 83 | 6.9 |
| 20 | 20.4 ± 1.3 | 102 | 6.5 |
| 50 | 55.7 ± 4.9 | 111 | 8.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Fan, Q.; Chen, Y.; Sun, X.; Shi, G. Production and Selection of Antibody–Antigen Pairs for the Development of Immunoenzyme Assay and Lateral Flow Immunoassay Methods for Carbofuran and Its Analogues. Biosensors 2022, 12, 560. https://doi.org/10.3390/bios12080560
Wu Y, Fan Q, Chen Y, Sun X, Shi G. Production and Selection of Antibody–Antigen Pairs for the Development of Immunoenzyme Assay and Lateral Flow Immunoassay Methods for Carbofuran and Its Analogues. Biosensors. 2022; 12(8):560. https://doi.org/10.3390/bios12080560
Chicago/Turabian StyleWu, Yuxiang, Qi Fan, Yinuo Chen, Xia Sun, and Guoqing Shi. 2022. "Production and Selection of Antibody–Antigen Pairs for the Development of Immunoenzyme Assay and Lateral Flow Immunoassay Methods for Carbofuran and Its Analogues" Biosensors 12, no. 8: 560. https://doi.org/10.3390/bios12080560
APA StyleWu, Y., Fan, Q., Chen, Y., Sun, X., & Shi, G. (2022). Production and Selection of Antibody–Antigen Pairs for the Development of Immunoenzyme Assay and Lateral Flow Immunoassay Methods for Carbofuran and Its Analogues. Biosensors, 12(8), 560. https://doi.org/10.3390/bios12080560





