Living Sample Viability Measurement Methods from Traditional Assays to Nanomotion
Abstract
1. Introduction
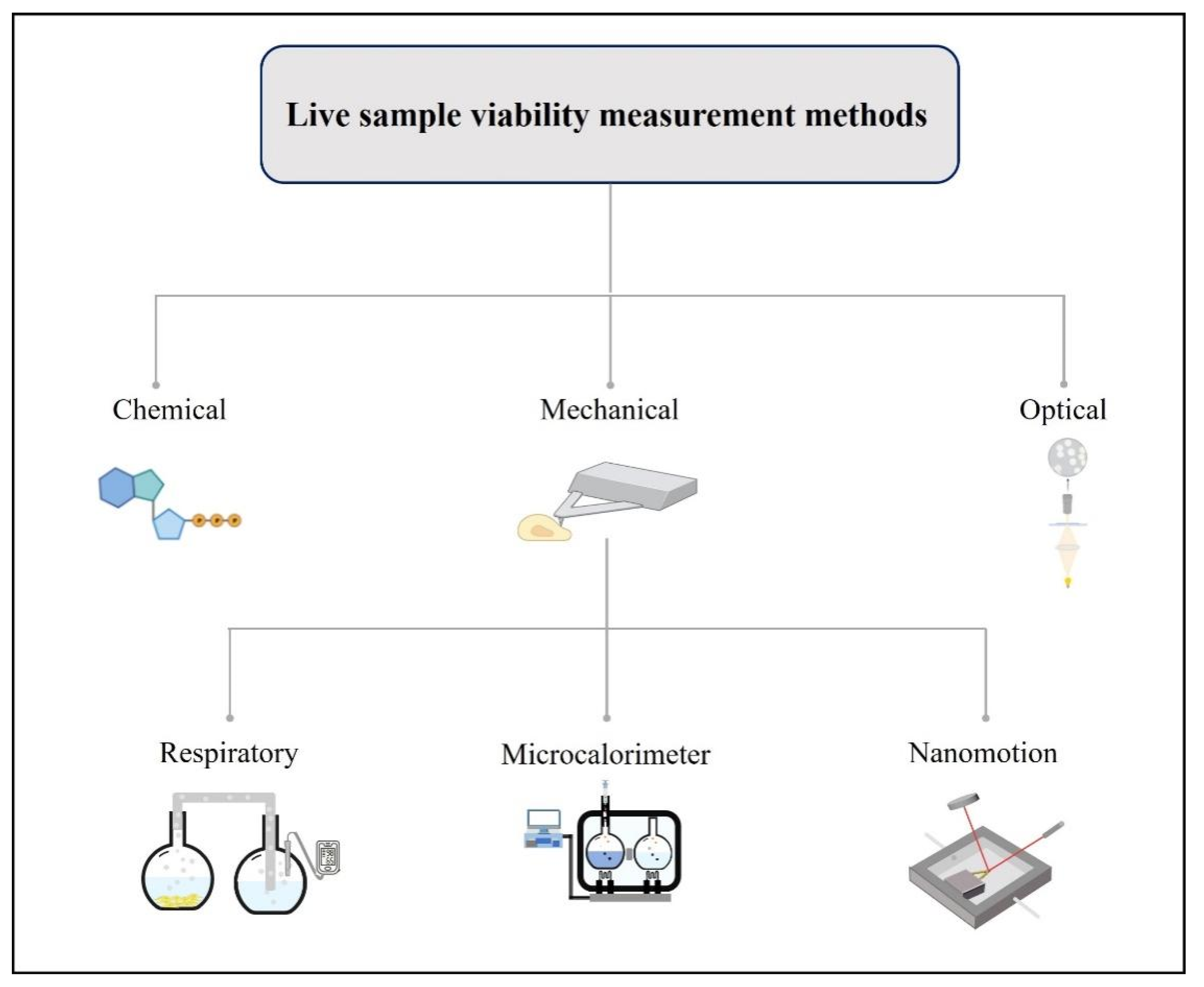
2. Living Sample Viability Measurement Methods
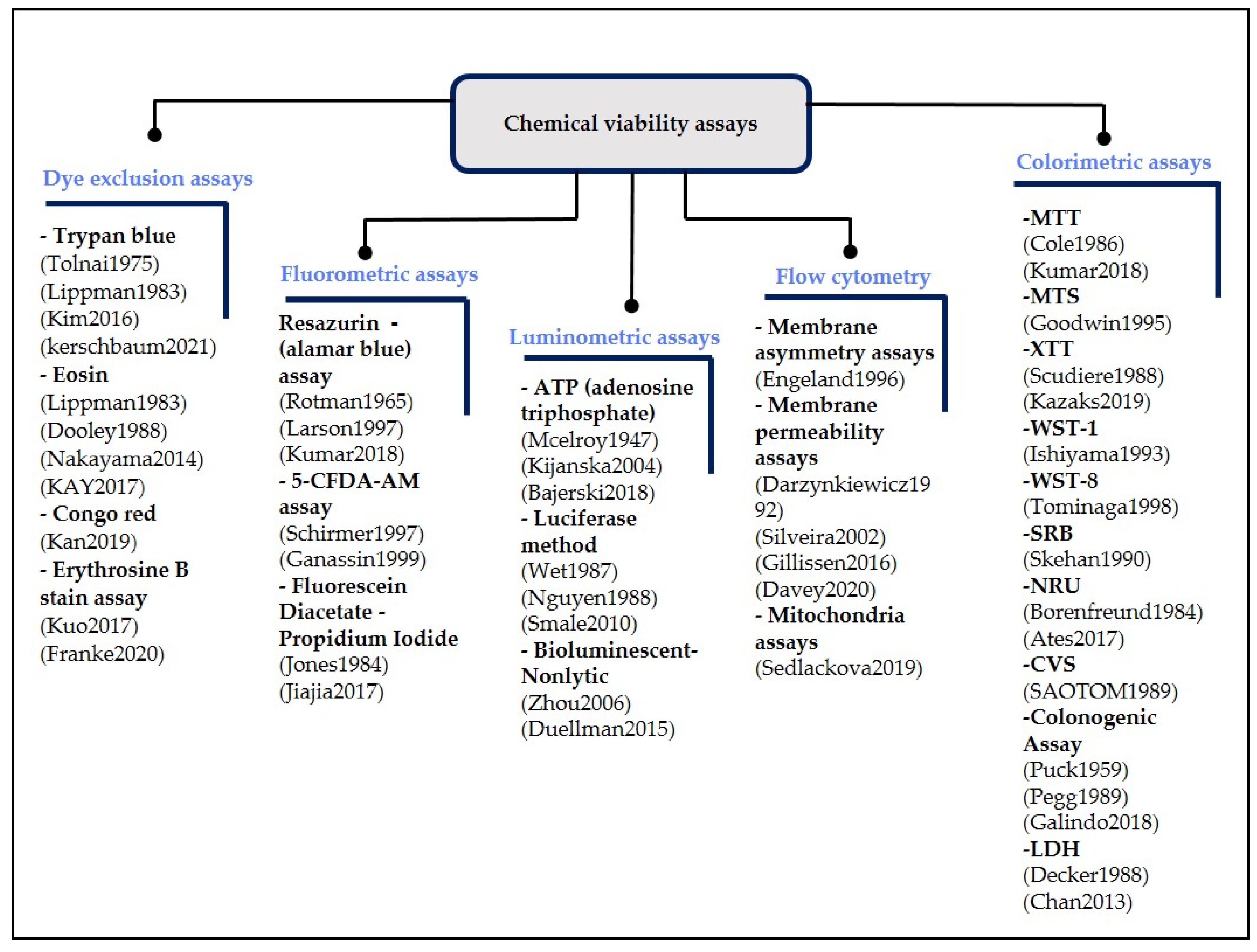
2.1. Chemical Viability Assays
2.2. Optical Measurement Methods
2.2.1. Raman Spectroscopy
2.2.2. Flow Imaging Microscopy
2.2.3. Holography
2.2.4. On-Chip, Lensless Video Microscopy Technology
2.3. Mechanical Measuring Methods
2.3.1. Respiratory Measuring Methods
2.3.2. Microcalorimeter Measurement Methods
2.3.3. Micro-Nanomechanical Oscillator Sensors
3. The AFM Oscillating Sensor Mode (Nanomotion)
3.1. Nanomotion Introduction
3.2. Nanomotion Application
| Attachment Protocol | Results Display | Application | Cell Type | Time | Agent | Cantilever Type | Cantilever Functionalization | Ref. |
|---|---|---|---|---|---|---|---|---|
| Inject sample medium inside AFM test room | Variance value | Antibiotic resistance | E. coli and S. aureus | 60–90 min | Ampicillin | DNP-10, Bruker | APTES (0.2%, 1.5 min) | [126] |
| Cantilever incubates in sample medium outside of the AFM test room | Variance value | Antibiotic resistance | E. Coli | 2 h | Ampicillin | DNP-10, Bruker | Glutaraldehyde (0.5%, 7 min) | [154] |
| Cantilever incubates in sample medium outside of the AFM test room | Variance value; power spectral density | Protein conformational changes | Ligands, such as ATP | <10 min | Topo II enzymes with Pbr322 DNA (200 nm) | DNP-10, Bruker | APTES (0.1%, 1 min) | [153] |
| Cantilever incubates in sample medium outside of the AFM test room and Micrometric motors of the AFM (AFM single-cell force spectroscopy) | Variance value | Life-searching experiments on Earth and interplanetary missions | E. coli | >190 min | Bactericidal dose (10 μg/mL) | DNP-10, Bruker | Glutaraldehyde (0.5%, 7 min) | [120] |
| S. aureus | >190 min | Bactericidal dose (2 μg/mL) | Glutaraldehyde (0.5%, 7 min) | |||||
| C. albicans | >190 min | Fungicidal dose (20 μg/mL) | Glutaraldehyde (0.5%, 7 min) | |||||
| MC3T3-E1 | >190 min | 5% glutaraldehyde | Fibronection (10 μg/mL, 15 min) | |||||
| M17 | >190 min | Salt concentration increasing | Poly-L-lysine (10%, 30 min) | |||||
| Cantilever incubates in sample medium outside of the AFM test room | Variance value | Cell viability | MCF7 | 7 h | Paclitaxel | DNP-10, Bruker | APTES (10%, 30 min) | [144] |
| Inject sample medium inside AFM test room | Damping value | Cell viability | Hela and MCF7 | 4–5 h | Au NPs | SNL-10, Bruker | - | [127] |
| Micrometric motors of the AFM (AFM single-cell force spectroscopy) | Variance value | Single-cell cytotoxicity assays | M17 | 7 h | Extracellular monomeric and amyloid α-synuclein species | DNP-10, Bruker | Poly-L-lysine (10%, 30 min) | [152] |
| Cantilever incubates in sample medium outside of the AFM test room | Variance value | Bloodstream infection | E. coli | 90 min | Ceftriaxone, ciprofloxacin and ampicillin | NP-O10, Bruker | Glutaraldehyde (0.5%, 7 min) | [149] |
| Cantilever incubates in sample medium outside of the AFM test room | Variance value | Mitochondrial activity detected | Mitochondria- embryonic kidney cells | 110 min | Malate, pyruvate, ADP, sodium azide, and rotenone | NP-O10, Bruker | Glutaraldehyde (5%, 10 min) | [145] |
| Inject sample medium inside AFM test room | Variance value | Sperm motility | Semen | - | Alcohol, spermagic | - | APTES (10%, 15 min) | [150] |
| Cantilever incubates in sample medium outside of the AFM test room | Variance value | Antibiotic resistance | B. pertussis | 100 min | Erythromycin (Sigma- E6376); clarithromycin (Sigma -A3487), trimthoprim-sulfamethoxazole | - | Glutaraldehyde (0.5%, 10 min) | [148] |
| Cantilever incubates in sample medium outside of the AFM test room | Variance value | Antibiotic resistance | Bacillus Calmette-Guérin (BCG) and M. abscessus | 200 min | BCG vs. Isoniazid and rifampicin M. abscessus vs. Amikacin | DNP-10, Bruker and SD-qp-CONT, NanoandMore | Glutaraldehyde (0.5%, 15 min) | [155] |
| The micrometric motors of the AFM (AFM single-cell force spectroscopy) | Variance value | Cell metabolic changes | HEK293 | 40 min | Frataxin overexpression | DNP-10, Bruker | Poly-D-lysine (20 μg/mL, 15 min) | [151] |
| Inject sample medium inside AFM test room | Variance value | Antibiotic resistance | E. coli | 120 min | Bacteriophage T7 | RC800PSA, Olympus | Poly-L-lysine (0.01%, 15 min) | [156] |
| Cantilever incubates in sample medium outside of the AFM test room | Variance value | Yeast resistance to antifungal drugs | C. albicans | >2 h | Fibronectin | Qp-CONT, nanoandmore | Con A (2 mg/mL, 30 min) | [157] |
| Cantilever incubates in sample medium outside of the AFM test room | Violin plots | Bacterial virulence | B. pertussis | 5 min | Mgso4 | SD-qp-CONT, nanoandmore | Poly-L-lysine (0.1%, 5 min) | [158] |
| Cantilever incubates in sample medium outside of the AFM test room | Variance value | Viability and susceptibility of microorganisms | E. coli and S. aureus | 4 h | Ampicillin, glutaraldehyde | SD-qp-CONT, nanoandmore | Glutaraldehyde (0.5%, 10 min) | [159] |
3.3. Attachment Protocol
3.4. Results Display
3.5. Challenges and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mahto, S.K.; Chandra, P.; Rhee, S.W. In vitro models, endpoints and assessment methods for the measurement of cytotoxicity. Toxicol. Environ. Health Sci. 2010, 2, 87–93. [Google Scholar] [CrossRef]
- Hu, C.; He, S.; Lee, Y.J.; He, Y.R.; Anastasio, M.; Popescu, G. Label-free cell viability assay using phase imaging with computational specificity (PICS). Quant. Phase Imaging VII 2021, 11653, 48. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Single, A.; Beetham, H.; Telford, B.J.; Guilford, P.; Chen, A. A comparison of real-time and endpoint cell viability assays for improved synthetic lethal drug validation. J. Biomol. Screen. 2015, 20, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zhang, R.; Zhang, F.; Zhang, Y.; Li, G.; Miao, R.; Shao, S. An Evaluation Approach of Cell Viability Based on Cell Detachment Assay in a Single-Channel Integrated Microfluidic Chip. ACS Sens. 2019, 4, 2654–2661. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, R.; Zhang, F.; Zhang, Y. Evaluating cell viability heterogeneity based on information fusion of multiple adhesion strengths. Biotechnol. Bioeng. 2021, 118, 2360–2367. [Google Scholar] [CrossRef]
- Venturelli, L.; Kohler, A.C.; Stupar, P.; Villalba, M.I.; Kalauzi, A.; Radotic, K.; Bertacchi, M.; Dinarelli, S.; Girasole, M.; Pešić, M.; et al. A perspective view on the nanomotion detection of living organisms and its features. J. Mol. Recognit. 2020, 33, e2849. [Google Scholar] [CrossRef]
- Gilbert, D.F. Cell Viability Assays; Springer: New York, NY, USA, 2017. [Google Scholar]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Duellman, S.J.; Zhou, W.; Meisenheimer, P.; Vidugiris, G.; Cali, J.J.; Gautam, P.; Wennerberg, K.; Vidugiriene, J. Bioluminescent, Nonlytic, Real-Time Cell Viability Assay and Use in Inhibitor Screening. Assay Drug Dev. Technol. 2015, 13, 456–465. [Google Scholar] [CrossRef]
- Kerschbaum, H.H.; Tasa, B.A.; Schürz, M.; Oberascher, K.; Bresgen, N. Trypan blue—Adapting a dye used for labelling dead cells to visualize pinocytosis in viable cells. Cell. Physiol. Biochem. 2021, 55, 171–184. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, H.J.; Lee, H.J.; Lee, K.; Hong, D.; Lim, H.; Cho, K.; Jung, N.; Yi, Y.W. Application of a non-hazardous vital dye for cell counting with automated cell counters. Anal. Biochem. 2016, 492, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lippman, M.E. Comparison of dye exclusion assays with a clonogenic assay in the determination of drug-Induced cytotoxicity. Cancer Res. 1983, 43, 258–264. [Google Scholar]
- Tolnai, S. A method for viable cell count. Tissue Cult. Assoc. Man. 1975, 1, 37–38. [Google Scholar] [CrossRef]
- Dooley, M.P. The use of eosin B to assess the viability and developmental potential of rat embryos. Retrosp. Theses Diss. 1988, 8839, 1–256. [Google Scholar]
- Nakayama, Y.; Tsujinaka, T. Acceleration of robust ‘biotube’ vascular graft fabrication by in-body tissue architecture technology using a novel eosin Y-releasing mold. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 231–238. [Google Scholar] [CrossRef]
- Kay, A.B. Paul ehrlich and the early history of granulocytes. Myeloid Cells Health Dis. A Synth. 2017, 4, 3–15. [Google Scholar] [CrossRef]
- Kan, A.; Birnbaum, D.P.; Praveschotinunt, P.; Joshi, N.S. Congo red fluorescence for rapid in situ characterization of synthetic curli systems. Appl. Environ. Microbiol. 2019, 85, e00434-19. [Google Scholar] [CrossRef]
- Kuo, C.T.; Chen, Y.L.; Hsu, W.T.; How, S.C.; Cheng, Y.H.; Hsueh, S.S.; Liu, H.S.; Lin, T.H.; Wu, J.W.; Wang, S.S.S. Investigating the effects of erythrosine B on amyloid fibril formation derived from lysozyme. Int. J. Biol. Macromol. 2017, 98, 159–168. [Google Scholar] [CrossRef]
- Franke, J.D.; Braverman, A.L.; Cunningham, A.M.; Eberhard, E.E.; Perry, G.A. Erythrosin B: A versatile colorimetric and fluorescent vital dye for bacteria. Biotechnol. J. 2020, 68, 7–13. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018, 2018, 465–468. [Google Scholar] [CrossRef]
- Rotman, B.; Papermaster, B.W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc. Natl. Acad. Sci. USA 1966, 55, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.M.; Doughman, D.J.; Gregerson, D.S.; Obritsch, W.F. A new, simple, nonradioactive, nontoxic in vitro assay to monitor corneal endothelial cell viability. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1929–1933. [Google Scholar]
- Schirmer, K.; Chan, A.G.J.; Greenberg, B.M.; Dixon, D.G.; Bols, N.C. Methodology for demonstrating and measuring the photocytotoxicity of fluoranthene to fish cells in culture. Toxicol. In Vitro 1997, 11, 107–113. [Google Scholar] [CrossRef]
- Ganassin, R.C.; Bols, N.C. Growth of rainbow trout hemopoietic cells in methylcellulose and methods of monitoring their proliferative response in this matrix. Methods Cell Sci. 2000, 22, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Jiajia, L.; Shinghung, M.; Jiacheng, Z.; Jialing, W.; Dilin, X.; Shengquan, H.; Zaijun, Z.; Qinwen, W.; Yifan, H.; Wei, C. Assessment of neuronal viability using fluorescein diacetate-propidium iodide double staining in cerebellar granule neuron culture. J. Vis. Exp. 2017, 2017, e55442. [Google Scholar] [CrossRef]
- Jones, K.H.; Senft, J.A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J. Histochem. Cytochem. 1985, 33, 77–79. [Google Scholar] [CrossRef]
- Mecelroy, W.D. The energy source for bioluminescence in an isolated system. Zoology 1947, 33, 342–345. [Google Scholar] [CrossRef]
- Bajerski, F.; Stock, J.; Hanf, B.; Darienko, T.; Heine-Dobbernack, E.; Lorenz, M.; Naujox, L.; Keller, E.R.J.; Schumacher, H.M.; Friedl, T.; et al. ATP content and cell viability as indicators for cryostress across the diversity of life. Front. Physiol. 2018, 9, 921. [Google Scholar] [CrossRef]
- Kijanska, M.; Kelm, J. In vitro 3D Spheroids and Microtissues: ATP-based Cell Viability and Toxicity Assays. Assay Guid. Man. 2004, 1, 1–13. [Google Scholar]
- Smale, S.T. Luciferase assay. Cold Spring Harb. Protoc. 2010, 5, 2008–2011. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Morange, M.; Bensaude, O. Firefly luciferase luminescence assays using scintillation counters for quantitation in transfected mammalian cells. Anal. Biochem. 1988, 171, 404–408. [Google Scholar] [CrossRef]
- de Wet, J.R.; Wood, K.V.; DeLuca, M.; Helinski, D.R.; Subramani, S. Firefly luciferase gene: Structure and expression in mammalian cells. Mol. Cell. Biol. 1987, 7, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Valley, M.P.; Shultz, J.; Hawkins, E.M.; Bernad, L.; Good, T.; Good, D.; Riss, T.L.; Klaubert, D.H.; Wood, K.V. New bioluminogenic substrates for monoamine oxidase assays. J. Am. Chem. Soc. 2006, 128, 3122–3123. [Google Scholar] [CrossRef] [PubMed]
- van Engeland, M.; Ramaekers, F.C.S.; Schutte, B.; Reutelingsperger, C.P.M. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996, 24, 131–139. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Bruno, S.; Del Bino, G.; Gorczyca, W.; Hotz, M.A.; Lassota, P.; Traganos, F. Features of apoptotic cells measured by flow cytometry. Cytometry 1992, 13, 795–808. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, M.G.; Romão, M.V.S.; Loureiro-Dias, M.C.; Rombouts, F.M.; Abee, T. Flow cytometric assessment of membrane integrity of ethanol-stressed Oenococcus oeni cells. Appl. Environ. Microbiol. 2002, 68, 6087–6093. [Google Scholar] [CrossRef]
- Gillissen, M.A.; Yasuda, E.; De Jong, G.; Levie, S.E.; Go, D.; Spits, H.; van Helden, P.M.; Hazenberg, M.D. The modified FACS calcein AM retention assay: A high throughput flow cytometer based method to measure cytotoxicity. J. Immunol. Methods 2016, 434, 16–23. [Google Scholar] [CrossRef]
- Davey, H.; Guyot, S. Estimation of Microbial Viability Using Flow Cytometry. Curr. Protoc. Cytom. 2020, 93, e72. [Google Scholar] [CrossRef]
- Sedlackova, L.; Korolchuk, V.I. Mitochondrial quality control as a key determinant of cell survival. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 575–587. [Google Scholar] [CrossRef]
- Cole, S.P.C. Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemother. Pharmacol. 1986, 17, 259–263. [Google Scholar] [CrossRef]
- Goodwin, C.J.; Holt, S.J.; Downes, S.; Marshall, N.J. Microculture tetrazolium assays: A comparison between two new tetrazolium salts, XTT and MTS. J. Immunol. Methods 1995, 179, 95–103. [Google Scholar] [CrossRef]
- Kazaks, A.; Collier, M.; Conley, M. Cytotoxicity of Caffeine on MCF-7 Cells Measured by XTT Cell Proliferation Assay (P06-038-19). Curr. Dev. Nutr. 2019, 3, 548. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a Soluble Tetrazolium/Formazan Assay for Cell Growth and Drug Sensitivity in Culture Using Human and Other Tumor Cell Lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Scarcello, E.; Lambremont, A.; Vanbever, R.; Jacques, P.J.; Lison, D. Mind your assays: Misleading cytotoxicity with the WST-1 assay in the presence of manganese. PLoS ONE 2020, 15, e0231634. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Seifabadi, Z.S.; Rezaei-Tazangi, F.; Azarbarz, N.; Nejad, D.B.; Mohammadiasl, J.; Darabi, H.; Pezhmanlarki-Tork, S. Assessment of viability of wharton’s jelly mesenchymal stem cells encapsulated in alginate scaffold by WST-8 assay kit. Med. J. Cell Biol. 2021, 9, 42–47. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Vajrabhaya, L.o.; Korsuwannawong, S. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. J. Anal. Sci. Technol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Ates, G.; Vanhaecke, T.; Rogiers, V.; Rodrigues, R.M. Assaying cellular viability using the neutral red uptake assay. Methods Mol. Biol. 2017, 1601, 19–26. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90). J. Tissue Cult. Methods 1985, 9, 7–9. [Google Scholar] [CrossRef]
- Saotome, K.; Morita, H.; Umeda, M. Cytotoxicity test with simplified crystal violet staining method using microtitre plates and its application to injection drugs. Toxicol. In Vitro 1989, 3, 317–321. [Google Scholar] [CrossRef]
- Puck, T.T. Quantitaive Studies on Mammalian Cells in Vitro. Rev. Moderen Phys. 1993, 46, 177–188. [Google Scholar]
- Pegg, D.E. Viability assays for preserved cells, tissues, and organs. Cryobiology 1989, 26, 212–231. [Google Scholar] [CrossRef]
- Galindo, C.C.; Lozano, D.M.V.; Rodríguez, B.C.; Perdomo-Arciniegas, A.M. Improved cord blood thawing procedure enhances the reproducibility and correlation between flow cytometry CD34+ cell viability and clonogenicity assays. Cytotherapy 2018, 20, 891–894. [Google Scholar] [CrossRef]
- Decker, T.; Lohmann-Matthes, M.L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1988, 115, 61–69. [Google Scholar] [CrossRef]
- Chan, F.K.M.; Moriwaki, K.; de Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [CrossRef]
- Ahmad, T.; Aggarwal, K.; Pattnaik, B.; Mukherjee, S.; Sethi, T.; Tiwari, B.K.; Kumar, M.; Micheal, A.; Mabalirajan, U.; Ghosh, B.; et al. Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis. 2013, 4, e461. [Google Scholar] [CrossRef]
- Karbowski, M.; Youle, R.J. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003, 10, 870–880. [Google Scholar] [CrossRef]
- Arnoult, D. Mitochondrial fragmentation in apoptosis. Trends Cell Biol. 2006, 17, 6–12. [Google Scholar] [CrossRef]
- Liu, X.; Hajnoczky, G. Altered fusion dynamics underlie unique morphological changes in mitochondria during hypoxia—Reoxygenation stress. Cell Death Differ. 2011, 18, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Mondol, A.S.; Töpfer, N.; Rüger, J.; Neugebauer, U.; Popp, J.; Schie, I.W. New perspectives for viability studies with high-content analysis Raman spectroscopy (HCA-RS). Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, K.; Hu, H.; Qie, X.; Huang, W.E.; Cui, Z.; Gong, Y.; Song, Y. In vitro anticancer drug sensitivity sensing through single-cell raman spectroscopy. Biosensors 2021, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Ou, Y.C.; Bogatcheva, G.; Thomas, G.; Mahadevan-Jansen, A.; Singh, B.; Lin, E.C.; Bardhan, R. Probing metabolic alterations in breast cancer in response to molecular inhibitors with Raman spectroscopy and validated with mass spectrometry. Chem. Sci. 2020, 11, 9863–9874. [Google Scholar] [CrossRef] [PubMed]
- Botelho, C.M.; Gonçalves, O.; Marques, R.; Thiagarajan, V.; Vorum, H.; Gomes, A.C.; Neves-Petersen, M.T. Photonic modulation of epidermal growth factor receptor halts receptor activation and cancer cell migration. J. Biophotonics 2018, 11, e201700323. [Google Scholar] [CrossRef]
- Czamara, K.; Petko, F.; Baranska, M.; Kaczor, A. Raman microscopy at the subcellular level: Study on early apoptosis in endothelial cells induced by Fas ligand and cycloheximide. Analyst 2016, 141, 1390–1397. [Google Scholar] [CrossRef]
- Abramczyk, H. Double face of cytochrome c in cancers by Raman imaging. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Pansare, K.; Singh, S.R.; Chakravarthy, V.; Gupta, N.; Hole, A.; Gera, P.; Sarin, R.; Krishna, C.M. Raman Spectroscopy: An Exploratory Study to Identify Post Radiation Cell Survival. Appl Spectrosc 2020, 2, 553–562. [Google Scholar] [CrossRef]
- Schie, I.W.; Rüger, J.; Mondol, A.S.; Ramoji, A.; Neugebauer, U.; Krafft, C.; Popp, J. High-Throughput Screening Raman Spectroscopy Platform for Label-Free Cellomics. Anal. Chem. 2018, 90, 2023–2030. [Google Scholar] [CrossRef]
- Jayan, H.; Pu, H.; Sun, D. Recent developments in Raman spectral analysis of microbial single cells: Techniques and applications. Crit. Rev. Food Sci. Nutr. 2021, 62, 4294–4308. [Google Scholar] [CrossRef]
- Goldrick, S.; Umprecht, A.; Tang, A.; Zakrzewski, R.; Cheeks, M.; Turner, R.; Charles, A.; Les, K.; Hulley, M.; Spencer, C.; et al. High-throughput raman spectroscopy combined with innovate data analysis workflow to enhance biopharmaceutical process development. Processes 2020, 8, 1179. [Google Scholar] [CrossRef]
- Verrier, S.; Zoladek, A.; Notingher, I. Raman Micro-Spectroscopy as a Non-invasive Cell Viability Test. In Mammalian Cell Viability. Methods in Molecular Biology (Methods and Protocols); Stoddart, M., Ed.; Humana Press, Springer: New York, NY, USA, 2011; Volume 740, pp. 179–189. [Google Scholar] [CrossRef]
- Grabarek, A.D.; Senel, E.; Menzen, T.; Hoogendoorn, K.H.; Pike-Overzet, K.; Hawe, A.; Jiskoot, W. Particulate impurities in cell-based medicinal products traced by flow imaging microscopy combined with deep learning for image analysis. Cytotherapy 2021, 23, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.J.; Cicalese, S.M.; Davis, H.B.; Dogdas, B.; Shah, T.; Culp, T.; Hoang, V.M. Cell confluency analysis on microcarriers by micro-flow imaging. Cytotechnology 2016, 68, 2469–2478. [Google Scholar] [CrossRef]
- Sediq, A.S.; Klem, R.; Nejadnik, M.R.; Meij, P.; Jiskoot, W. Label-Free, Flow-Imaging Methods for Determination of Cell Concentration and Viability. Pharm. Res. 2018, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Martin, T.; Li, Y.; Yang, L.; Halpenny, M.; Giulivi, A.; Allan, D.S. Cell aggregation in thawed haematopoietic stem cell products visualised using micro-flow imaging. Transfus. Med. 2012, 22, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Grabarek, A.D.; Jiskoot, W.; Hawe, A.; Pike-overzet, K.; Menzen, T. Forced degradation of cell-based medicinal products guided by flow imaging microscopy: Explorative studies with Jurkat cells. Eur. J. Pharm. Biopharm. 2021, 167, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Gambe-gilbuena, A.; Shibano, Y.; Krayukhina, E.; Torisu, T.; Uchiyama, S. Automatic Identi fi cation of the Stress Sources of Protein Aggregates Using Flow Imaging Microscopy Images. J. Pharm. Sci. 2020, 109, 614–623. [Google Scholar] [CrossRef]
- Kühn, J. Digital holographic microscopy real-time monitoring of cytoarchitectural alterations during simulated microgravity. J. Biomed. Opt. 2010, 15, 026021. [Google Scholar] [CrossRef]
- Pais, D.A.M.; Galrão, P.R.S.; Kryzhanska, A.; Barbau, J.; Isidro, I.A.; Alves, P.M. Holographic imaging of insect cell cultures: Online non-invasive monitoring of adeno-associated virus production and cell concentration. Processes 2020, 8, 487. [Google Scholar] [CrossRef]
- Kemper, B.; Carl, D.D.; Schnekenburger, J.; Bredebusch, I.; Schäfer, M.; Domschke, W.; von Bally, G. Investigation of living pancreas tumor cells by digital holographic microscopy. J. Biomed. Opt. 2006, 11, 034005. [Google Scholar] [CrossRef]
- Odete, M.A.; Philips, L. Label-free Viability Assay using Holographic Video Microscopy Label-free Viability Assay using Holographic Video Microscopy. Res. Sq. preprint. 2021. [Google Scholar] [CrossRef]
- Pala, M.A.; Çimen, M.E.; Akgül, A.; Yıldız, M.Z.; Boz, A.F. Fractal dimension-based viability analysis of cancer cell lines in lens-free holographic microscopy via machine. Eur. Phys. J. 2021, 123, 1–12. [Google Scholar] [CrossRef]
- Dubois, F.; Yourassowsky, C.; Monnom, O.; Legros, J.C.; Debeir IV, O.; Van Ham, P.; Kiss, R.; Decaestecker, C. Digital holographic microscopy for the three-dimensional dynamic analysis of in vitro cancer cell migration. J. Biomed. Opt. 2006, 11, 054032. [Google Scholar] [CrossRef]
- Moon, I.; Daneshpanah, M.; Javidi, B.; Stern, A. Automated three-dimensional identification and tracking of micro/nanobiological organisms by computational holographic microscopy. Proc. IEEE 2009, 97, 990–1010. [Google Scholar] [CrossRef]
- Pushkarsky, I.; Liu, Y.; Weaver, W.; Su, T.W.; Mudanyali, O.; Ozcan, A.; Di Carlo, D. Automated single-cell motility analysis on a chip using lensfree microscopy. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Jin, G.; Yoo, I.; Pil, S.; Yang, J.; Ha, U. Biosensors and Bioelectronics Lens-free shadow image based high-throughput continuous cell monitoring technique. Biosens. Bioelectron. 2012, 38, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Bae, H.; Cha, J.M.; Moon, S.J.; Dokmeci, M.R.; Cropek, D.M.; Khademhosseini, A. A cell-based biosensor for real-time detection of cardiotoxicity using lensfree imaging. Lab Chip 2011, 11, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Lee, S.A.; Yang, S.; Yang, C. Sub-pixel resolving optofluidic microscope for on-chip cell imaging. Lab Chip 2010, 10, 3125–3129. [Google Scholar] [CrossRef]
- Cui, X.; Lee, L.M.; Heng, X.; Zhong, W.; Sternberg, P.W.; Psaltis, D.; Yang, C. Lensless high-resolution on-chip optofluidic microscopes for Caenorhabditis elegans and cell imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 10670–10675. [Google Scholar] [CrossRef]
- Ozcan, A.; Demirci, U. Ultra wide-field lens-free monitoring of cells on-chip. Lab Chip 2007, 8, 98–106. [Google Scholar] [CrossRef]
- Kesavan, S.V.; Momey, F.; Cioni, O.; David-Watine, B.; Dubrulle, N.; Shorte, S.; Sulpice, E.; Freida, D.; Chalmond, B.; Dinten, J.M.; et al. High-throughput monitoring of major cell functions by means of lensfree video microscopy. Sci. Rep. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Nablo, B.J.; Ahn, J.J.; Bhadriraju, K.; Lee, J.M.; Reyes, D.R. Lens-Free Imaging as a Sensor for Dynamic Cell Viability Detection Using the Neutral Red Uptake Assay. ACS Appl. Bio Mater. 2020, 3, 6633–6638. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Y.; Xu, X.; Wang, R.; Yao, J.; Han, W.; Wei, M.; Chen, J.; Xuan, W.; Sun, L. High-precision lensless microscope on a chip based on in-line holographic imaging. Sensors 2021, 21, 720. [Google Scholar] [CrossRef] [PubMed]
- Rothbauer, M.; Ertl, P.; Mayr, T. Measurement of respiration and acidification rates of mammalian cells in thermoplastic microfluidic devices. Sens. Actuators B Chem. 2021, 334, 129664. [Google Scholar] [CrossRef]
- O’Riordan, T.C.; Buckley, D.; Ogurtsov, V.; O’Connor, R.; Papkovsky, D.B. A cell viability assay based on monitoring respiration by optical oxygen sensing. Anal. Biochem. 2000, 278, 221–227. [Google Scholar] [CrossRef]
- Bäckman, P.; Wadsö, I. Cell growth experiments using a microcalorimetric vessel equipped with oxygen and pH electrodes. J. Biochem. Biophys. Methods 1991, 23, 283–293. [Google Scholar] [CrossRef]
- Halpern, H.J.; Yu, C.; Peric, M.; Barth, E.D.; Karczmar, G.S.; River, J.N.; Grdina, D.J.; Teicher, B.A. Measurement of differences in pO2 in response to perfluorocarbon/carbogen in FSa and NFSa murine fibrosarcomas with low-frequency electron paramagnetic resonance oximetry. Radiat. Res. 1996, 145, 610–618. [Google Scholar] [CrossRef]
- Braissant, O.; Astasov-frauenhoffer, M.; Waltimo, T. A Review of Methods to Determine Viability, Vitality, and Metabolic Rates in Microbiology. Front. Microbiol. 2020, 11, 547458. [Google Scholar] [CrossRef]
- Randers-Eichhorn, L.; Bartlett, R.A.; Frey, D.D.; Rao, G. Noninvasive oxygen measurements and mass transfer considerations in tissue culture flasks. Biotechnol. Bioeng. 1996, 51, 466–478. [Google Scholar] [CrossRef]
- Wodnicka, M.; Guarino, R.D.; Hemperly, J.J.; Timmins, M.R.; Stitt, D.; Pitner, J.B. Novel fluorescent technology platform for high throughput cytotoxicity and proliferation assays. J. Biomol. Screen. 2000, 5, 141–150. [Google Scholar] [CrossRef]
- Guarino, R.D.; Dike, L.E.; Haq, T.A.; Rowley, J.A.; Pitner, J.B.; Timmins, M.R. Method for determining oxygen consumption rates of static cultures from microplate measurements of pericellular dissolved oxygen concentration. Biotechnol. Bioeng. 2004, 86, 775–787. [Google Scholar] [CrossRef]
- Mishra, A.; Starly, B. Real time in vitro measurement of oxygen uptake rates for HEPG2 liver cells encapsulated in alginate matrices. Microfluid. Nanofluidics 2009, 6, 373–381. [Google Scholar] [CrossRef]
- Super, A.; Jaccard, N.; Marques, M.P.C.; Macown, R.J.; Griffin, L.D.; Veraitch, F.S.; Szita, N. Real-time monitoring of specific oxygen uptake rates of embryonic stem cells in a microfluidic cell culture device. Biotechnol. J. 2016, 11, 1179–1189. [Google Scholar] [CrossRef]
- Mahfouzi, S.H.; Amoabediny, G.; Doryab, A.; Safiabadi-Tali, S.H.; Ghanei, M. Noninvasive Real-Time Assessment of Cell Viability in a Three-Dimensional Tissue. Tissue Eng. Part C Methods 2018, 24, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lei, J.; Xu, X.; Ding, L.; Zhai, C.; Yan, F.; Ju, H. Real-time monitoring of cell viability by its nanoscale height change with oxygen as endogenous indicator. Chem. Commun. 2010, 46, 7388–7390. [Google Scholar] [CrossRef] [PubMed]
- Wadsö, I. Microcalorimetric techniques for characterization of living cellular systems. Will there be any important practical applications? Thermochim. Acta 1995, 269–270, 337–350. [Google Scholar] [CrossRef]
- Braissant, O.; Wirz, D.; Göpfert, B.; Daniels, A.U. Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol. Lett. 2010, 303, 1–8. [Google Scholar] [CrossRef]
- Yang, N.; Shi, Q.; Zhu, X.; Wei, M.; Ullah, I.; Kwabena, P.O.; Kulik, E.; Mao, H.; Zhang, R. A Cell Viability Evaluation Method Based on Respiratory Thermodynamic Feature Detected by Microscopic Infrared Thermal Imaging Sensor. IEEE Sens. J. 2020, 20, 637–647. [Google Scholar] [CrossRef]
- Tan, A.M.; Lu, J.H. Microcalorimetric study of antiviral effect of drug. J. Biochem. Biophys. Methods 1999, 38, 225–228. [Google Scholar] [CrossRef]
- Spaepen, P.; de Boodt, S.; Aerts, J.; Sloten, J.V. Chapter 21 Digital Image Processing of Live/Dead Staining. Mamm. Cell Viability Methods Protoc. Methods Mol. Biol. 2011, 740, 209–230. [Google Scholar] [CrossRef]
- Lemos, D.; Oliveira, T.; Martins, L.; De Azevedo, V.R.; Rodrigues, M.F.; Ketzer, L.A.; Rumjanek, F.D. Isothermal Microcalorimetry of Tumor Cells: Enhanced Thermogenesis by Metastatic Cells. Front. Oncol. 2019, 9, 1430. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Gu, N. Cell Temperature Measurement for Biometabolism Monitoring. ACS Sens. 2021, 6, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Feng, J.; Neuzil, P. Recent advances of microcalorimetry for studying cellular metabolic heat. Trends Anal. Chem. 2021, 143, 116353. [Google Scholar] [CrossRef]
- Ilic, B.; Czaplewski, D.; Craighead, H.G.; Neuzil, P.; Campagnolo, C.; Batt, C. Mechanical resonant immunospecific biological detector. Appl. Phys. Lett. 2000, 77, 450–452. [Google Scholar] [CrossRef]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef]
- Ramos, D.; Tamayo, J.; Mertens, J.; Calleja, M.; Villanueva, L.G.; Zaballos, A. Detection of bacteria based on the thermomechanical noise of a nanomechanical resonator: Origin of the response and detection limits. Nanotechnology 2008, 19, 035503. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Nakajima, M.; Kojima, M.; Kojima, S.; Homma, M.; Fukuda, T. Instantaneous and quantitative single cells viability determination using dual nanoprobe inside ESEM. IEEE Trans. Nanotechnol. 2012, 11, 298–306. [Google Scholar] [CrossRef]
- Shen, Y.; Nakajima, M.; Kojima, S.; Homma, M.; Kojima, M.; Fukuda, T. Single cell adhesion force measurement for cell viability identification using an AFM cantilever-based micro putter. Meas. Sci. Technol. 2011, 22, 944–947. [Google Scholar] [CrossRef]
- Kasas, S.; Ruggeri, F.S.; Benadiba, C.; Maillard, C.; Stupar, P.; Tournu, H.; Dietler, G.; Longo, G. Detecting nanoscale vibrations as signature of life. Proc. Natl. Acad. Sci. USA 2015, 112, 378–381. [Google Scholar] [CrossRef]
- Mader, A.; Gruber, K.; Castelli, R.; Hermann, B.A.; Seeberger, P.H.; Rädler, J.O.; Leisner, M. Discrimination of Escherichia coli strains using glycan cantilever array sensors. Nano Lett. 2012, 12, 420–423. [Google Scholar] [CrossRef]
- Sharma, H.; Mutharasan, R. Rapid and sensitive immunodetection of Listeria monocytogenes in milk using a novel piezoelectric cantilever sensor. Biosens. Bioelectron. 2013, 45, 158–162. [Google Scholar] [CrossRef]
- Ndieyira, J.W.; Kappeler, N.; Logan, S.; Cooper, M.A.; Abell, C.; McKendry, R.A.; Aeppli, G. Surface-stress sensors for rapid and ultrasensitive detection of active free drugs in human serum. Nat. Nanotechnol. 2014, 9, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Maciaszek, J.L.; Andemariam, B.; Abiraman, K.; Lykotrafitis, G. AKAP-dependent modulation of BCAM/Lu adhesion on normal and sickle cell disease RBCs revealed by force nanoscopy. Biophys. J. 2014, 106, 1258–1267. [Google Scholar] [CrossRef]
- Liu, Y.; Schweizer, L.M.; Wang, W.; Reuben, R.L.; Schweizer, M.; Shu, W. Chemical Label-free and real-time monitoring of yeast cell growth by the bending of polymer microcantilever biosensors. Sens. Actuators B. Chem. 2013, 178, 621–626. [Google Scholar] [CrossRef]
- Longo, G.; Alonso-Sarduy, L.; Rio, L.M.; Bizzini, A.; Trampuz, A.; Notz, J.; Dietler, G.; Kasas, S. Rapid detection of bacterial resistance to antibiotics using AFM cantilevers as nanomechanical sensors. Nat. Nanotechnol. 2013, 8, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Riedel, R.; Del Pino, P.; Pelaz, B.; Said, A.H.; Soliman, M.; Pinnapireddy, S.R.; Feliu, N.; Parak, W.J.; Bakowsky, U.; et al. Real-time, label-free monitoring of cell viability based on cell adhesion measurements with an atomic force microscope. J. Nanobiotechnol. 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bennett, I.; Pyne, A.L.B.; McKendry, R.A. Cantilever Sensors for Rapid Optical Antimicrobial Sensitivity Testing. ACS Sensors 2020, 5, 3133–3139. [Google Scholar] [CrossRef]
- Linna, E.; BinAhmed, S.; Stottrup, B.L.; Castrill, S.R.V. Effect of Graphene Oxide Packing on Bacterial Adhesion using Single Cell Force Spectroscopy. Biophys. J. 2018, 114, 352a–353a. [Google Scholar] [CrossRef]
- Evans, E.A.; Calderwood, D.A. Forces and bond dynamics in cell adhesion. Science 2007, 316, 1148–1153. [Google Scholar] [CrossRef]
- Huang, H.; Dai, C.; Shen, H.; Gu, M.; Wang, Y.; Liu, J.; Chen, L.; Sun, L. Recent advances on the model, measurement technique, and application of single cell mechanics. Int. J. Mol. Sci. 2020, 21, 6248. [Google Scholar] [CrossRef]
- Müller, D.J.; Dufrêne, Y.F. Atomic force microscopy: A nanoscopic window on the cell surface. Trends Cell Biol. 2011, 21, 461–469. [Google Scholar] [CrossRef]
- Ungai-Salánki, R.; Peter, B.; Gerecsei, T.; Orgovan, N.; Horvath, R.; Szabó, B. A practical review on the measurement tools for cellular adhesion force. Adv. Colloid Interface Sci. 2019, 269, 309–333. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Hodel, A.W.; Spielhofer, A.; Cattin, C.J.; Müller, D.J.; Helenius, J. Wedged AFM-cantilevers for parallel plate cell mechanics. Methods 2013, 60, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.B.; Collinsworth, A.M.; Reichert, W.M.; Kraus, W.E.; Truskey, G.A. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J. Biomech. 2001, 34, 1545–1553. [Google Scholar] [CrossRef]
- Yang, S.P.; Yang, C.Y.; Lee, T.M.; Lui, T.S. Effects of calcium-phosphate topography on osteoblast mechanobiology determined using a cytodetacher. Mater. Sci. Eng. C 2012, 32, 254–262. [Google Scholar] [CrossRef]
- Sagvolden, G.; Giaever, I.; Pettersen, E.O.; Feder, J. Cell adhesion force microscopy. Proc. Natl. Acad. Sci. USA 1999, 96, 471–476. [Google Scholar] [CrossRef]
- Yamamoto, A.; Mishima, S.; Maruyama, N.; Sumita, M. Quantitative evaluation of cell attachment to glass, polystyrene, and fibronectin- or collagen-coated polystyrene by measurement of cell adhesive shear force and cell detachment energy. J. Biomed. Mater. Res. 2000, 50, 114–124. [Google Scholar] [CrossRef]
- Lee, C.C.; Wu, C.C.; Su, F.C. The Technique for Measurement of Cell Adhesion Force. J. Med. Biol. Eng. 2004, 24, 51–56. [Google Scholar]
- Marcotte, L.; Tabrizian, M. Sensing surfaces: Challenges in studying the cell adhesion process and the cell adhesion forces on biomaterials. Itbm-Rbm 2008, 29, 77–88. [Google Scholar] [CrossRef]
- Elbourne, A.; Chapman, J.; Gelmi, A.; Cozzolino, D.; Crawford, R.J.; Truong, V.K. Bacterial-nanostructure interactions: The role of cell elasticity and adhesion forces. J. Colloid Interface Sci. 2019, 546, 192–210. [Google Scholar] [CrossRef]
- Friedrichs, J.; Legate, K.R.; Schubert, R.; Bharadwaj, M.; Werner, C.; Müller, D.J.; Benoit, M. A practical guide to quantify cell adhesion using single-cell force spectroscopy. Methods 2013, 60, 169–178. [Google Scholar] [CrossRef]
- Ramos, D.; Tamayo, J.; Mertens, J.; Calleja, M.; Zaballos, A. Origin of the response of nanomechanical resonators to bacteria adsorption. J. Appl. Phys. 2006, 100, 106105. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Zhou, X.; Liang, X.M.; Gao, D.; Liu, H.; Zhao, G.; Zhang, Q.; Wu, X. Quantification of cell viability and rapid screening anti-cancer drug utilizing nanomechanical fluctuation. Biosens. Bioelectron. 2016, 77, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Stupar, P.; Chomicki, W.; Maillard, C.; Mikeladze, D.; Kalauzi, A.; Radotić, K.; Dietler, G.; Kasas, S. Mitochondrial activity detected by cantilever based sensor. Mech. Sci. 2017, 8, 23–28. [Google Scholar] [CrossRef]
- Kohler, A.C.; Venturelli, L.; Longo, G.; Dietler, G.; Kasas, S. Nanomotion detection based on atomic force microscopy cantilevers. Cell Surf. 2019, 5, 100021. [Google Scholar] [CrossRef] [PubMed]
- Lissandrello, C.; Inci, F.; Francom, M.; Paul, M.R.; Demirci, U.; Ekinci, K.L. Nanomechanical motion of Escherichia coli adhered to a surface. Appl. Phys. Lett. 2014, 105, 113701. [Google Scholar] [CrossRef]
- Villalba, M.I.; Stupar, P.; Chomicki, W.; Bertacchi, M.; Dietler, G.; Arnal, L.; Vela, M.E.; Yantorno, O.; Kasas, S. Nanomotion Detection Method for Testing Antibiotic Resistance and Susceptibility of Slow-Growing Bacteria. Small 2018, 14, 1702671. [Google Scholar] [CrossRef]
- Stupar, P.; Opota, O.; Longo, G.; Prod’Hom, G.; Dietler, G.; Greub, G.; Kasas, S. Nanomechanical sensor applied to blood culture pellets: A fast approach to determine the antibiotic susceptibility against agents of bloodstream infections. Clin. Microbiol. Infect. 2017, 23, 400–405. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Z.; Zhou, X.; Liu, H.; Xue, C.; Zhao, G.; Cao, Y.; Zhang, Q.; Wu, X. Nanomechanical sensors for direct and rapid characterization of sperm motility based on nanoscale vibrations. Nanoscale 2017, 9, 18258–18267. [Google Scholar] [CrossRef]
- Vannocci, T.; Dinarelli, S.; Girasole, M.; Pastore, A.; Longo, G. A new tool to determine the cellular metabolic landscape: Nanotechnology to the study of Friedreich’s ataxia. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Ruggeri, F.S.; Mahul-Mellier, A.L.; Kasas, S.; Lashuel, H.A.; Longo, G.; Dietler, G. Amyloid single-cell cytotoxicity assays by nanomotion detection. Cell Death Discov. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Alonso-Sarduy, L.; De Los Rios, P.; Benedetti, F.; Vobornik, D.; Dietler, G.; Kasas, S.; Longo, G. Real-time monitoring of protein conformational changes using a nano-mechanical sensor. PLoS ONE 2014, 9, e103674. [Google Scholar] [CrossRef] [PubMed]
- Aghayee, S.; Benadiba, C.; Notz, J.; Kasas, S.; Dietler, G.; Longo, G. Combination of fluorescence microscopy and nanomotion detection to characterize bacteria. J. Mol. Recognit. 2013, 26, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Mustazzolu, A.; Venturelli, L.; Dinarelli, S.; Brown, K.; Floto, R.A.; Dietler, G.; Fattorini, L.; Kasas, S.; Girasole, M.; Longo, G. A rapid unraveling of the activity and antibiotic susceptibility of mycobacteria. Antimicrob. Agents Chemother. 2019, 63, e02194-18. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.; Cuervo, A.; Carrascosa, J.L. Nanomechanical detection of: Escherichia coli infection by bacteriophage T7 using cantilever sensors. Nanoscale 2019, 11, 17689–17698. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.C.; Venturelli, L.; Kannan, A.; Sanglard, D.; Dietler, G.; Willaert, R.; Kasas, S. Yeast nanometric scale oscillations highlights fibronectin induced changes in C. Albicans. Fermentation 2020, 6, 28. [Google Scholar] [CrossRef]
- Villalba, M.I.; Venturelli, L.; Willaert, R.; Vela, M.E.; Yantorno, O.; Dietler, G.; Longo, G.; Kasas, S. Nanomotion spectroscopy as a new approach to characterize bacterial virulence. Microorganisms 2021, 9, 1545. [Google Scholar] [CrossRef]
- Venturelli, L.; Harrold, Z.R.; Murray, A.E.; Villalba, M.I.; Lundin, E.M.; Dietler, G.; Kasas, S.; Foschia, R. Nanomechanical bio-sensing for fast and reliable detection of viability and susceptibility of microorganisms. Sensors Actuators B Chem. 2021, 348, 130650. [Google Scholar] [CrossRef]
- Stupar, P. Atomic Force Microscopy of Biological Systems: Quantitative Imaging and Nanomotion Detection. EPFL 2018, 8334, 1–133. [Google Scholar]
- Lukacs, G.; Maloney, N.; Hegner, M. Ink-jet printing: Perfect tool for cantilever array sensor preparation for microbial growth detection. J. Sens. 2012, 3, 276–283. [Google Scholar] [CrossRef]
- Maciaszek, J.L.; Partola, K.; Zhang, J.; Andemariam, B.; Lykotrafitis, G. Single-cell force spectroscopy as a technique to quantify human red blood cell adhesion to subendothelial laminin. J. Biomech. 2014, 47, 3855–3861. [Google Scholar] [CrossRef]
- Zanetti, M.; Chen, S.N.; Conti, M.; Taylor, M.R.; Sbaizero, O.; Mestroni, L.; Lazzarino, M. Microfabricated cantilevers for parallelized cell—cell adhesion measurements. Eur. Biophys. J. 2021, 51, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.L.; Proctor, D.T.; Ghasemloonia, A.; Lama, S.; Zareinia, K.; Ahn, Y.; Al-Saiedy, M.R.; Green, F.H.; Amrein, M.W.; Sutherland, G.R. Vibrational profiling of brain tumors and cells. Theranostics 2017, 7, 2417–2430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Braun, T.; Ghatkesar, M.K.; Backmann, N.; Grange, W.; Boulanger, P.; Letellier, L.; Lang, H.P.; Bietsch, A.; Gerber, C.; Hegner, M. Quantitative time-resolved measurement of membrane protein-ligand interactions using microcantilever array sensors. Nat. Nanotechnol. 2009, 4, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Pelling, A.E.; Sehati, S.; Gralla, E.B.; Gimzewski, J.K. Time dependence of the frequency and amplitude of the local nanomechanical motion of yeast. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 178–183. [Google Scholar] [CrossRef] [PubMed]





| Attachment Protocol | Incubation Condition | Advantages | Drawbacks | Ref. |
|---|---|---|---|---|
| Cantilever incubated in sample medium outside of the AFM test room | The adhesion process is carried out under different conditions of the chemical effect process | Easy and no need for expensive equipment | The location and number of cells or bacteria cannot be controlled; When handling and installing the cantilever, there is a risk of contamination, sample death, or cantilever damage | [143,148,149,151,153,154,155,157,158,159] |
| Inject sample medium inside the test room | The adhesion and chemical effect processes are carried out in the same test room and under the same conditions | All measurement processes are carried out under the same conditions; There is no risk of contamination or death of cells or bacteria | The location and number of cells or bacteria cannot be controlled; Requires high sample concentration | [126,127,150,156] |
| The micrometric motors of the AFM—AFM single-cell force spectroscopy | The adhesion and chemical effect processes are carried out in the same test room and under the same conditions | The location and number of cells or bacteria can be controlled; It is a single-cell and multi-cell measurement process | Complex and expensive equipment; There is a risk of cell injury during the adhesion process; A sample is limited by its size and by cantilever size | [120,151,152] |
| Ink-jet printing | The adhesion and chemical effect processes are carried out in the same test room and under the same conditions | The location of cells or bacteria can be controlled; There is no risk of contamination or death of cells or bacteria | Complex and expensive equipment is needed; The number of cells or bacteria cannot be controlled | [161,165] |
| Measurement Method | Principle | Features |
|---|---|---|
| Chemical viability assays | Injection of chemical compound(s) into living samples and evaluation of sample interaction with these compound(s) |
|
| Raman spectroscopy | Detection of morphological changes |
|
| Flow imaging microscopy | Detection of morphological changes of living samples while the sample fluid is in a continuous flow |
|
| Holography | Detection of rapid changes in living sample structure parameters resulting from mechanical or morphological changes |
|
| On-chip, lensless video microscopy technology | Detection and evaluation of changes in the shadows of living samples |
|
| Respiratory measuring methods | Detection of the oxygen absorbed and consumed by a living sample |
|
| Microcalorimeter measuring methods | Detection of the resulting heat from a living sample |
|
| Nanomotion | Take advantage of the AFM cantilever’s high sensitivity to changes in mass caused by sample adherence to the cantilever surface |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-madani, H.; Du, H.; Yao, J.; Peng, H.; Yao, C.; Jiang, B.; Wu, A.; Yang, F. Living Sample Viability Measurement Methods from Traditional Assays to Nanomotion. Biosensors 2022, 12, 453. https://doi.org/10.3390/bios12070453
Al-madani H, Du H, Yao J, Peng H, Yao C, Jiang B, Wu A, Yang F. Living Sample Viability Measurement Methods from Traditional Assays to Nanomotion. Biosensors. 2022; 12(7):453. https://doi.org/10.3390/bios12070453
Chicago/Turabian StyleAl-madani, Hamzah, Hui Du, Junlie Yao, Hao Peng, Chenyang Yao, Bo Jiang, Aiguo Wu, and Fang Yang. 2022. "Living Sample Viability Measurement Methods from Traditional Assays to Nanomotion" Biosensors 12, no. 7: 453. https://doi.org/10.3390/bios12070453
APA StyleAl-madani, H., Du, H., Yao, J., Peng, H., Yao, C., Jiang, B., Wu, A., & Yang, F. (2022). Living Sample Viability Measurement Methods from Traditional Assays to Nanomotion. Biosensors, 12(7), 453. https://doi.org/10.3390/bios12070453






