Dual Molecular Design toward a Lysosome-Tagged AIEgen and Heavy-Atom-Free Photosensitizers for Hypoxic Cancer Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
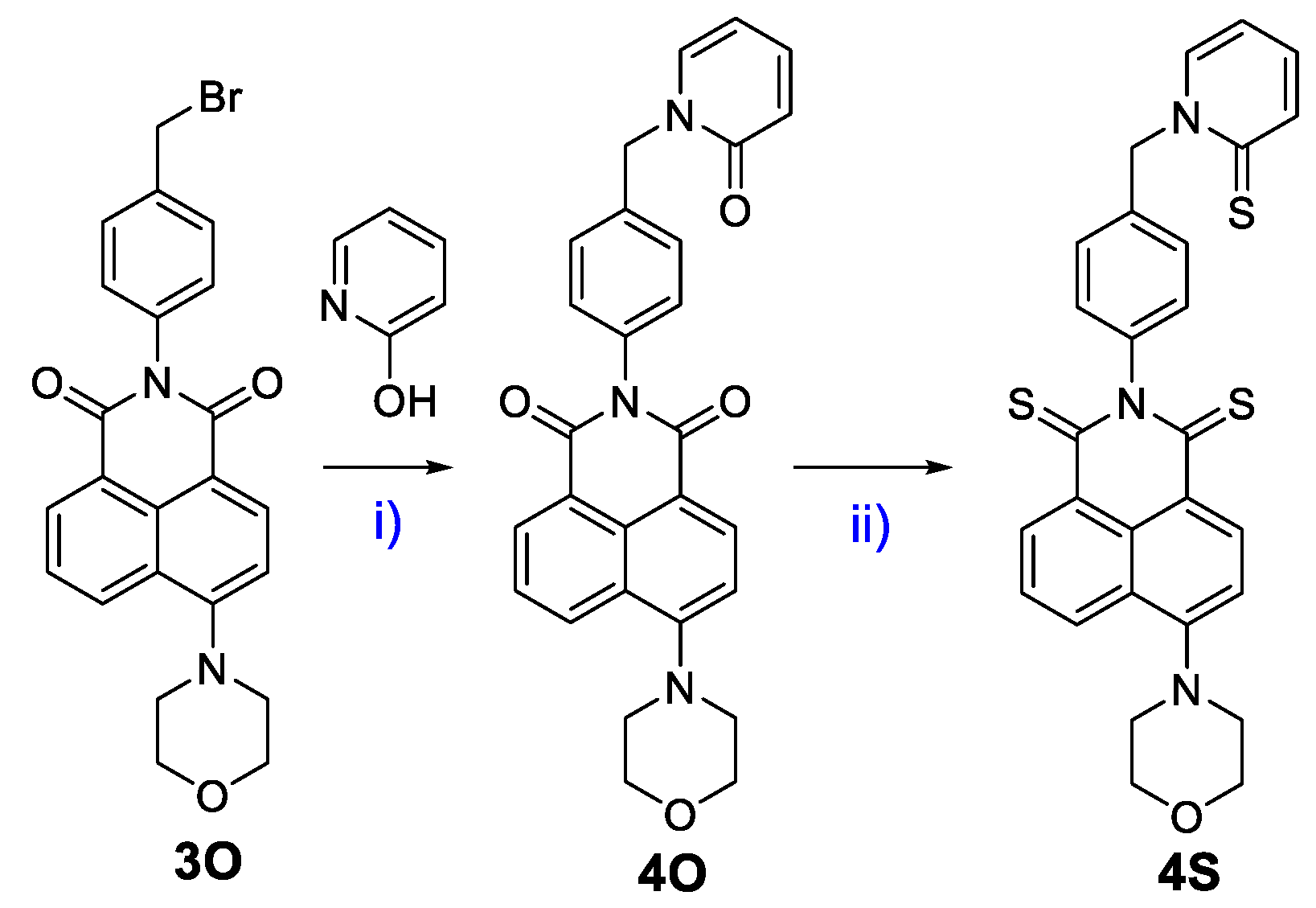
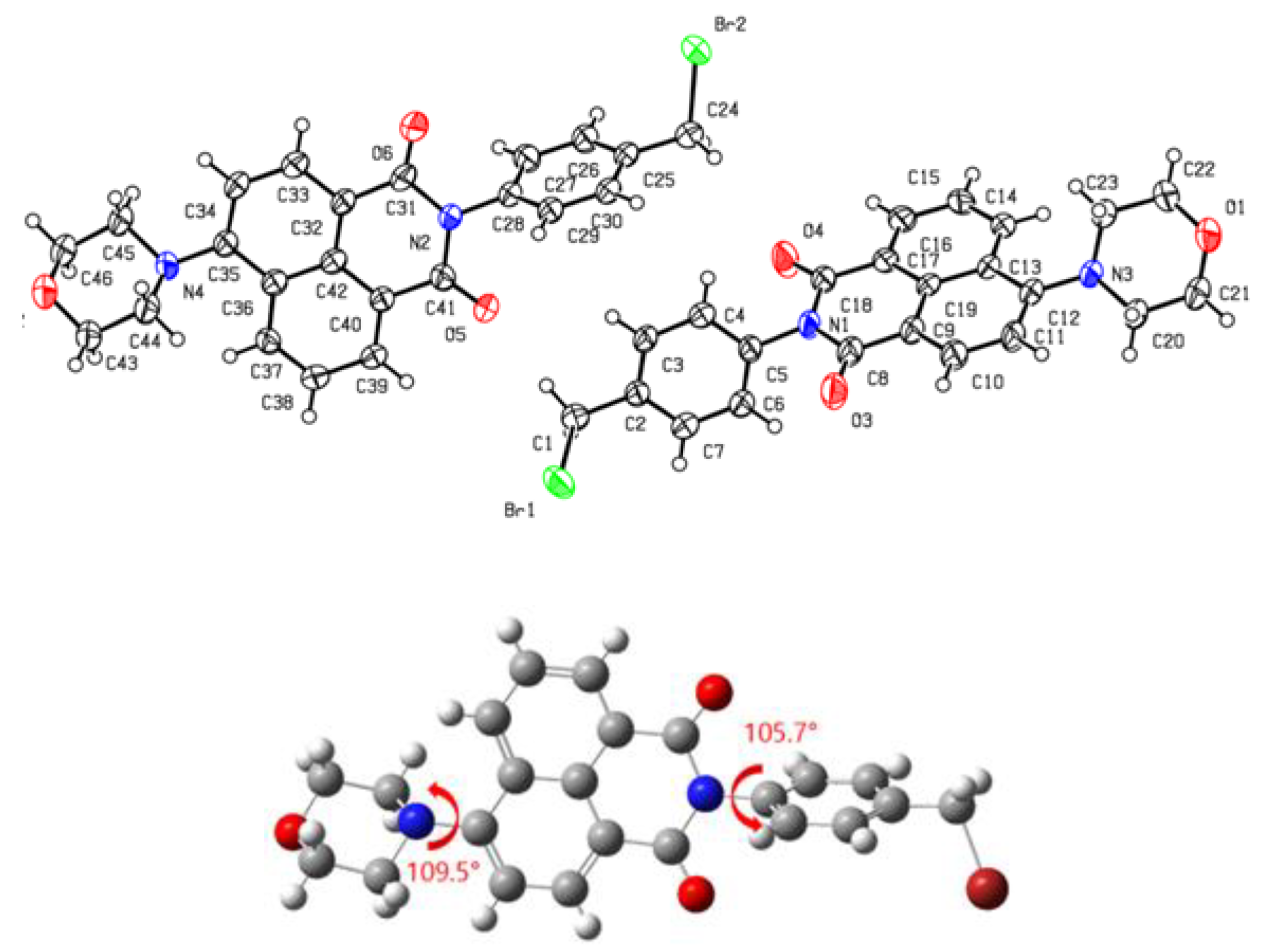
2.1. Synthesis of 4S
2.2. Cell Experiments
2.3. Confocal Microscopy Cell Imaging
2.4. Cell Viability
2.5. Theoretical Calculation
3. Results and Discussion
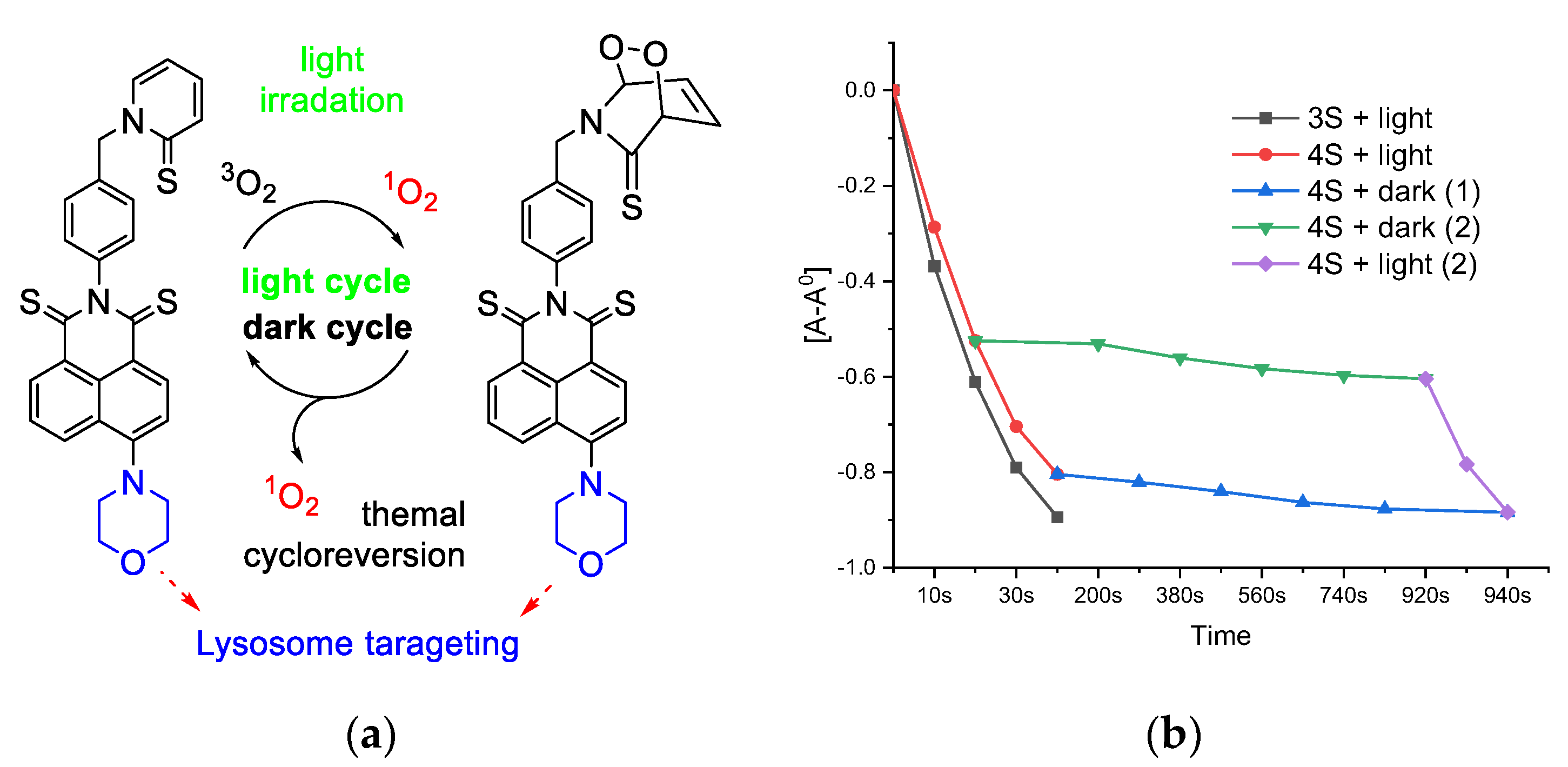
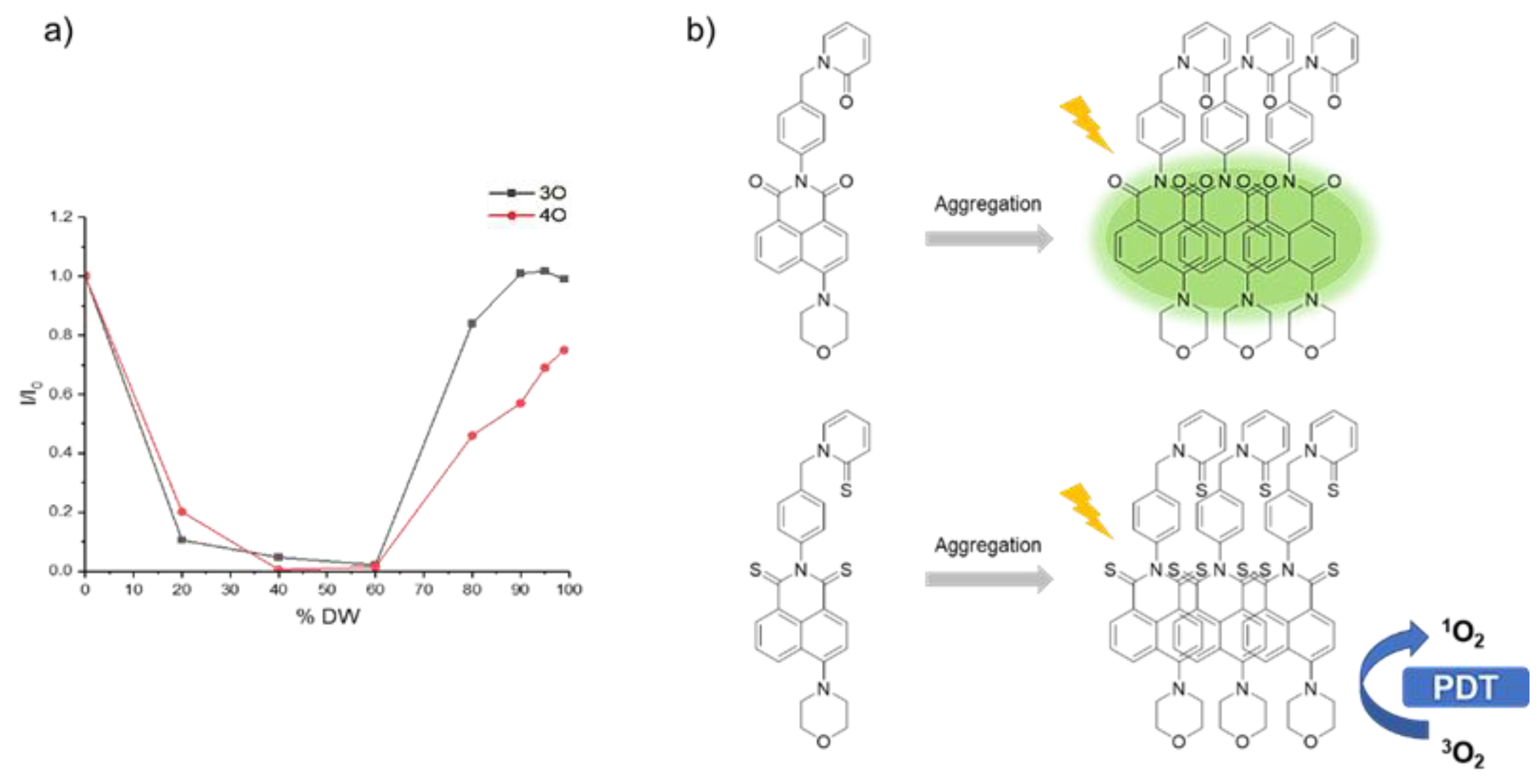
3.1. Molecular Design, Synthesis Process, and Photophysical Properties
3.2. In Vitro Experiment
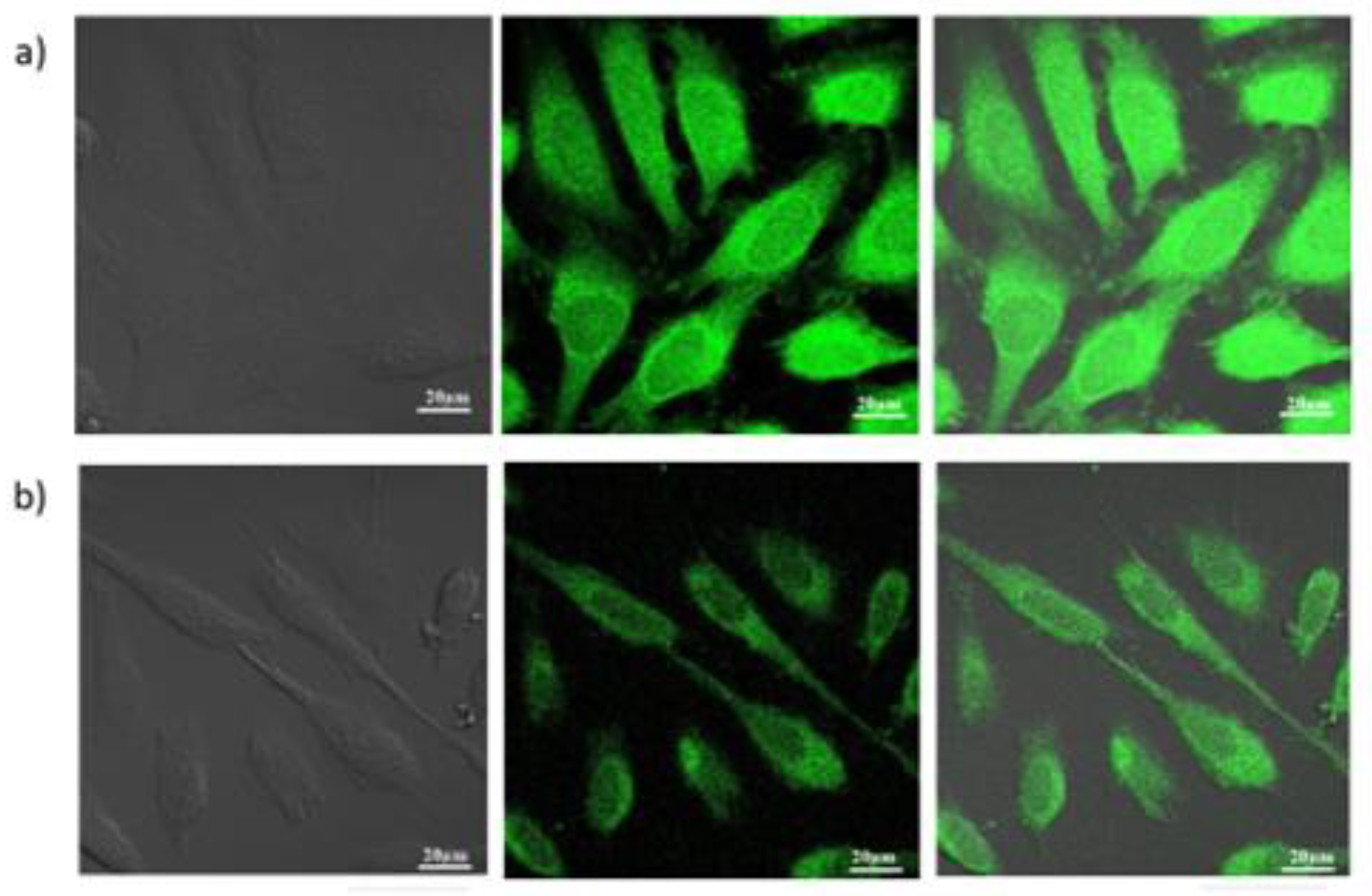
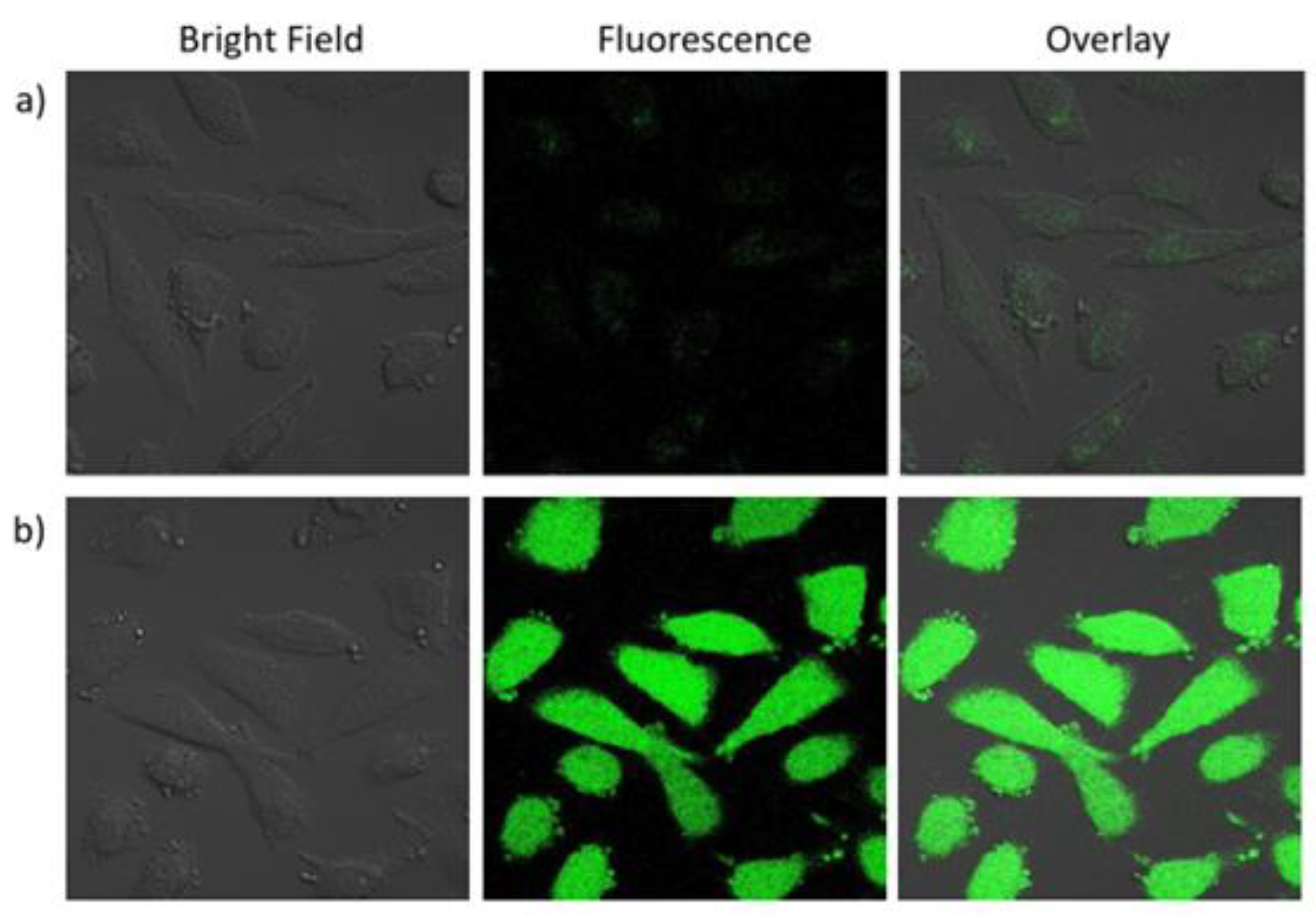
3.2.1. Cell Imaging
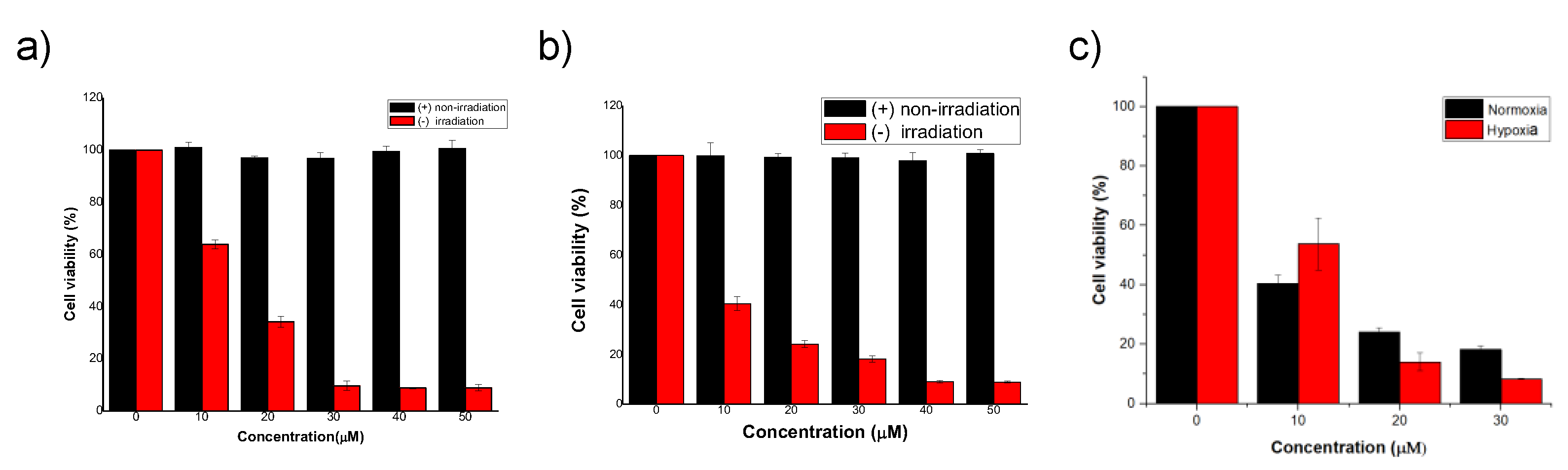
3.2.2. PDT in Normoxia and Hypoxia
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farrell, K.M.; Brister, M.M.; Pittelkow, M.; Sølling, T.I.; Crespo-Hernandez, C.E. Heavy-Atom-Substituted Nucleobases in Photodynamic Applications: Substitution of Sulfur with Selenium in 6-Thioguanine Induces a Remarkable Increase in the Rate of Triplet Decay in 6-Selenoguanine. J. Am. Chem. Soc. 2018, 140, 11214–11218. [Google Scholar] [CrossRef] [PubMed]
- Karran, P.; Attard, N. Thiopurines in current medical practice: Molecular mechanisms and contributions to therapy-related cancer. Rev. Cancer 2008, 8, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.Z.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Feng, G.; Qin, W.; Tang, B.Z.; Liu, B. Targeted and image-guided photodynamic cancer therapy based on organic nanoparticles with aggregation-induced emission characteristics. Chem. Commun. 2014, 50, 8757–8760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.; Qin, A.; Tang, T.; Tang, B.Z. Aggregation-induced emission: The whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Hu, F.; Huang, Y.; Zhang, G.; Zhao, G.; Yang, H.; Zhang, D. Targeted Bioimaging and Photodynamic Therapy of Cancer Cells with an Activatable Red Fluorescent Bioprobe. Anal. Chem. 2014, 86, 7987–7995. [Google Scholar] [CrossRef]
- Chang, C.C.; Hsieh, M.C.; Lin, J.C.; Chang, T.C. Selective photodynamic therapy based on aggregation-induced emission enhancement of fluorescent organic nanoparticles. Biomaterials 2012, 33, 897–906. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Chien, C.-H.; Chang, C.-C.; Chang, T.-C. Aggregation induced photodynamic therapy enhancement based on linear and nonlinear excited FRET of fluorescent organic nanoparticles. J. Mater. Chem. B 2013, 1, 2350–2357. [Google Scholar] [CrossRef] [Green Version]
- Pandey, N.K.; Xiong, W.; Wang, L.; Chen, W.; Bui, B.; Yang, J.; Amador, E.; Chen, M.; Xing, C.; Athavale, A.A.; et al. Aggregation-induced emission luminogens for highly effective microwave dynamic therapy. Bioact. Mater. 2022, 7, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Liu, H.-W.; Hu, X.-X.; Li, K.; Liu, Y.; Rong, Q.; Zhu, L.; Yuan, L.; Qu, F.-L.; Zhang, X.-B.; Tan, W. A mitochondrial-targeted prodrug for NIR imaging guided and synergetic NIR photodynamic-chemo cancer therapy. Chem. Sci. 2017, 8, 7689–7695. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.-N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Yim, Y.; Kim, S.; Ryu, B.; Swamy, K.M.K.; Kim, G.; Kwon, N.; Kim, C.-Y.; Park, S.; Yoon, J. Molecular Design of Highly Efficient Heavy-Atom-Free Triplet BODIPY Derivatives for Photodynamic Therapy and Bioimaging. Angew. Chem. Int. Ed. 2020, 59, 8957–8962. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- Li, X.; Kolemen, S.; Yoon, J.; Akkaya, E.U. Activatable Photosensitizers: Agents for Selective Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1604053–1604063. [Google Scholar] [CrossRef]
- Luby, B.M.; Walsh, C.D.; Zheng, G. Advanced Photosensitizer Activation Strategies for Smarter Photodynamic Therapy Beacons. Angew. Chem. Int. Ed. 2019, 58, 2558–2569. [Google Scholar] [CrossRef]
- Gorman, A.; Killoran, J.; O’Shea, C.; Kenna, T.; Gallagher, W.M.; O’Shea, D.F. In Vitro Demonstration of the Heavy-Atom Effect for Photodynamic Therapy. J. Am. Chem. Soc. 2004, 126, 10619–10631. [Google Scholar] [CrossRef] [PubMed]
- Bertout, J.A.; Patel, S.A.; Simon, M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 2008, 8, 967–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northcott, J.M.; Dean, I.S.; Mouw, J.K.; Weaver, V.M. Feeling Stress: The Mechanics of Cancer Progression and Aggression. Front. Cell Dev. Biol. 2018, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Xu, S.; Liu, B. Photosensitizers with Aggregation-Induced Emission: Materials and Biomedical Applications. Adv. Mater. 2018, 30, 1801350. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef] [PubMed]
- Lawetz, V.; Orlandi, G.; Siebrand, W. Theory of Intersystem Crossing in Aromatic Hydrocarbons. J. Chem. Phys. 1971, 56, 4058. [Google Scholar] [CrossRef]
- Beljonne, D.; Shuai, Z.; Pourtois, G.; Bredas, J.L. Spin-Orbit Coupling and Intersystem Crossing in Conjugated Polymers: A Configuration Interaction Description. J. Phys. Chem. A 2001, 105, 3899–3907. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Baek, G.; Qi, S.; Heo, S.; Yim, Y.; Yoon, J. A lysosome-localized thionaphthalimide as a potential heavy-atom-free photosensitizer for selective photodynamic therapy. Dyes Pigm. 2020, 177, 108265. [Google Scholar] [CrossRef]
- Turan, I.S.; Yildiz, D.; Turksoy, A.; Gunaydin, G.; Akkaya, E.U. A Bifunctional Photosensitizer for Enhanced Fractional Photodynamic Therapy: Singlet Oxygen Generation in the Presence and Absence of Light. Angew. Chem. Int. Ed. 2016, 55, 2875–2878. [Google Scholar] [CrossRef]
- Pham, T.C.; Heo, S.; Nguyen, V.-N.; Lee, M.W.; Yoon, J.; Lee, S. Molecular Design toward Heavy-Atom-free Photosensitizers Based on the C=S Bond and their Dual Functions in Hypoxia Photodynamic Cancer Therapy and ClO− Detection. ACS Appl. Mater. Interfaces 2021, 13, 13949–13957. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Kim, D.; Jung, O.-S.; Lee, D.J.; Kim, H.J.; Lee, M.W.; Yoon, J.; Kim, H.M.; et al. Hypochlorite-Activated Fluorescence Emission and Antibacterial Activities of Imidazole Derivatives for Biological Applications. Front. Chem. 2021, 9, 713078. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-N.; Qi, S.; Kim, S.; Kwon, N.; Kim, G.; Yim, Y.; Park, S.; Yoon, J. An Emerging Molecular Design Approach to Heavy-Atom-Free Photosensitizers for Enhanced Photodynamic Therapy under Hypoxia. J. Am. Chem. Soc. 2019, 141, 16243–16248. [Google Scholar] [CrossRef] [PubMed]
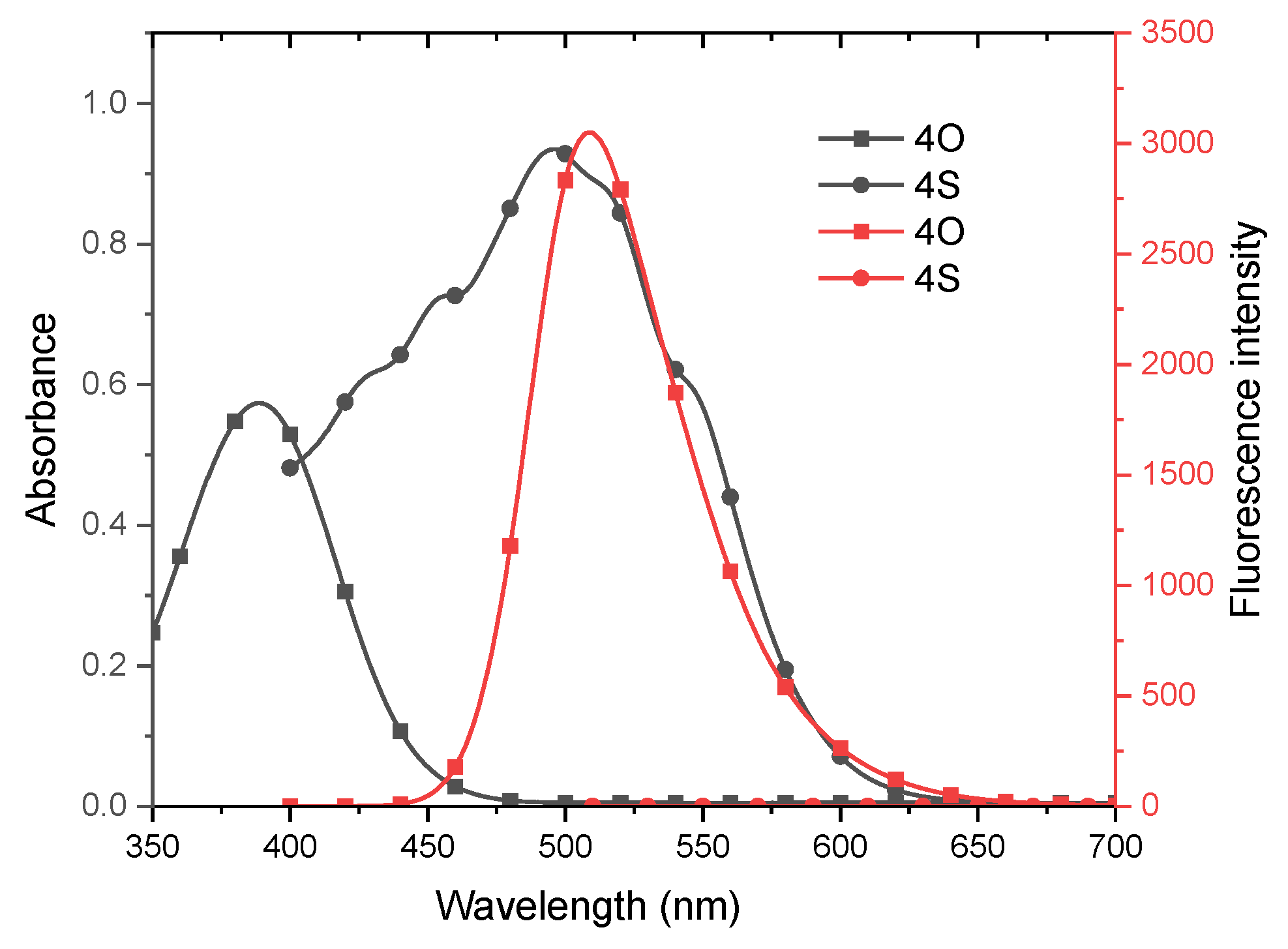

| λabsa (nm) | ԑ × 103 (M−1 cm−1) | λems a (nm) | ∆v b (nm) | ՓF a,c | Eg d (eV) | kISCe | Փ∆ f | |
|---|---|---|---|---|---|---|---|---|
| 3O | 388 | 11.96 | 509 | 121 | 0.94 | 3.58 | 5.1 × 1010 | - |
| 3S | 496 | 20.20 | - | - | - | 2.47 | 9.7 × 1012 | 0.56 |
| 4O | 389 | 13.48 | 509 | 120 | 0.98 | 3.40 | 3.9 × 108 | - |
| 4S | 496 | 23.37 | - | - | - | 2.29 | 5.6 × 1012 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.C.; Hoang, T.T.H.; Choi, Y.; Lee, S.; Joo, S.-W.; Kim, G.; Kim, D.; Jung, O.-S.; Lee, S. Dual Molecular Design toward a Lysosome-Tagged AIEgen and Heavy-Atom-Free Photosensitizers for Hypoxic Cancer Photodynamic Therapy. Biosensors 2022, 12, 420. https://doi.org/10.3390/bios12060420
Pham TC, Hoang TTH, Choi Y, Lee S, Joo S-W, Kim G, Kim D, Jung O-S, Lee S. Dual Molecular Design toward a Lysosome-Tagged AIEgen and Heavy-Atom-Free Photosensitizers for Hypoxic Cancer Photodynamic Therapy. Biosensors. 2022; 12(6):420. https://doi.org/10.3390/bios12060420
Chicago/Turabian StylePham, Thanh Chung, Thi Thuy Hang Hoang, Yeonghwan Choi, Seongman Lee, Sang-Woo Joo, Gun Kim, Dongwon Kim, Ok-Sang Jung, and Songyi Lee. 2022. "Dual Molecular Design toward a Lysosome-Tagged AIEgen and Heavy-Atom-Free Photosensitizers for Hypoxic Cancer Photodynamic Therapy" Biosensors 12, no. 6: 420. https://doi.org/10.3390/bios12060420
APA StylePham, T. C., Hoang, T. T. H., Choi, Y., Lee, S., Joo, S.-W., Kim, G., Kim, D., Jung, O.-S., & Lee, S. (2022). Dual Molecular Design toward a Lysosome-Tagged AIEgen and Heavy-Atom-Free Photosensitizers for Hypoxic Cancer Photodynamic Therapy. Biosensors, 12(6), 420. https://doi.org/10.3390/bios12060420





