Naked-Eye Chromogenic Test Strip for Cyanide Sensing Based on Novel Phenothiazine Push–Pull Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
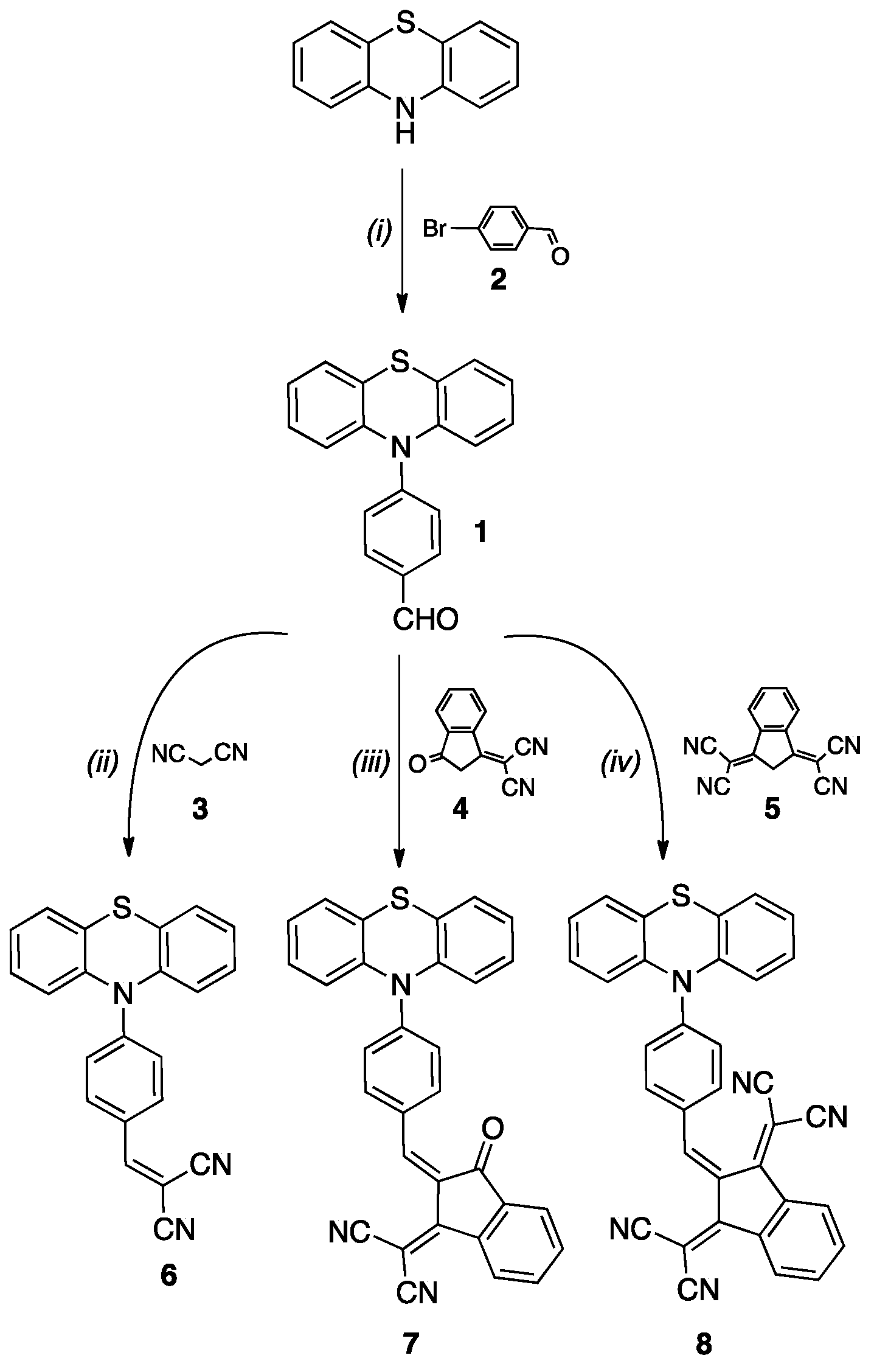
2.2. Synthesis
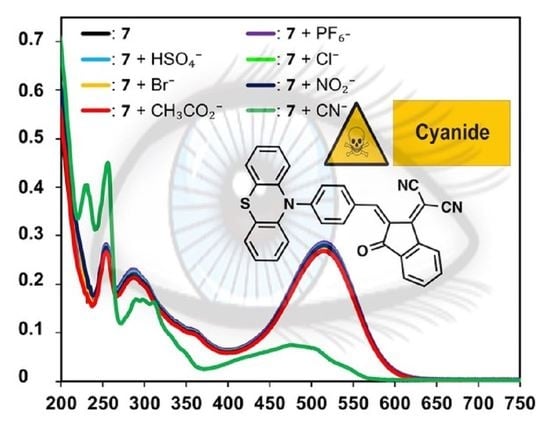
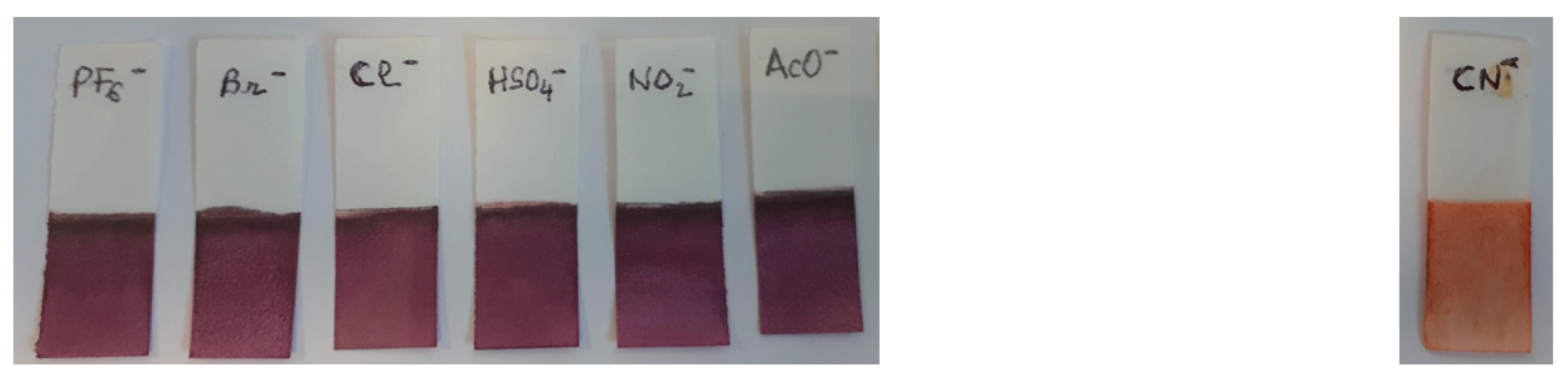
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mangani, S.; Ferraroni, M. Supramolecular Chemistry of Anions; Bianchi, A., Bowman-James, K., Garcia-Espana, E., Eds.; John Wiley-VCH: New York, NY, USA, 1997; Chapter 3; p. 63. [Google Scholar]
- Wu, J.; Kwon, B.; Liu, W.; Anslyn, E.V.; Wang, P.; Kim, J.S. Chromogenic/fluorogenic ensemble chemosensing systems. Chem. Rev. 2015, 115, 7893–7943. [Google Scholar] [CrossRef] [PubMed]
- Cliff, J.; Nzwalo, H.; Muquingue, H. Cyanide in the production of long-term adverse health effects in humans. In Toxicology of Cyanides and Cyanogens; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; Chapter 7; pp. 98–112. [Google Scholar]
- Puljak, L.; Kilic, G. Emerging roles of chloride channels in human diseases. Biochim. Biophys. Acta 2006, 1762, 404–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, M.L.; Salminen, W.F.; Larson, P.R.; Barter, R.A.; Kranetz, J.L.; Simon, G.S. Toxicological review of inorganic phosphates. Food Chem. Toxicol. 2001, 39, 759–786. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality 2017. Available online: https://apps.who.int/iris/handle/10665/254637 (accessed on 20 April 2022).
- Zagrobelny, M.; Bak, S.; Møller, B.L. Cyanogenesis in plants and arthropods. Phytochemistry 2008, 69, 1457–1468. [Google Scholar] [CrossRef]
- Jaszczak, E.; Kozioł, K.; Kiełbratowska, B.; Polkowska, Z. Appliation of ion chromatography with pulsed amperometric detection for the determination of trace cyanide in biological samples, including breast milk. J. Chromatogr. B 2019, 1110–1111, 36–42. [Google Scholar] [CrossRef]
- Zhang, Q.; Maddukuri, N.; Gong, M. A direct and rapid method to determine cyanide in urine by capillary electrophoresis. J. Chromatogr. A 2015, 1414, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Udhayakumari, D. Chromogenic and fluorogenic chemosensors for lethal cyanide ion. A comprehensive review of the year 2106. Sens. Actuators B Chem. 2018, 259, 1022–1057. [Google Scholar] [CrossRef]
- Molecular Recognition in Supramolecular Chemistry: From Molecules to Nanomaterials; Gale, P.; Steed, J. (Eds.) John Wiley & Sons, Ltd.: London, UK, 2012. [Google Scholar]
- Jackson, R.; Logue, B.A. A review of rapid and field-portable analytical techniques for the diagnosis of cyanide exposure. Anal. Chim. Acta 2017, 960, 18–39. [Google Scholar] [CrossRef]
- Bencini, A.; Lippolis, V. Metal-based optical chemosensors for CN− detection. Environ. Sci. Pollut. Res. 2016, 23, 24451–24475. [Google Scholar] [CrossRef]
- Touceda-Varela, A.; Stevenson, E.I.; Galve-Gasion, J.A.; Dryden, D.T.; Mareque-Rivas, J.C. Selective turn-on fluorescence detection cyanide in water using hydrophobic CdSe quantum dots. Chem. Commun. 2008, 17, 1998–2000. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Liu, X.; Valencia, M.A.; Sui, B.; Zhang, Y.; Belfield, K.D. Far-red-emitting TEG-substituted squaraine dye: Synthesis, optical properties, and selective detection of cyanide in aqueous solution. Eur. J. Org. Chem. 2017, 2017, 3957–3964. [Google Scholar] [CrossRef]
- Yang, Y.-K.; Tae, J. Acrinium salt based fluorescent and colorimetric chemosensor for the detection of cyanide in water. Org. Lett. 2006, 8, 5721–5723. [Google Scholar] [CrossRef]
- Mouradzadegun, A.; Abadast, F. An improved organic/inorganic solid receptor for colorimetric cyanide-chemosensing in water: Towards new mechanism aspects, simplistic use and portability. Chem. Commun. 2014, 50, 5983–15986. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, Z.; Pan, X.; Dong, W.; Jia, A.-Q. A novel colorimetric and fluorescent probe based on indolium salt for detection of cyanide in 100% aqueous solution. Dyes Pigm. 2019, 168, 175–179. [Google Scholar] [CrossRef]
- Prakash, K.; Ranjan Sahoo, P.; Kumar, S. A fast, highly selective and sensitive anion probe stemmed from anthracene-oxazine conjugation with CN- induced FRET. Dye. Pigm. 2017, 143, 393–400. [Google Scholar] [CrossRef]
- Hao, Y.; Nguyen, K.H.; Zhang, Y.; Zhang, G.; Fan, S.; Li, F.; Guo, C.; Lu, Y.; Song, X.; Qu, P.; et al. A highly selective and ratiometric fluorescent probe for cyanide by rationally altering the susceptible H-atom. Talanta 2018, 176, 234–241. [Google Scholar] [CrossRef]
- Orrego-Hernaández, J.; Portilla, J. Synthesis of dicyanovinyl-substituted 1-(2-pyridyl)pyrazoles: Design of a fluorescent chemosensor for selective recognition of cyanide. J. Org. Chem. 2017, 82, 13376–13385. [Google Scholar] [CrossRef]
- Garg, B.; Yan, L.; Bisht, T.; Zhu, C.; Ling, Y.-C. A phenothiazine-based colorimetric chemodosimeter for the rapid detection of cyanide anions in organic and aqueous media. RSC Adv. 2014, 4, 36344–36349. [Google Scholar] [CrossRef]
- Zou, Q.; Li, X.; Xu, Q.; Agren, H.; Zhao, W.; Qu, Y. A near-infrared “on-off” fluorescent and colourimetriccyanide chemodosimeter based on phenothiazine with applications in living cell imaging. RSC Adv. 2014, 4, 59809–59816. [Google Scholar] [CrossRef]
- Garg, B.; Ling, Y.-C. A highly selective phenothiazine-based fluorescence “turn-on” indicator based on cyaide-promoted novel protection/deprotection mechanism. Chem. Commun. 2015, 51, 8809–8812. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Wu, F.; Zhao, Y.; Liu, Y.; Zhu, L. Phenothiazine-cyanine-functionalized upconversion nanoparticles for LRET and colorimetric sensing of cyanide ions in water samples. J. Photochem. Photobiol. A 2016, 319–320, 53–61. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Al-Zahrani, F.A.M.; Al-Amshany, Z.M.; Asiri, A.M. Synthesis of a new fluorescent cyanide chemosensor based on phenothiazine derivative. Sens. Actuators B Chem. 2017, 240, 288–296. [Google Scholar] [CrossRef]
- Ramachandran, E.; Vandarkuzhali, S.A.A.; Sivaraman, G.; Dhamodharan, R. Phenothiazine based donor-acceptor compounds with solid-state emission in the yellow to NIR region and their highly selective and sensitive detection of cyanide ion in ppb level. Chem. Eur. J. 2018, 24, 11042–11050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-W.; Wu, G.; Sun, X.-Y.; Chao, J.-B.; Li, Y.Q.; Jiang, L.; Han, H. A highly selective and ratiometric molecular probe for cyanide sensing based on a phenothiazine-hemicyanine dye. J. Lumin. 2018, 201, 474–478. [Google Scholar] [CrossRef]
- Zhu, L.; Nie, J.; Li, Q.; Du, J.; Fan, X.; Bai, F.; Yang, Q.; Shan, Y.; Li, Y. Reaction-based fluorescent probe for differential detection of cyanide and bisulfite in the aqueous media. J. Lumin. 2019, 215, 116620. [Google Scholar] [CrossRef]
- Al-Zahrani, F.A.M. Selective “turn-on” fluorescent sensor for cyanide in aqueous environment and test strips. J. Fluoresc. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Al-Soliemy, A.M. Novel asymmetrical phenothiazine for fluorescent detection of cyanide anions. J. Mol. Struct. 2019, 1179, 525–531. [Google Scholar] [CrossRef]
- Suganya, S.; Ravindran, E.; Mahato, M.K.; Prasad, E. Orange emitting fluorescent probe for the selective detection of cyanide ion in solution and solid states. Sens. Actuators B Chem. 2019, 291, 426–432. [Google Scholar] [CrossRef]
- Al-Zahratni, F.A.M.; El-Shishtawy, R.M.; Asiri, A.M.; Al-Soliemy, A.M.; Mellah, K.A.; Ahmed, N.S.E.; Jedidi, A. A new phenothiazine-based selective visual and fluorescent sensor for cyanide. BMC Chem. 2020, 14, 2. [Google Scholar]
- Olivieri, A.C. Practical guidelines for reporting results in single-and multi-component analytical calibration: A tutorial. Anal. Chim. Acta 2015, 868, 10–22. [Google Scholar] [CrossRef]
- Allegrini, F.; Olivieri, A.C. IUPAC-consistent approach to the limit of detection in partial least-squares calibration. Anal. Chem. 2014, 86, 7858–7866. [Google Scholar] [CrossRef] [PubMed]
- Fédération Sciences Chimiques Marseille. Available online: https://fr-chimie.univ-amu.fr/spectropole/ (accessed on 20 April 2022).
- Hart, A.S.; Bikram, C.K.C.; Subbaiyan, N.K.; Karr, P.A.; D’Souza, F. Phenothiazine-sensitized organic solar cells: Effect of dye anchor group positioning on the cell performance. ACS Appl. Mater. Interfaces 2012, 4, 5813–5820. [Google Scholar] [CrossRef]
- Bello, K.A.; Cheng, L.; Griffiths, J. Near-infrared absorbing methine dyes based on dicyanovinyl derivatives of indan-1,3-dione. J. Chem. Soc. Perkin Trans. 1987, 2, 815–818. [Google Scholar] [CrossRef]
- Raimundo, J.-M.; Blanchard, P.; Gallego Planas, N.; Mercier, N.; Ledoux-Rak, I.; Hierle, R.; Roncali, J. Design and synthesis of push-pull chromophores for second-order nonlinear optics derived from rigidified thiophene pi-conjugating spacers. J. Org. Chem. 2002, 67, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Schottel, B.L.; Chifotides, H.T.; Dunbar, K.R. Anion-pi interactions. Chem. Soc. Rev. 2008, 37, 68–83. [Google Scholar] [CrossRef]
- Giese, M.; Albrecht, M.; Rissanen, K. Experimental investigation on anion-π-interactions-applications and biochemical relevance. Chem. Commun. 2016, 52, 1778–1795. [Google Scholar] [CrossRef] [Green Version]
- Kepler, S.; Zeller, M.; Rosokha, S.V. Anion—π Complexes of halides with p-benzoquinones: Structures, thermodynamics, and criteria of charge transfer to electron transfer transition. J. Am. Chem. Soc. 2019, 141, 9338–9348. [Google Scholar] [CrossRef]
- Sugimoto, H.; Miyake, H.; Tsukube, H. Receptor versatility of tris(pyridine-1-ium-2-ylmethyl)amine in anion binding through hydrogen bonding. J. Chem. Soc. Dalton Trans. 2002, 24, 4535–4540. [Google Scholar] [CrossRef]
- Thordason, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
- Martín Várguez, P.E.; Brunel, F.; Raimundo, J.-M. Recent electrochemical/electrical microfabricated sensor devices for ionic and polyionic analytes. ACS Omega 2020, 5, 4733–4742. [Google Scholar] [CrossRef]
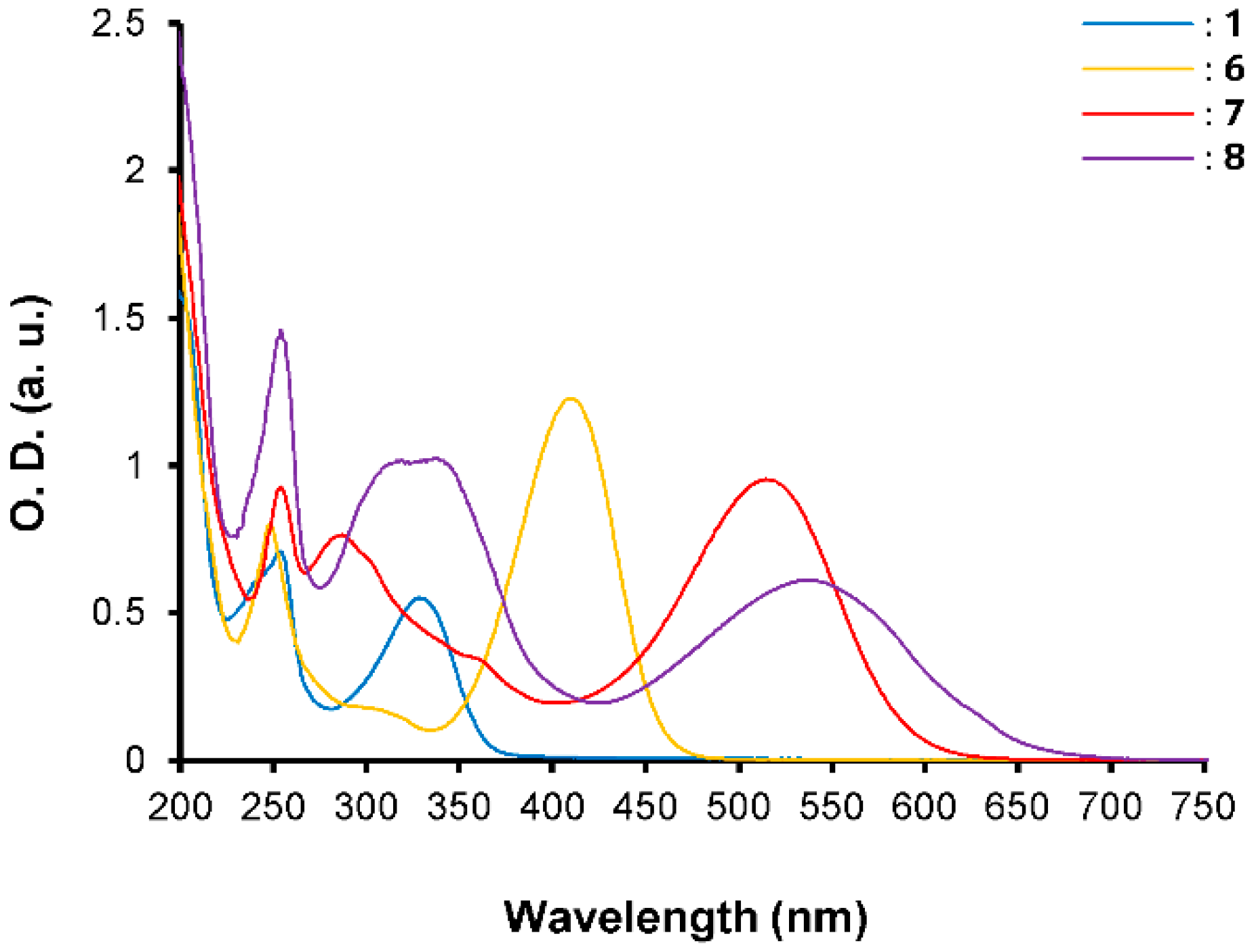
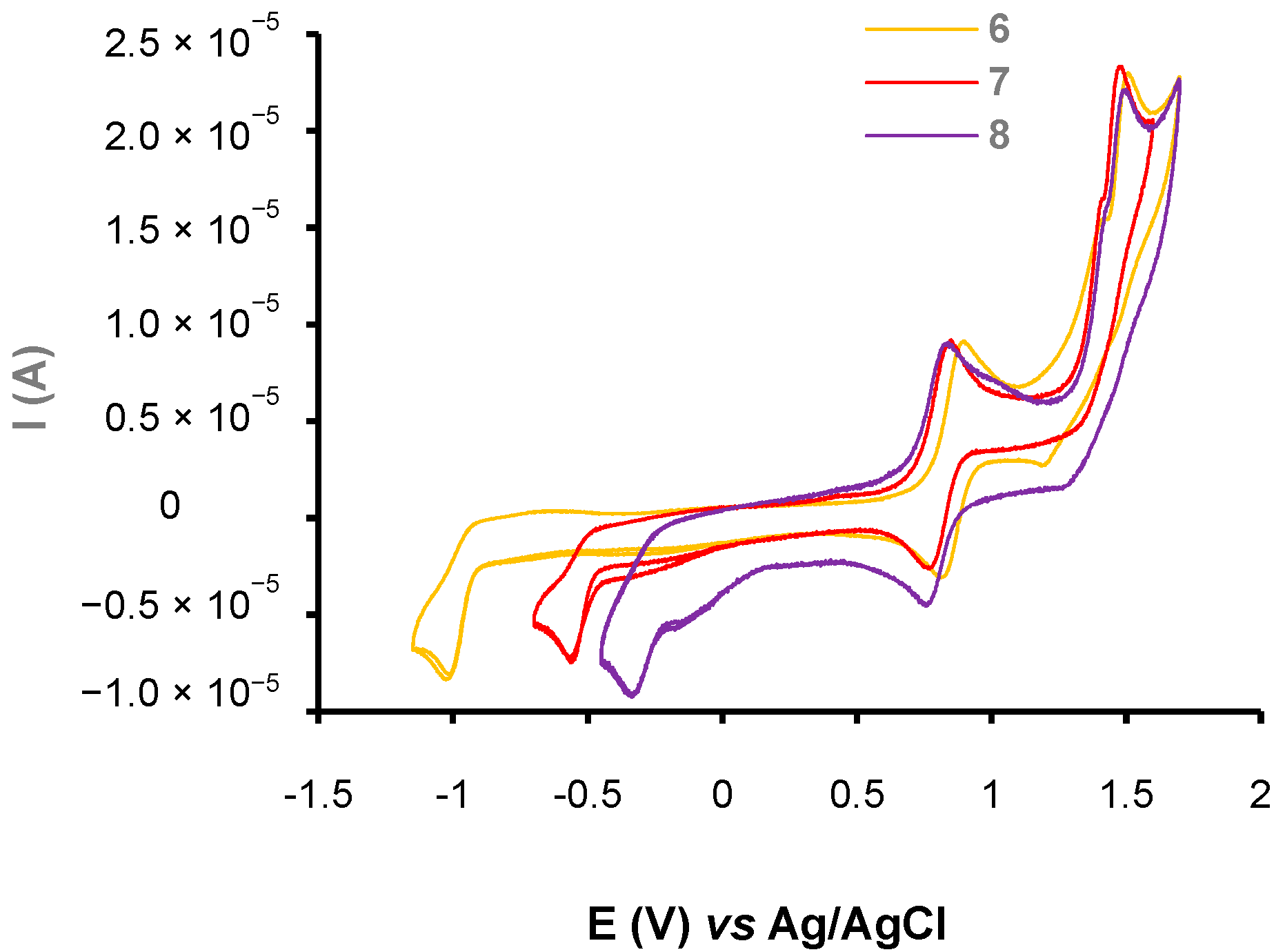
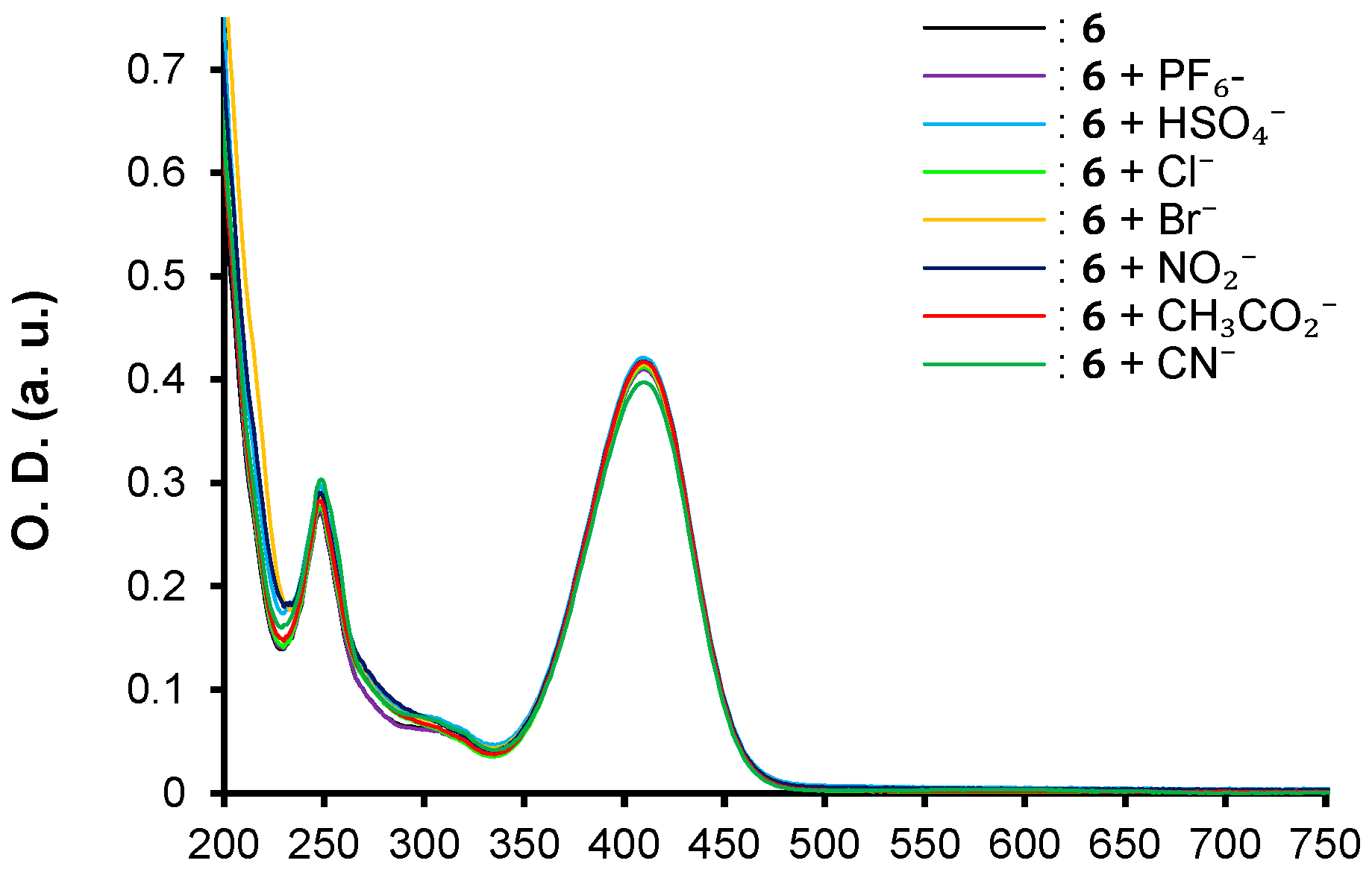

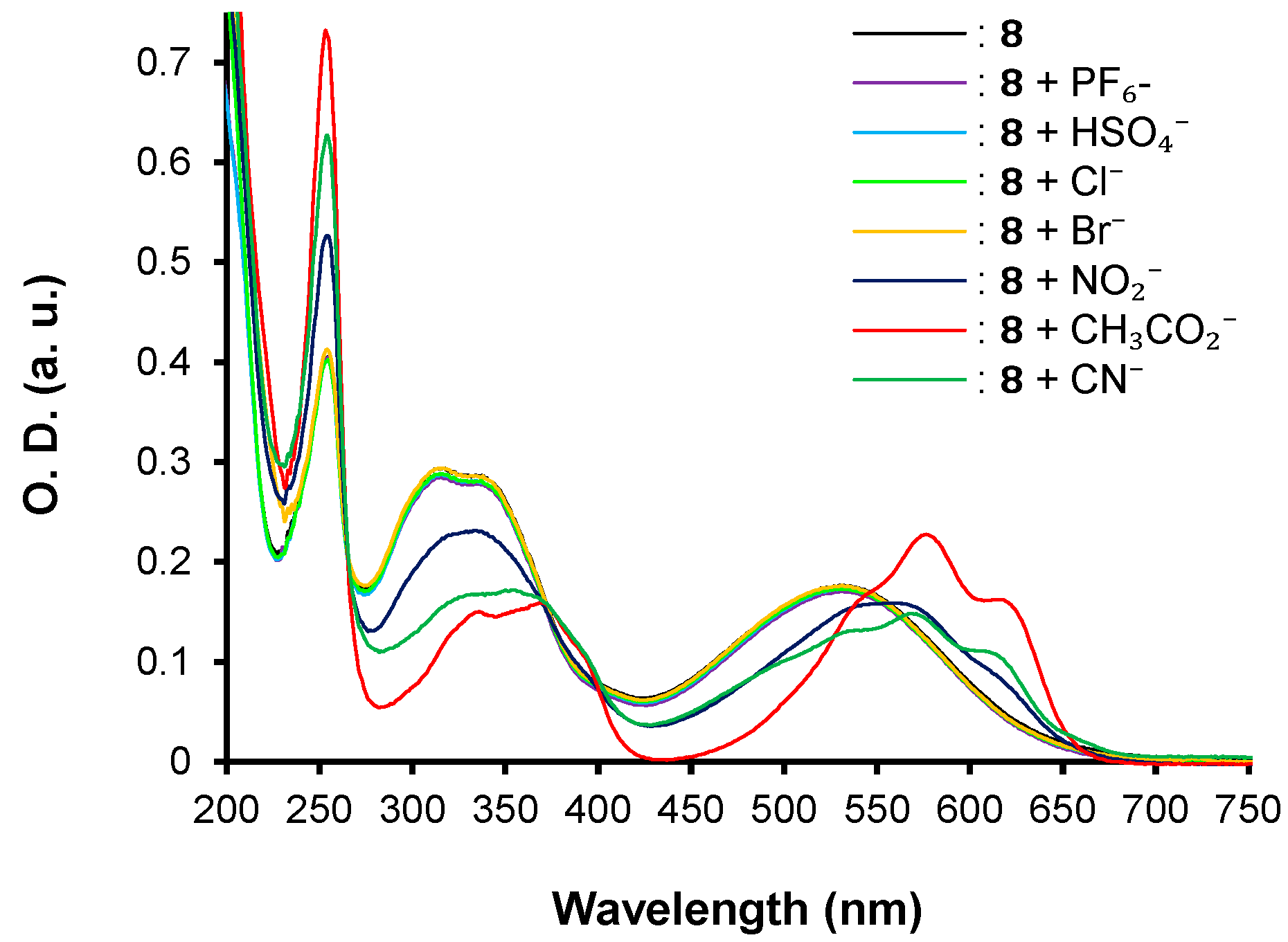
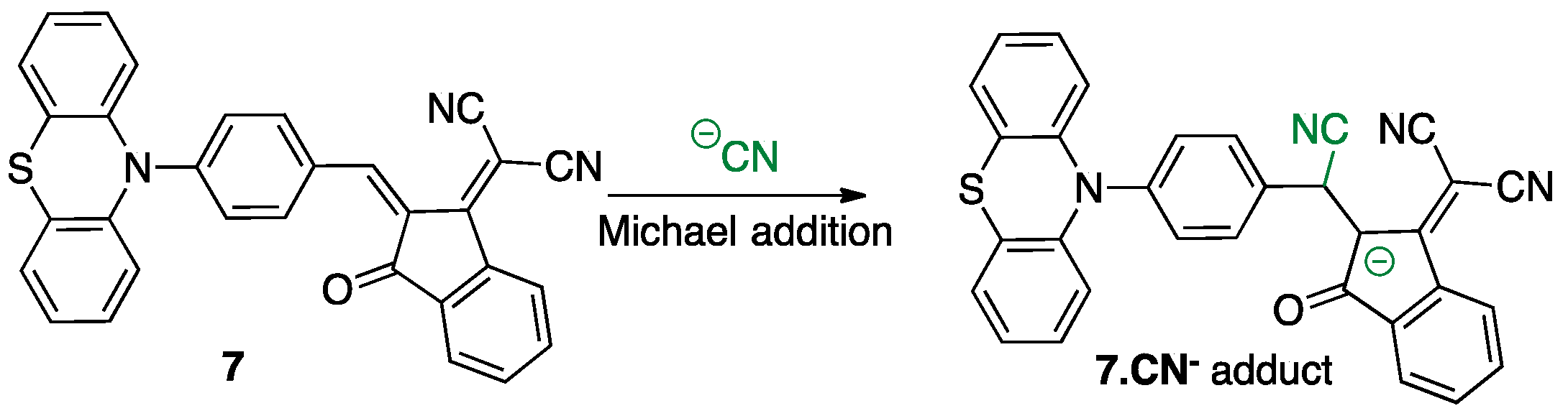
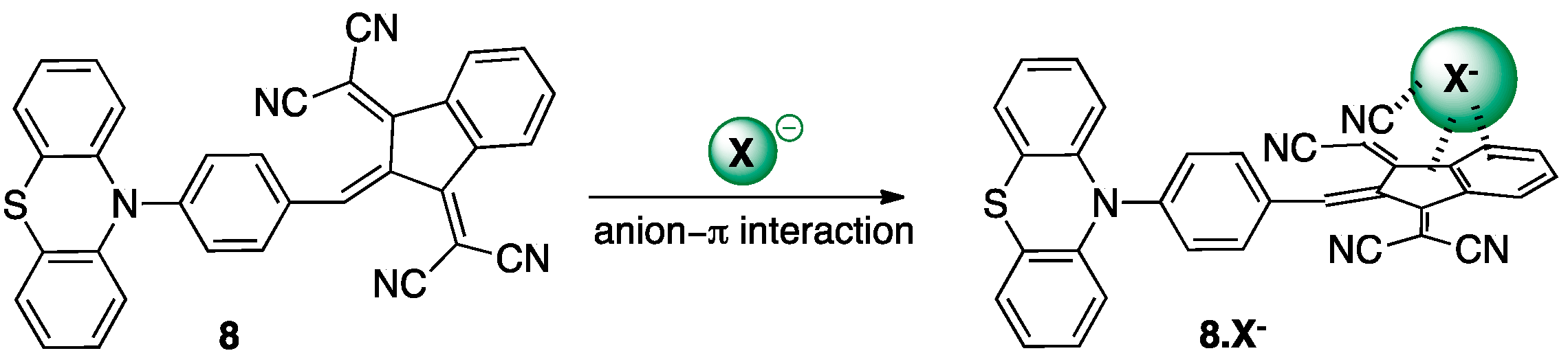
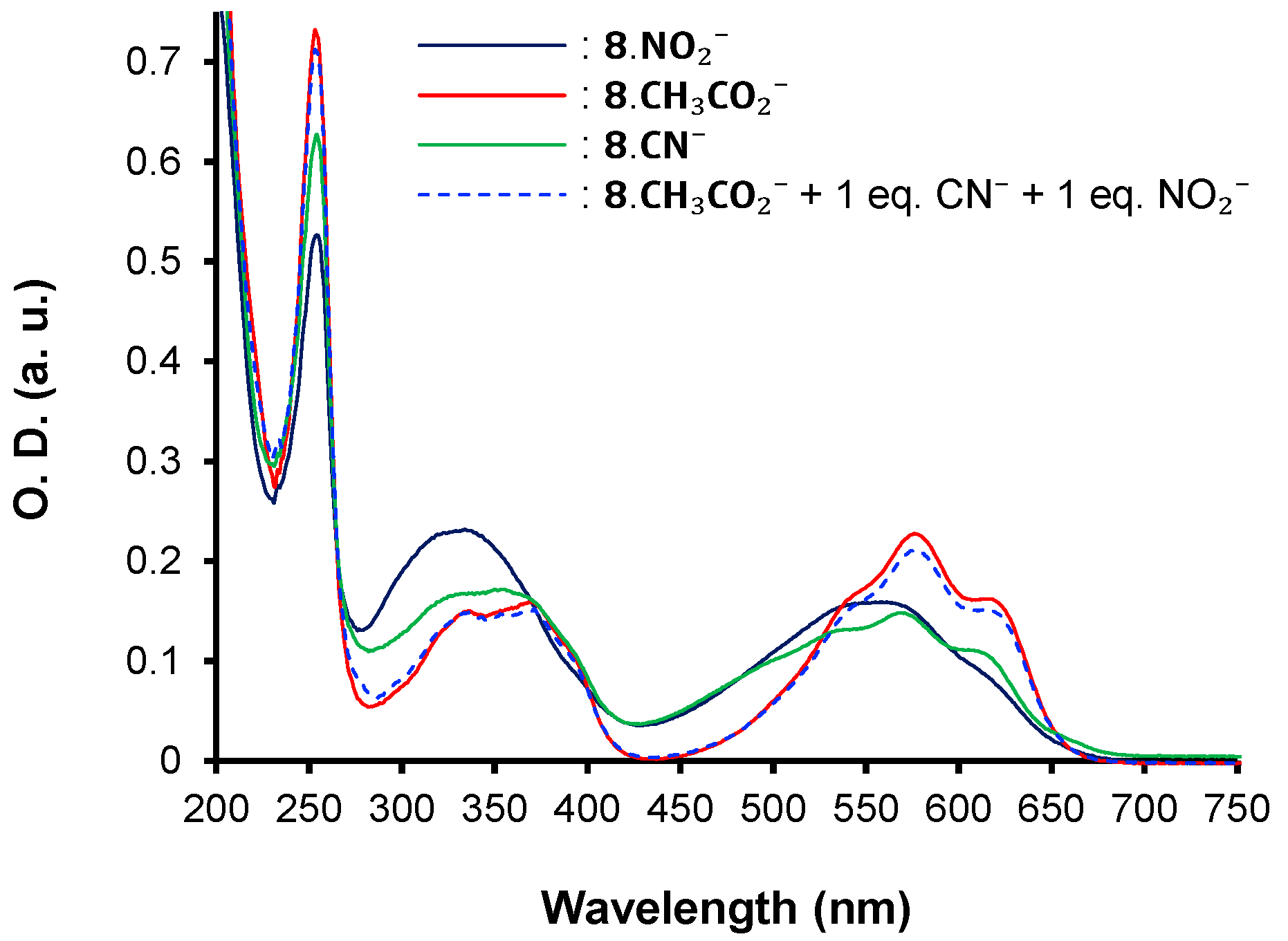

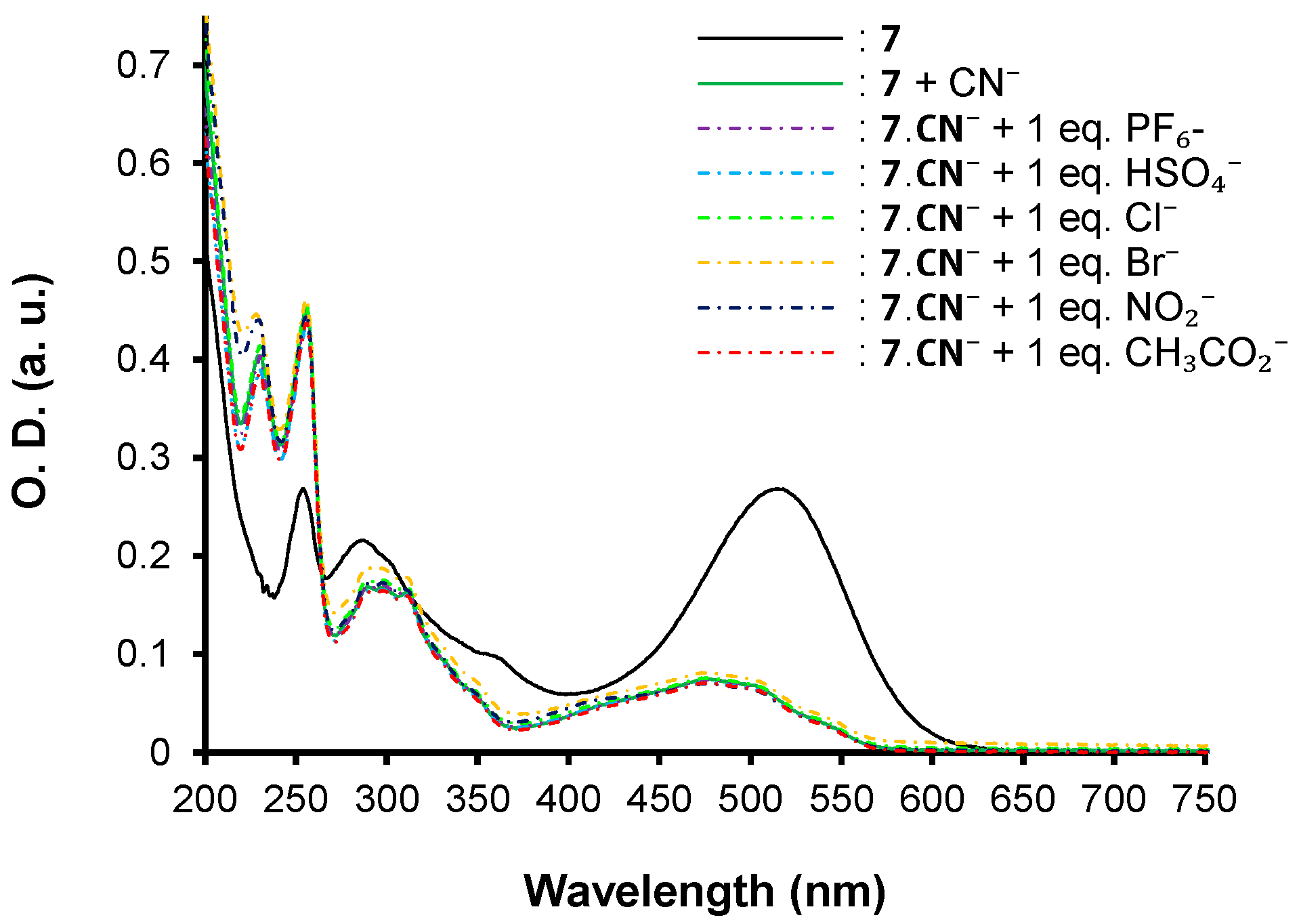
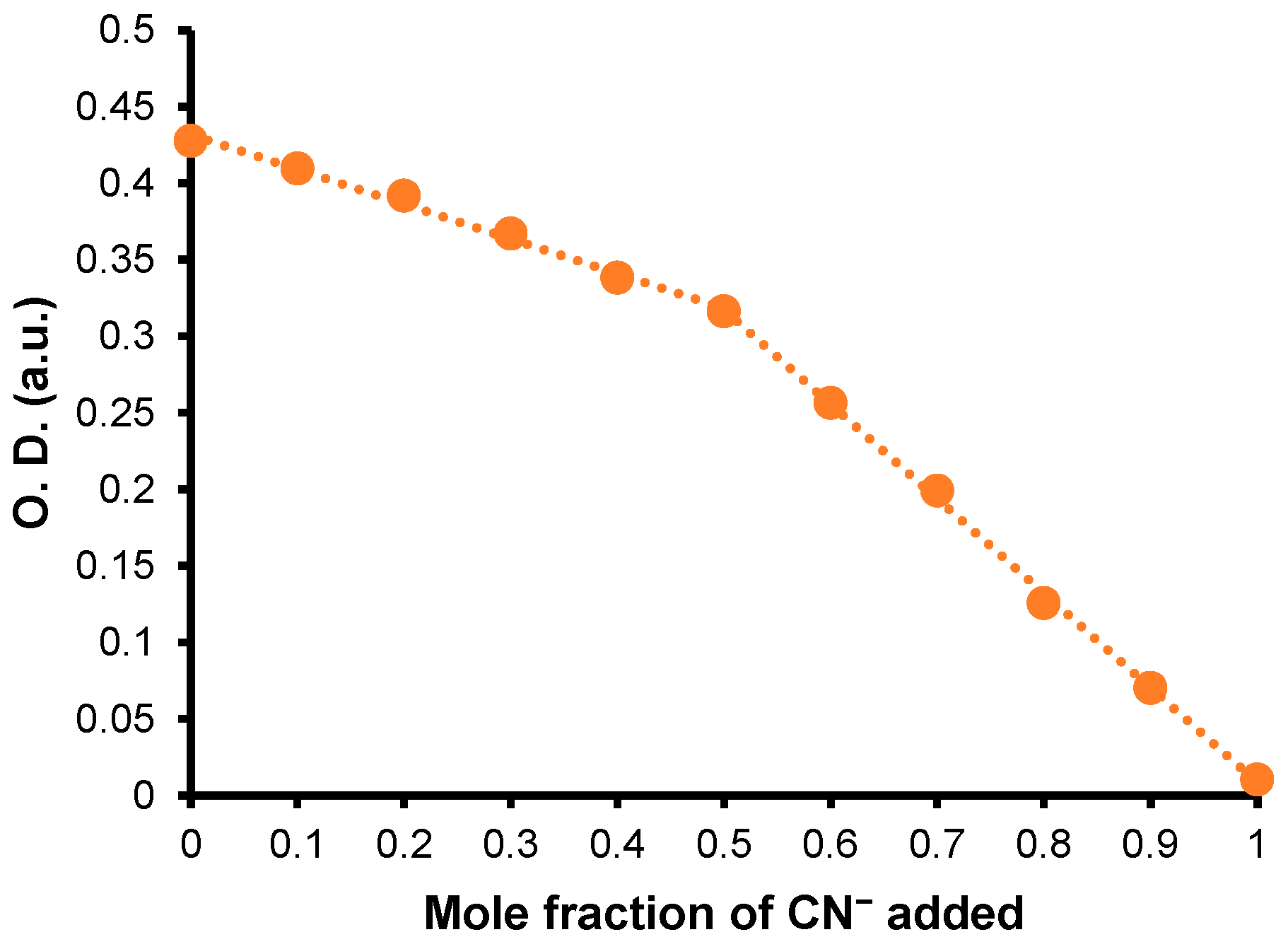
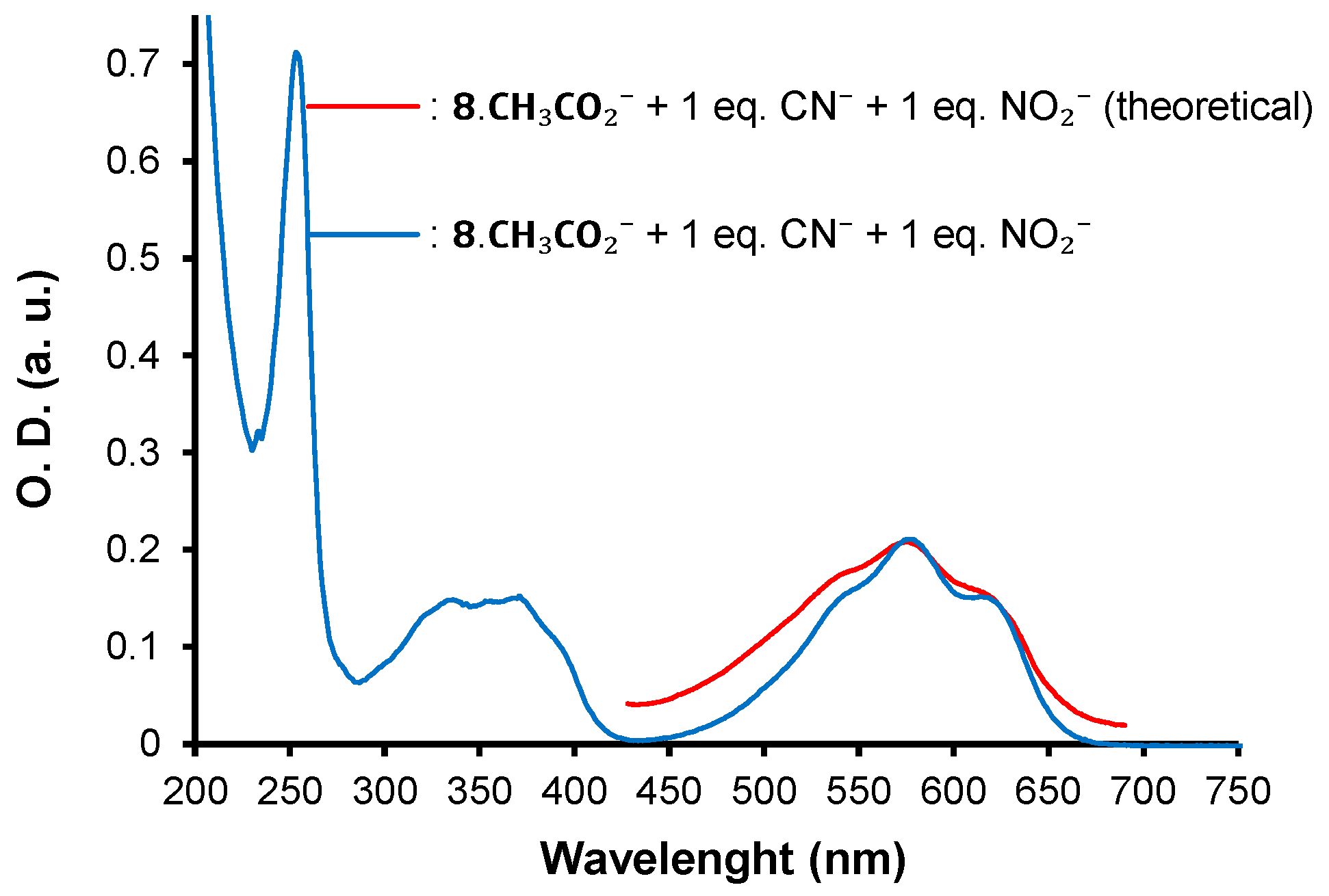
| λmax (nm) | E°Ox1 (V) | E°Ox2 c (V) | E°Red (V) | E°Ox1ONSET (V) | E°RedONSET (V) | Egelec (eV) | |
|---|---|---|---|---|---|---|---|
| 6 | 410 | 0.90 | 1.51 | −1.01 | 0.76 | −0.94 | 1.70 |
| 7 | 514 | 0.85 | 1.51 | −0.57 | 0.74 | −0.47 | 1.21 |
| 8 | 531 | 0.84 | 1.50 | −0.39 | 0.71 | −0.27 | 0.98 |
| HOMO (eV) | LUMO (eV) | |
|---|---|---|
| 6 | 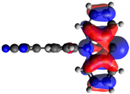 −5.32 eV | 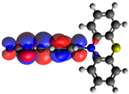 −3.13 eV |
| 7 |  −5.15 eV | 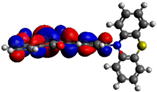 −3.38 eV |
| 8 | 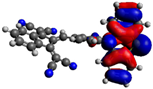 −5.15 eV | 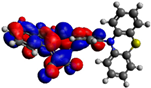 −3.60 eV |
| LogK1 | LogK2 | Stoichiometry | |
|---|---|---|---|
| 8.NO2− | 4.31 ± 0.10 | - | 1:1 |
| 8.CN− | 5.51 ± 0.12 | - | 1:1 |
| 8.CH3CO2− | 6.28 ± 0.10 | 5.19 ± 0.10 | 1:2 |
| Phenothiazine-Based Chemosensors | LOD Solvent [Ref] | Phenothiazine-Based Chemosensors | LOD Solvent [Ref] |
|---|---|---|---|
 | 1.56 µM b DCM [22] |  | 9.80 × 10−2 µM DMF/Tris-HCl buffer 1:99 v/v 10 mM, pH 9.3 [29] |
 | 6.70 × 10−2 µM a DMSO/H2O (9:1) [23] 3.21 × 10−3 µM aCH3CN [26] | 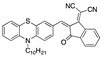 | 3.06–3.20 × 10−3 µM a CH3CN [30] |
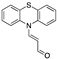 | 13.00 µM a CH3CN [24] | 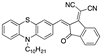 | 3.20 × 10−3 µM a CH3CN: water (9:1) [31] |
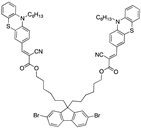 | 4.26 µM THF [32] |  | 9.85 µM EtOH/PBS (4:6) [25] |
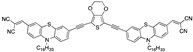 | 0.57 µM a DMSO [27] | 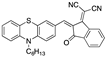 | 3.39 µM b, 0.011 µM a CH3CN: water (9:1) [33] |
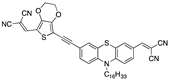 | 0.32 µM a DMSO [27] | 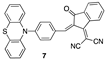 | 9.12 µM b CH3CN [this work] |
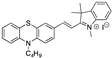 | 0.02 µM DMSO [28] |  | 4.59 µM b CH3CN (this work) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín Várguez, P.E.; Raimundo, J.-M. Naked-Eye Chromogenic Test Strip for Cyanide Sensing Based on Novel Phenothiazine Push–Pull Derivatives. Biosensors 2022, 12, 407. https://doi.org/10.3390/bios12060407
Martín Várguez PE, Raimundo J-M. Naked-Eye Chromogenic Test Strip for Cyanide Sensing Based on Novel Phenothiazine Push–Pull Derivatives. Biosensors. 2022; 12(6):407. https://doi.org/10.3390/bios12060407
Chicago/Turabian StyleMartín Várguez, Pedro E., and Jean-Manuel Raimundo. 2022. "Naked-Eye Chromogenic Test Strip for Cyanide Sensing Based on Novel Phenothiazine Push–Pull Derivatives" Biosensors 12, no. 6: 407. https://doi.org/10.3390/bios12060407
APA StyleMartín Várguez, P. E., & Raimundo, J.-M. (2022). Naked-Eye Chromogenic Test Strip for Cyanide Sensing Based on Novel Phenothiazine Push–Pull Derivatives. Biosensors, 12(6), 407. https://doi.org/10.3390/bios12060407