Portable Respiration Monitoring System with an Embroidered Capacitive Facemask Sensor
Abstract
:1. Introduction
2. Materials and Methods
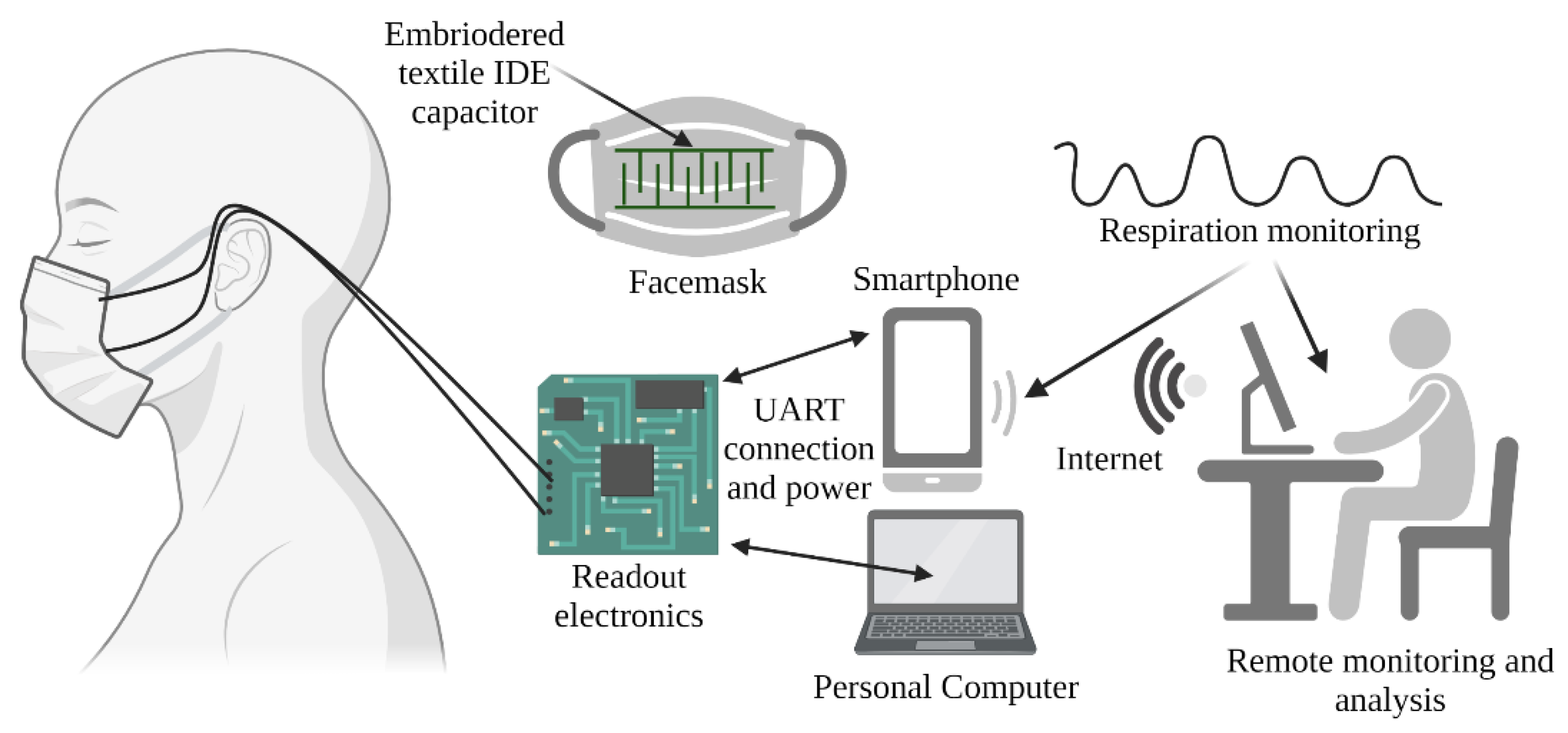
2.1. The System Architecture
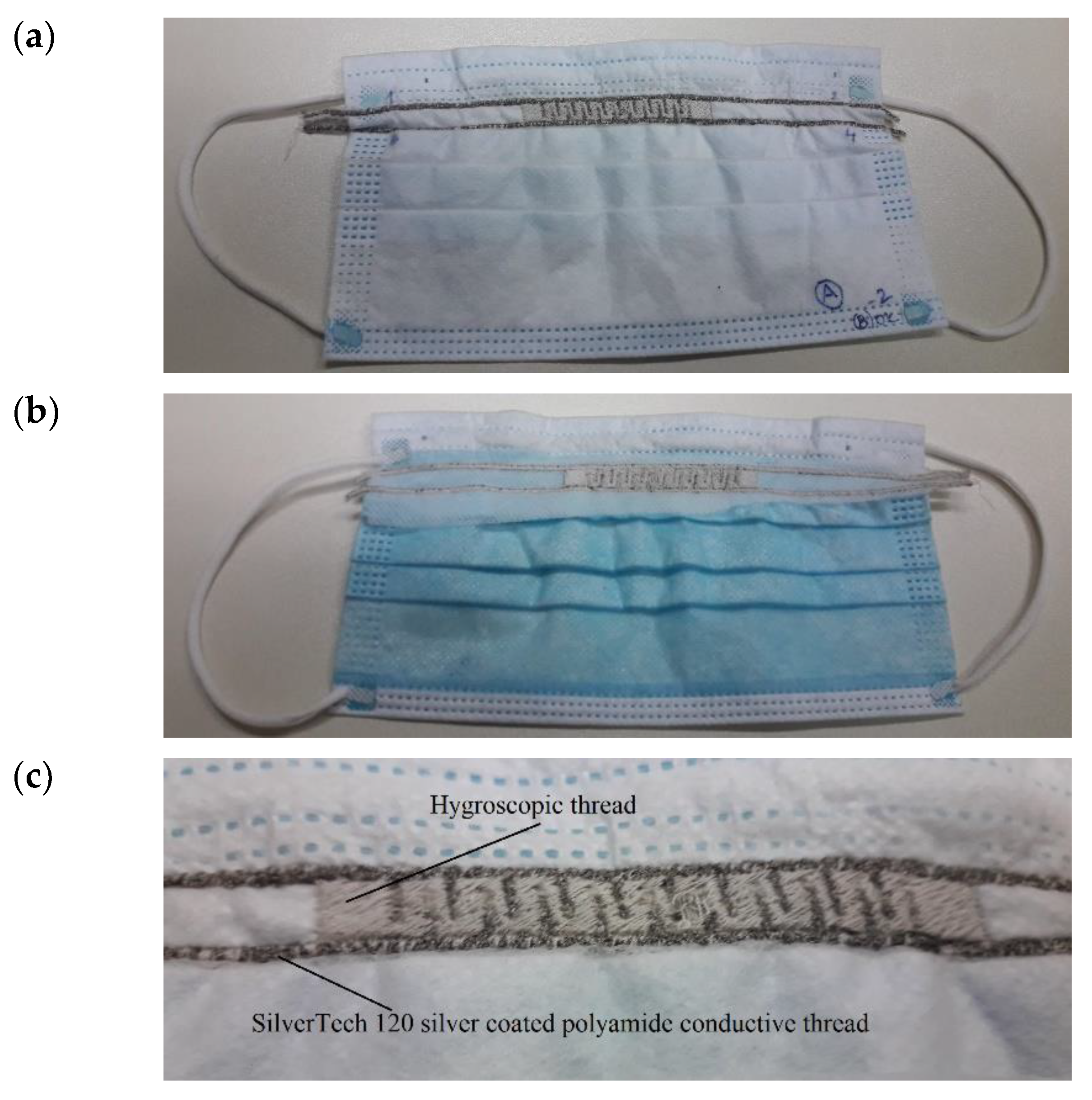
2.2. The Facemask Sensor
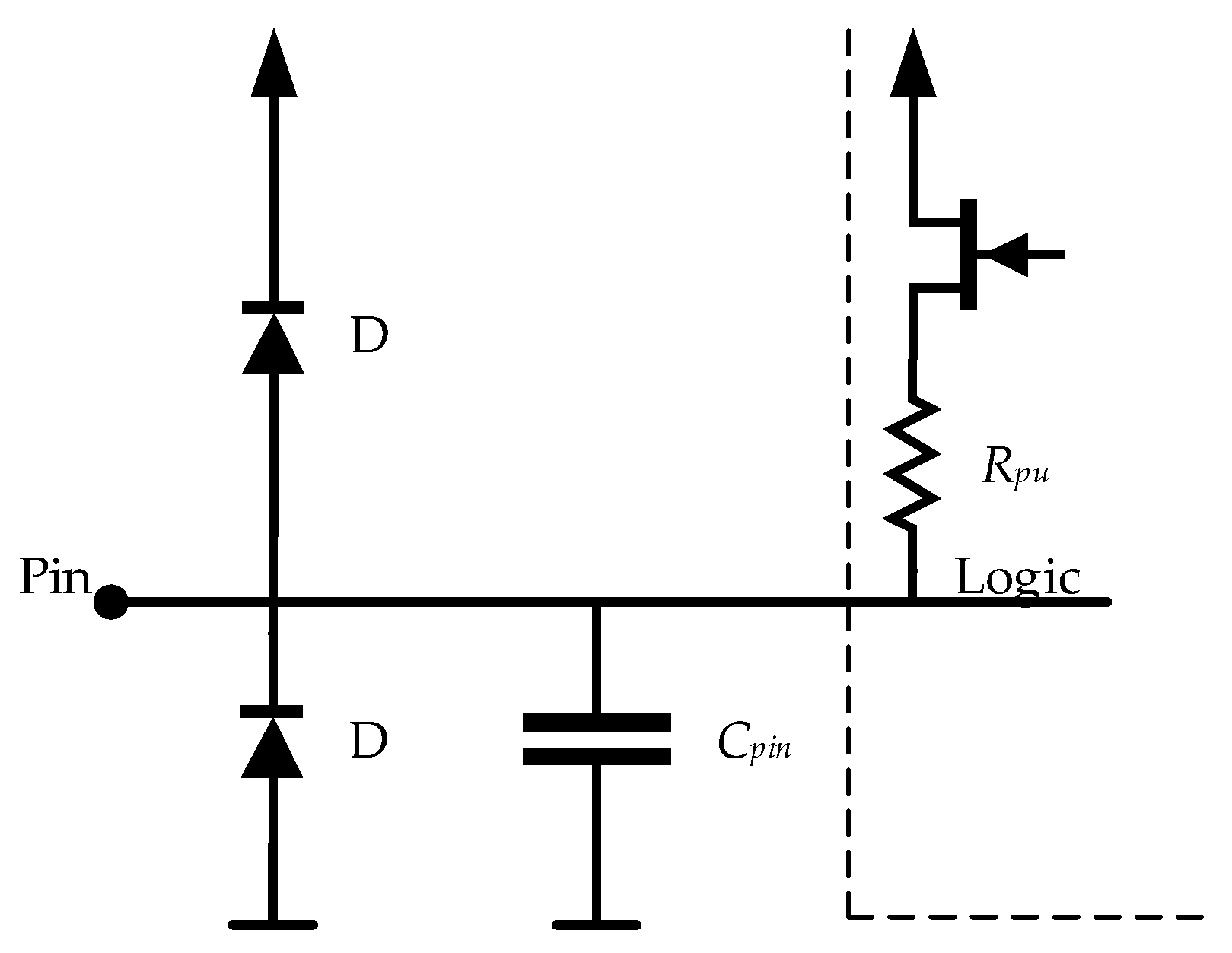
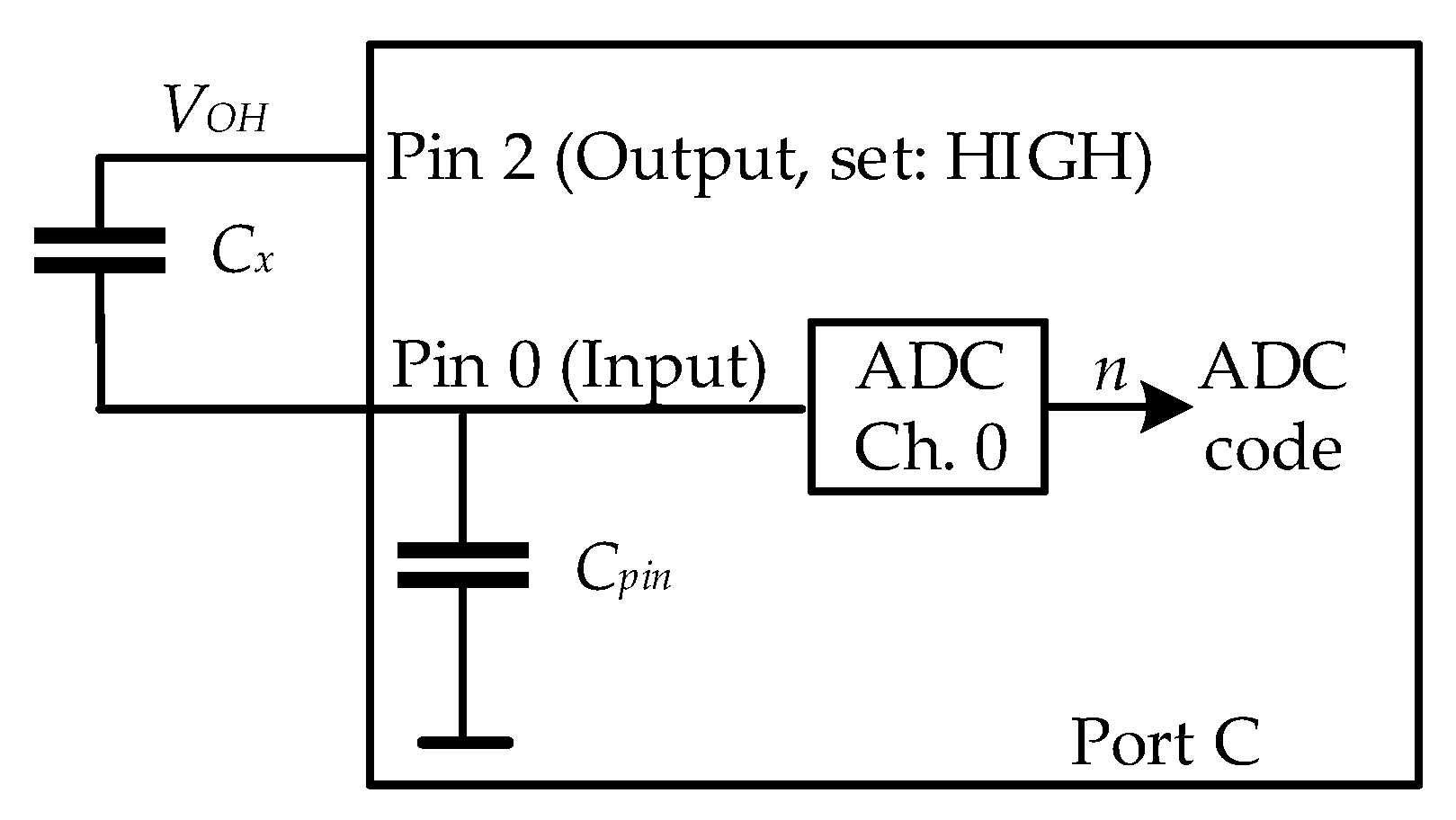
2.3. The Readout Electronics
2.4. Data Acquisition System
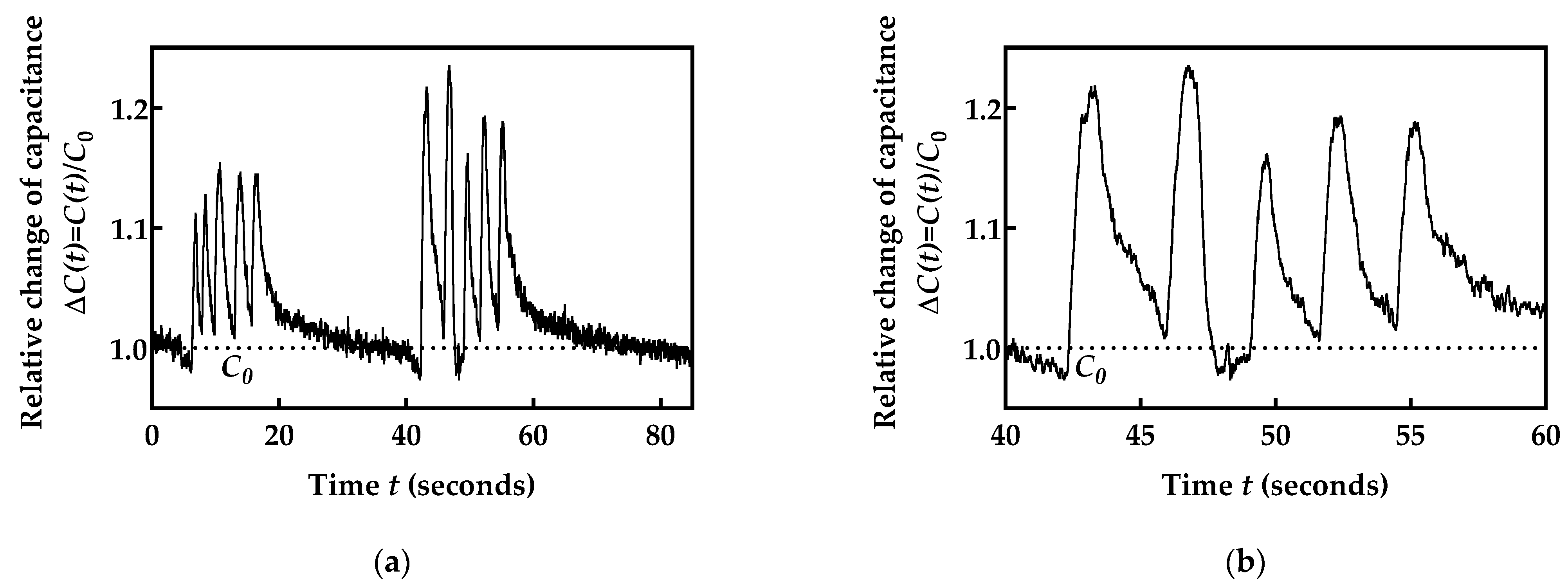
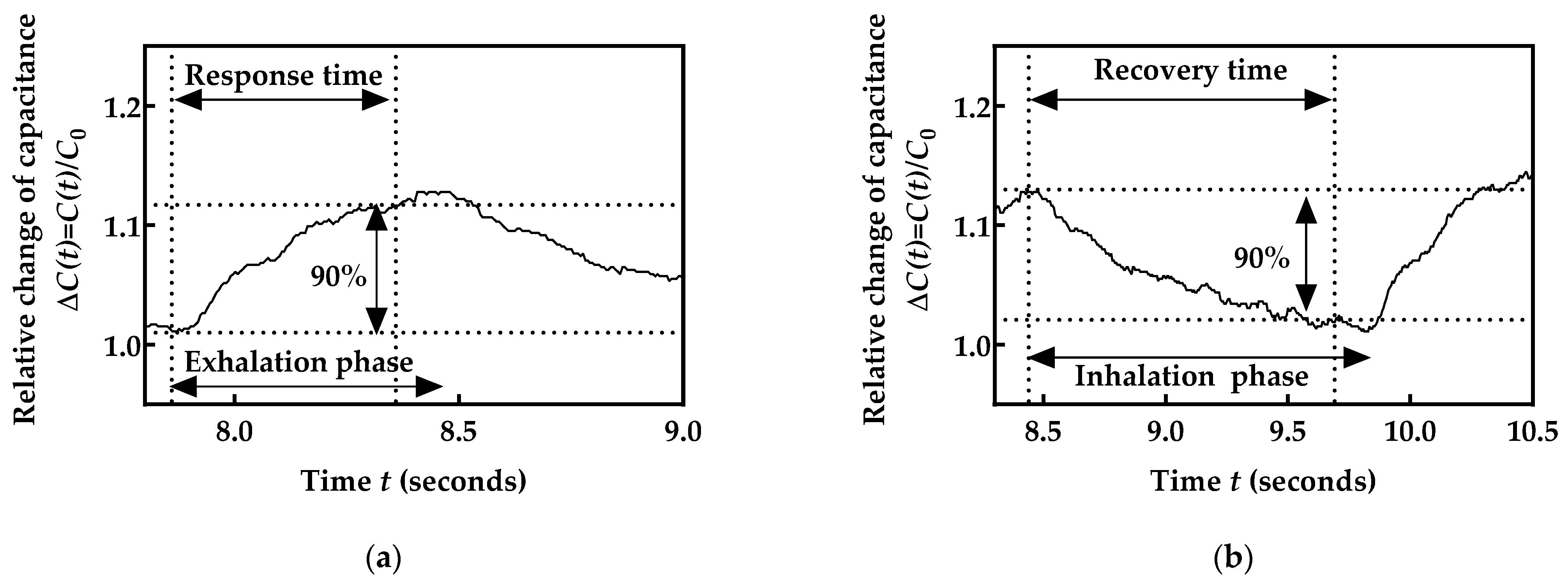
2.5. Validation of the Obtained Respiration Monitoring Data
- Artificial validation prototypes;
- Metronome as a reference;
- Validation against a reference device;
- Physical assessment.
3. Experimental Results and Discussion
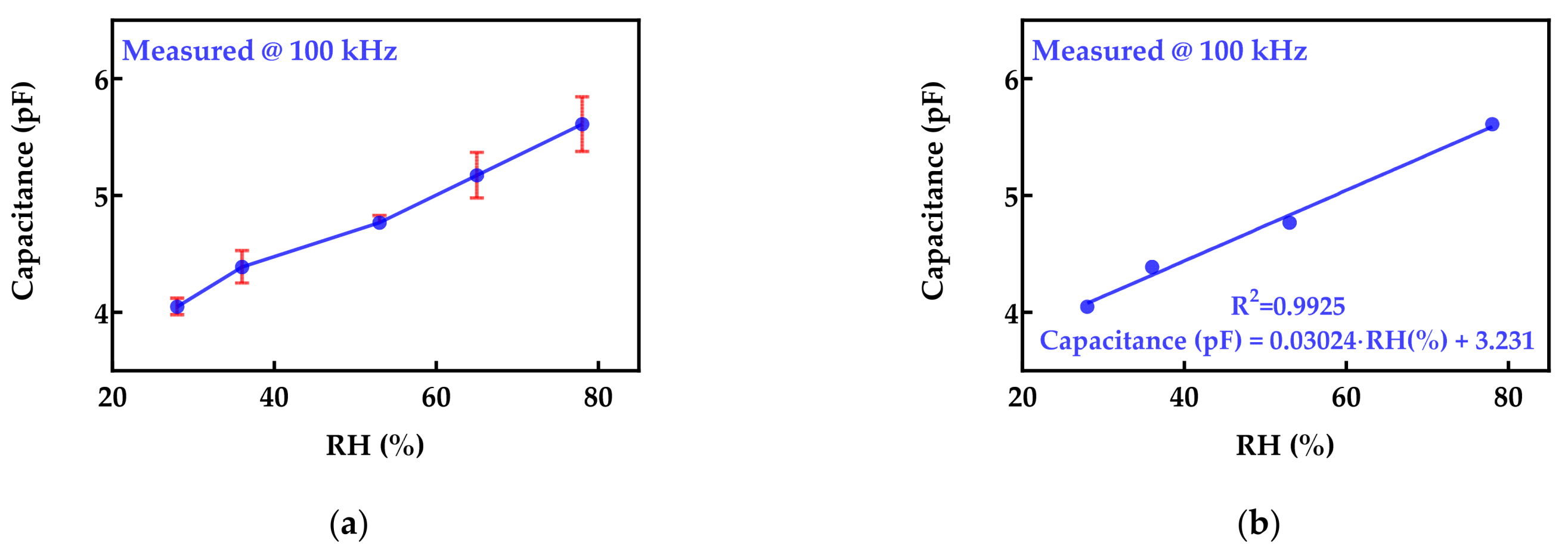
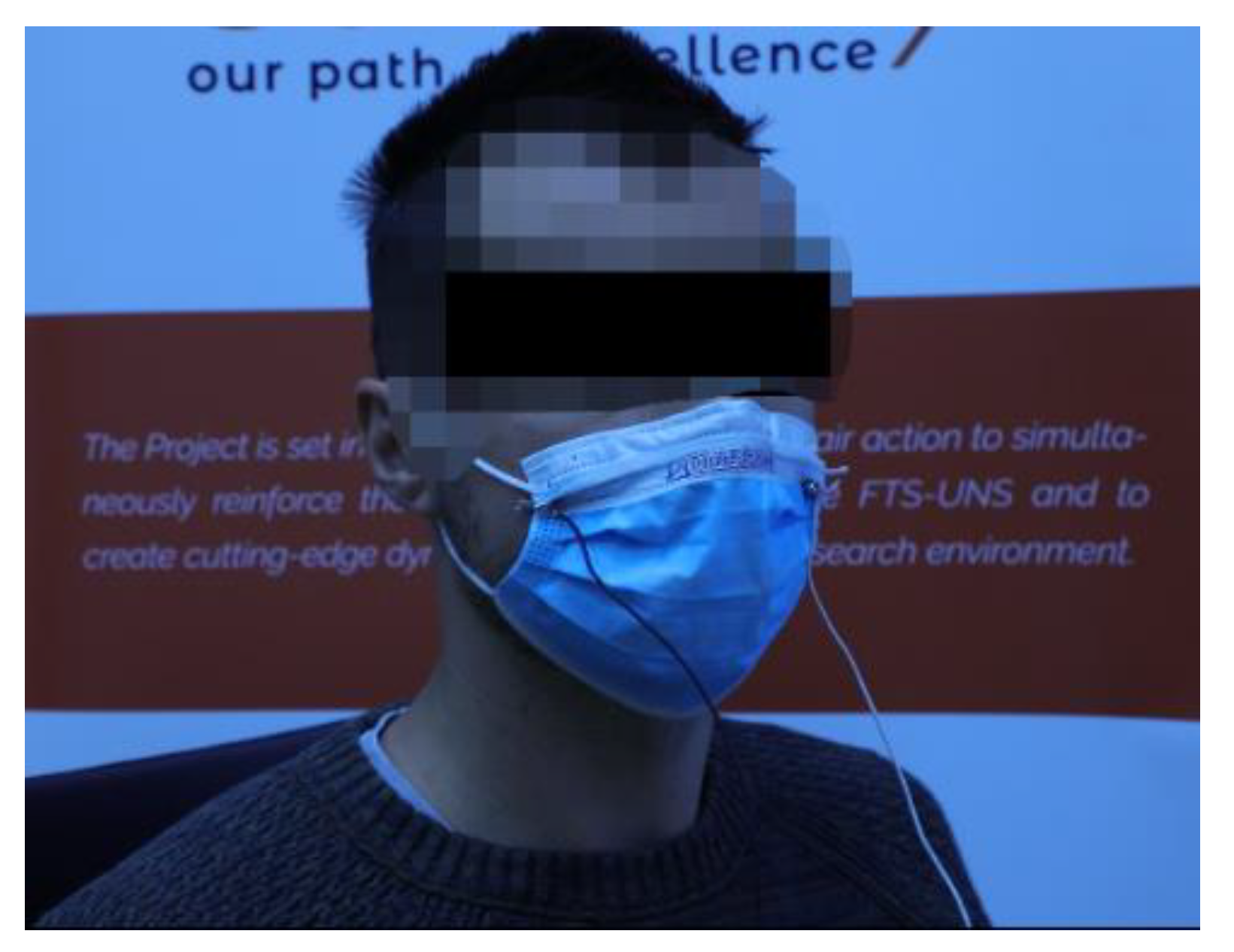
3.1. Sensor Embroidered on Protective Face Mask
3.2. The Readout Electronics
3.3. Implementation of Data Acquisition System
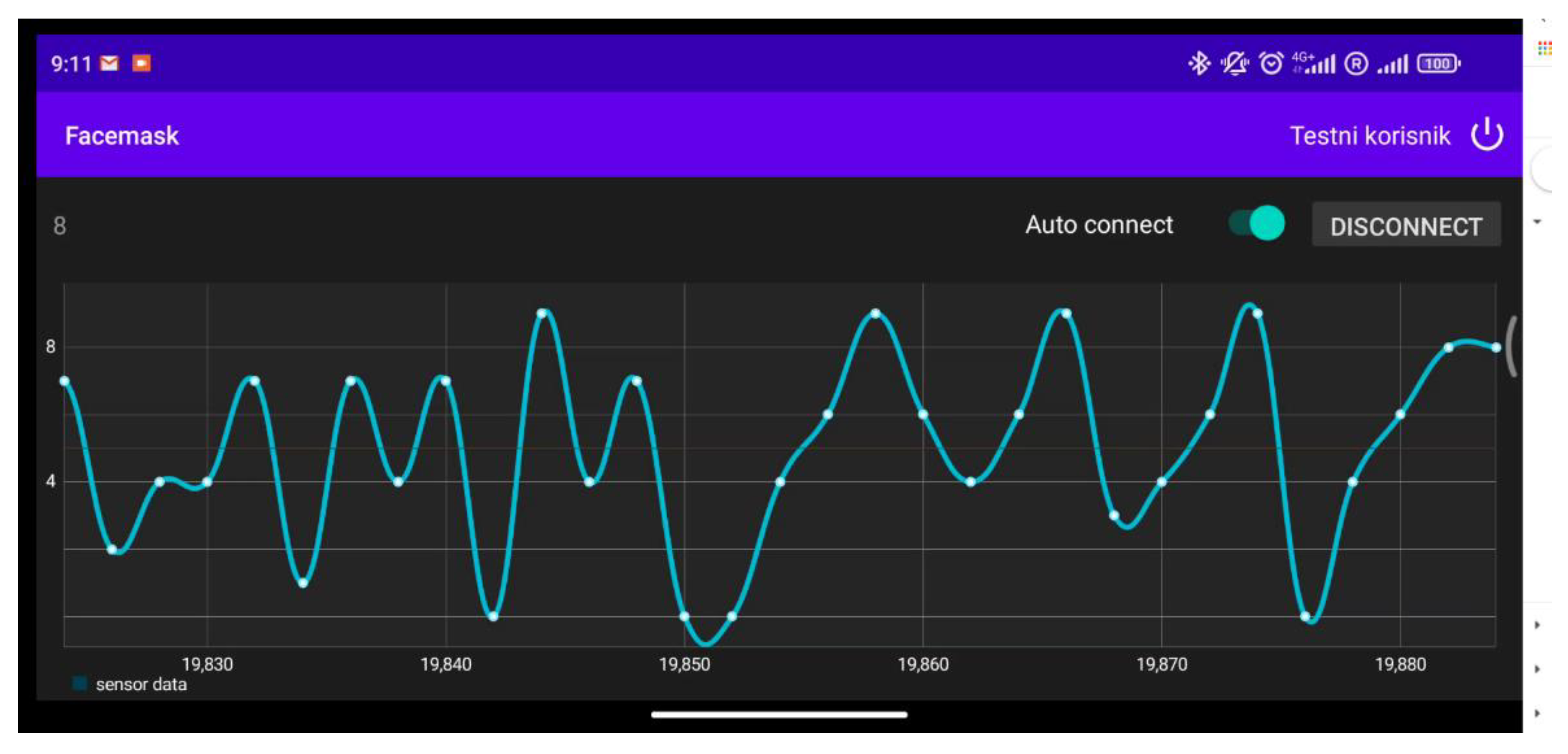
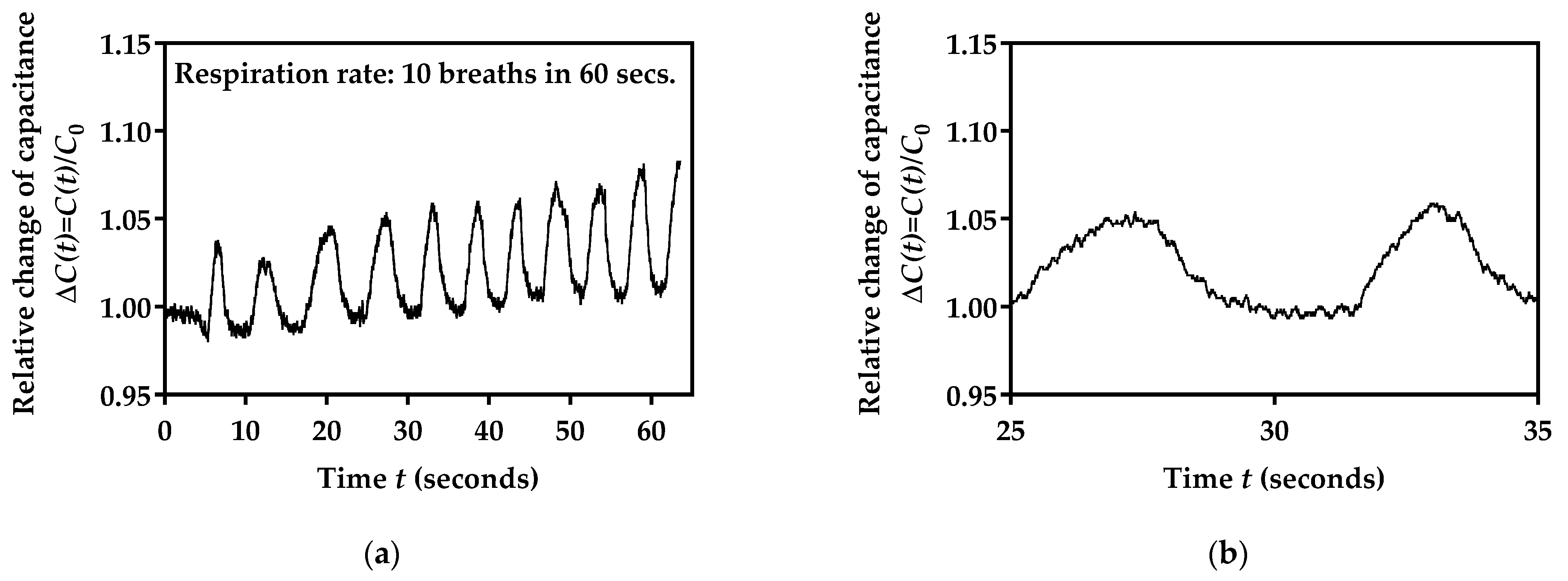
3.4. The Respiration Rate Monitoring
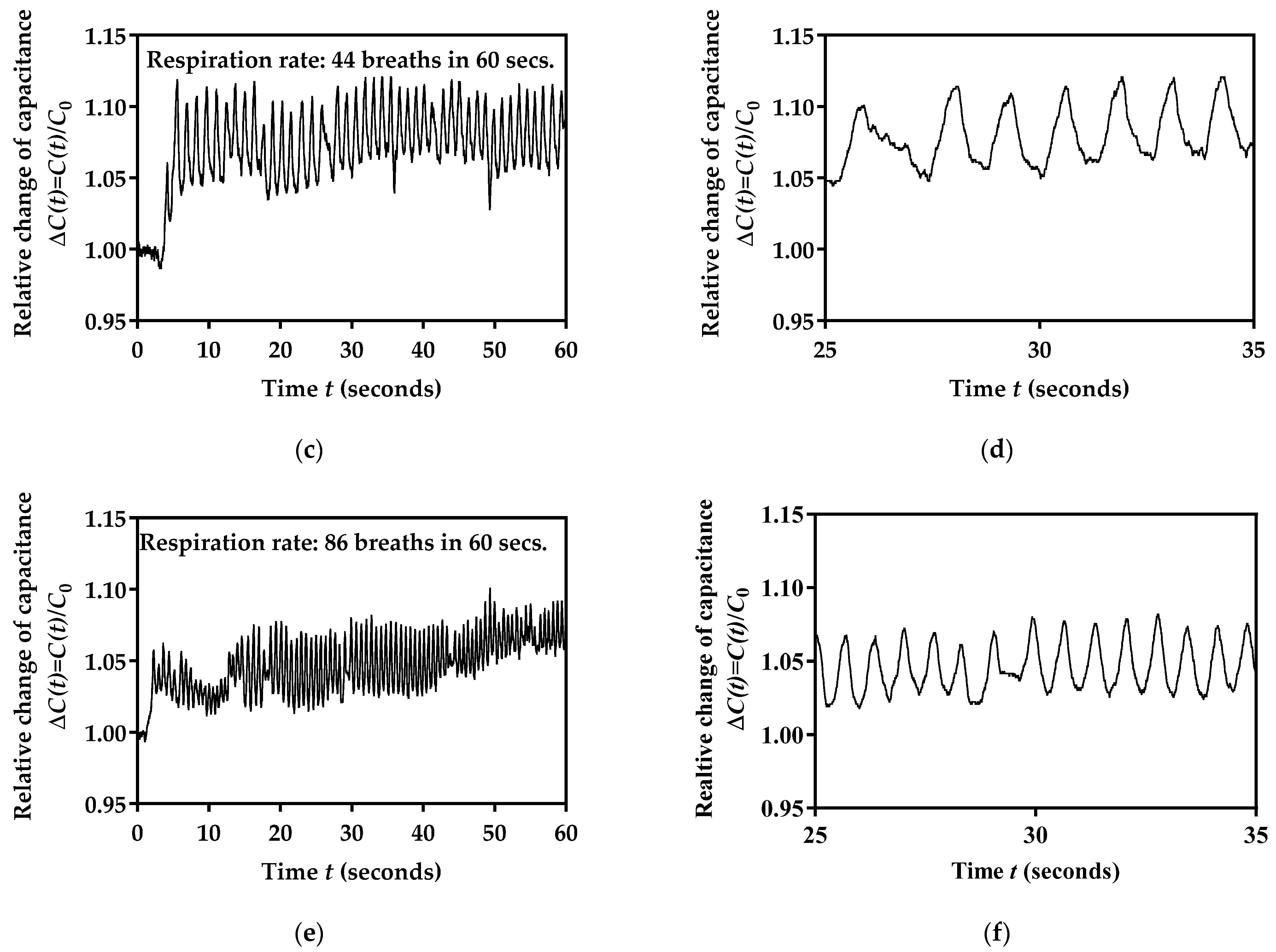
3.5. Performance Testing for Three Different Breathing Rates
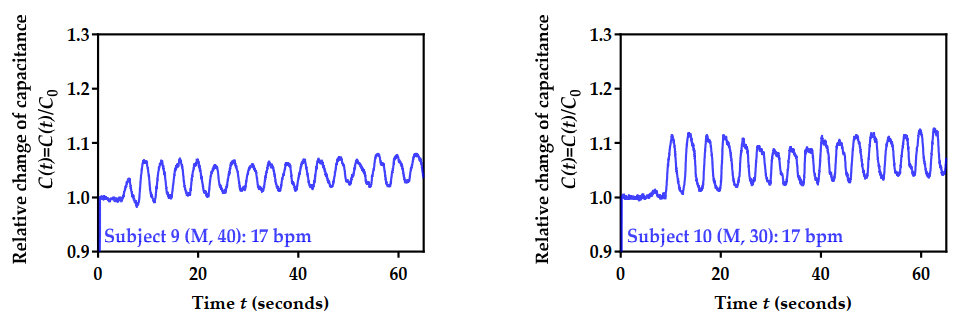
3.6. Performance Testing for 10 Volunteers
3.7. Power Consumption
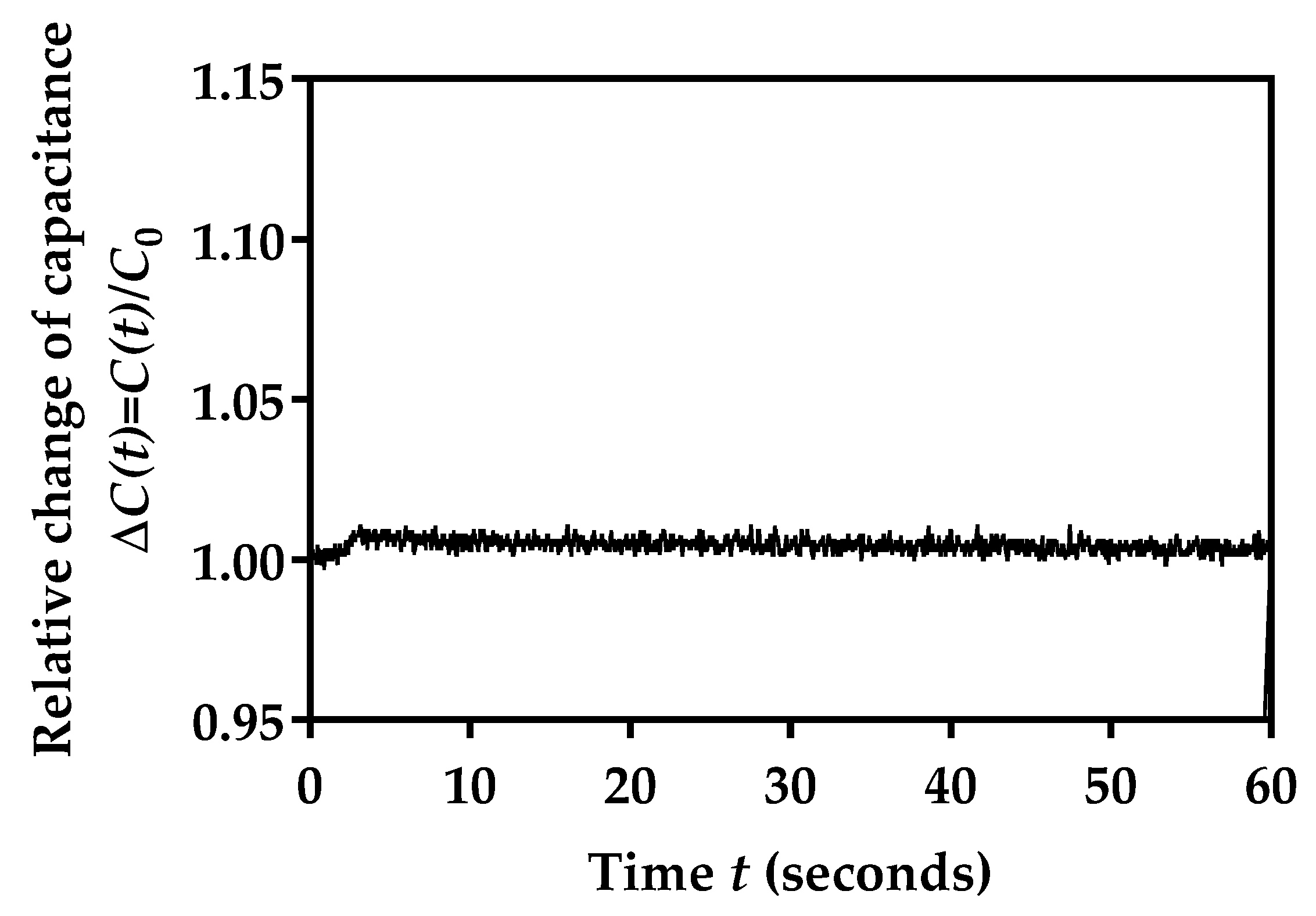
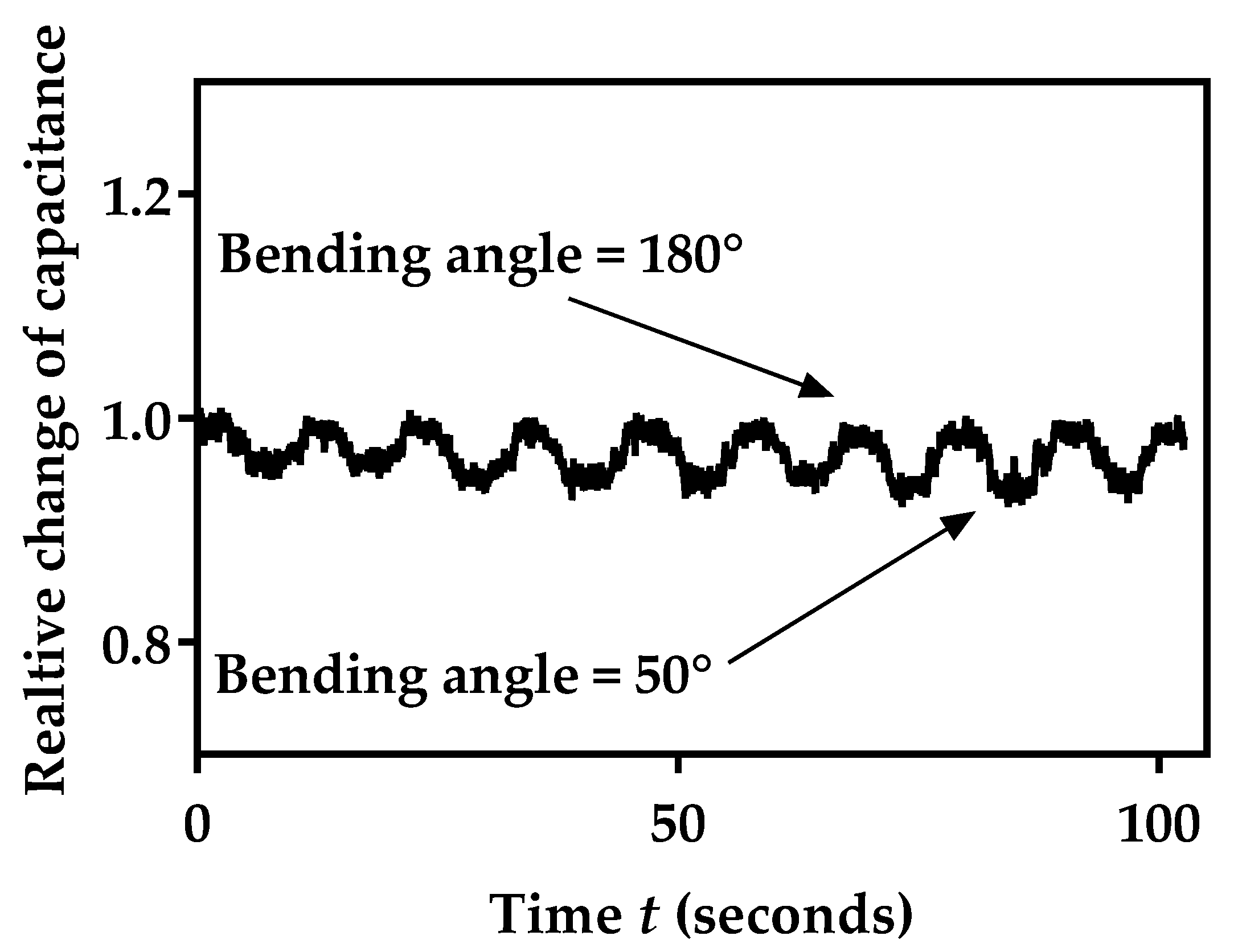
3.8. Mechanical Stability of Sensor Capacitance during Bending
3.9. Performance Comparison of the Proposed Device with the State of the Art
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of Breath Analysis Based on Humidity and Gas Sensors: Potential and Challenges. Sens. Actuators B Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Kano, S.; Jarulertwathana, N.; Mohd-Noor, S.; Hyun, J.K.; Asahara, R.; Mekaru, H. Respiratory Monitoring by Ultrafast Humidity Sensors with Nanomaterials: A Review. Sensors 2022, 22, 1251. [Google Scholar] [CrossRef] [PubMed]
- Güntner, A.T.; Abegg, S.; Königstein, K.; Gerber, P.A.; Schmidt-Trucksäss, A.; Pratsinis, S.E. Breath Sensors for Health Monitoring. ACS Sens. 2019, 4, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, Y.; Tai, H.; Liu, B.; Duan, Z.; Yuan, Z.; Pan, H.; Xie, G.; Du, X.; Su, Y. An integrated flexible self-powered wearable respiration sensor. Nano Energy 2019, 63, 103829. [Google Scholar] [CrossRef]
- Folke, M.; Cernerud, L.; Ekstrome, M.; Hök, B. Critical review of non-invasive respiratory monitoring in medical care. Med. Biol. Eng. Comput. 2003, 41, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalidi, F.Q.; Saatchi, R.; Burke, D.; Elphick, H.; Tan, S. Respiration rate monitoring methods: A review. Pediatr. Pulmonol. 2011, 46, 523–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbishley, P.; Rodriguez-Villegas, E. Breathing Detection: Towards a Miniaturized, Wearable, Battery-Operated Monitoring System. IEEE Trans. Biomed. Eng. 2007, 55, 196–204. [Google Scholar] [CrossRef]
- Mimoz, O.; Benard, T.; Gaucher, A.; Frasca, D.; Debaene, B. Accuracy of respiratory rate monitoring using a non-invasive acoustic method after general anaesthesia. Br. J. Anaesth. 2012, 108, 872–875. [Google Scholar] [CrossRef] [Green Version]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Huang, C.-T.; Shen, C.-L.; Tang, C.-F.; Chang, S.-H. A wearable yarn-based piezo-resistive sensor. Sens. Actuators A Phys. 2008, 141, 396–403. [Google Scholar] [CrossRef]
- Atalay, O.; Kennon, W.R.; Demirok, E. Weft-Knitted Strain Sensor for Monitoring Respiratory Rate and Its Electro-Mechanical Modeling. IEEE Sens. J. 2014, 15, 110–122. [Google Scholar] [CrossRef]
- Wijesiriwardana, R. Inductive fiber-meshed strain and displacement transducers for respiratory measuring systems and motion capturing systems. IEEE Sens. J. 2006, 6, 571–579. [Google Scholar] [CrossRef]
- Min, S.D.; Yun, Y.; Shin, H. Simplified Structural Textile Respiration Sensor Based on Capacitive Pressure Sensing Method. IEEE Sens. J. 2014, 14, 3245–3251. [Google Scholar] [CrossRef]
- Lin, X.; Seet, B.-C. A Linear Wide-Range Textile Pressure Sensor Integrally Embedded in Regular Fabric. IEEE Sens. J. 2015, 15, 5384–5385. [Google Scholar] [CrossRef]
- Li, Y.; Samad, Y.A.; Liao, K. From cotton to wearable pressure sensor. J. Mater. Chem. A 2014, 3, 2181–2187. [Google Scholar] [CrossRef]
- Kundu, S.K.; Kumagai, S.; Sasaki, M. A Wearable Capacitive Sensor for Monitoring Human Respiratory Rate. Jpn. J. Appl. Phys. 2013, 52, 04CL05. [Google Scholar] [CrossRef]
- Guo, L.; Berglin, L.; Wiklund, U.; Mattila, H. Design of a garment-based sensing system for breathing monitoring. Text. Res. J. 2012, 83, 499–509. [Google Scholar] [CrossRef]
- Güder, F.; Ainla, A.; Redston, J.; Mosadegh, B.; Glavan, A.; Martin, T.J.; Whitesides, G.M. Paper-Based Electrical Respiration Sensor. Angew. Chem. Int. Ed. 2016, 55, 5727–5732. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Li, Z.; Takakuwa, M.; Inoue, D.; Hashizume, D.; Jiang, Z.; Shi, Y.; Ou, L.; Nayeem, O.G.; Umezu, S.; et al. Smart Face Mask Based on an Ultrathin Pressure Sensor for Wireless Monitoring of Breath Conditions. Adv. Mater. 2021, 34, 2107758. [Google Scholar] [CrossRef]
- Tipparaju, V.V.; Wang, D.; Yu, J.; Chen, F.; Tsow, F.; Forzani, E.; Tao, N.; Xian, X. Respiration Pattern Recognition by Wearable Mask Device. Biosens. Bioelectron. 2020, 169, 112590. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. Paper-Based Sensors for Gas, Humidity, and Strain Detections: A Review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Yuan, Z.; Zhao, Q.; Sun, P.; Xie, G.; Du, X.; Tai, H. Facile, Flexible, Cost-Saving, and Environment-Friendly Paper-Based Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019, 11, 21840–21849. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Jiang, Y.; Tai, H. Recent advances in humidity sensors for human body related humidity detection. J. Mater. Chem. C 2021, 9, 14963–14980. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Huang, Q.; Yuan, Z.; Zhao, Q.; Wang, S.; Zhang, Y.; Tai, H. A do-it-yourself approach to achieving a flexible pressure sensor using daily use materials. J. Mater. Chem. C 2021, 9, 13659–13667. [Google Scholar] [CrossRef]
- Dai, J.; Li, L.; Shi, B.; Li, Z. Recent progress of self-powered respiration monitoring systems. Biosens. Bioelectron. 2021, 194, 113609. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Huang, Q.; Wang, S.; Wang, Y.; Pan, H.; Zhao, Q.; Xie, G.; Du, X.; Tai, H. Paper and carbon ink enabled low-cost, eco-friendly, flexible, multifunctional pressure and humidity sensors. Smart Mater. Struct. 2021, 30, 055012. [Google Scholar] [CrossRef]
- Stavrakis, A.; Simić, M.; Stojanović, G. Electrical Characterization of Conductive Threads for Textile Electronics. Electronics 2021, 10, 967. [Google Scholar] [CrossRef]
- Simic, M. Complex Impedance Measurement System for Environmental Sensors Characterization. In Proceedings of the 2014 22nd Telecommunications Forum Telfor (TELFOR), Belgrade, Serbia, 25–27 November 2014; pp. 660–663. [Google Scholar]
- Vasiljevic, D.Z.; Mansouri, A.; Anzi, L.; Sordan, R.; Stojanovic, G.M. Performance Analysis of Flexible Ink-Jet Printed Humidity Sensors Based on Graphene Oxide. IEEE Sens. J. 2018, 18, 4378–4383. [Google Scholar] [CrossRef]
- Reverter, F.; Gasulla, M.; Pallas-Areny, R. A Low-Cost Microcontroller Interface for Low-Value Capacitive Sensors. In Proceedings of the 21st IEEE Instrumentation and Measurement Technology Conference (IEEE Cat. No.04CH37510), Como, Italy, 18–20 May 2004; pp. 1771–1775. [Google Scholar]
- Reverter, F. The Art of Directly Interfacing Sensors to Microcontrollers. J. Low Power Electron. Appl. 2012, 2, 265–281. [Google Scholar] [CrossRef] [Green Version]
- Vanegas, E.; Igual, R.; Plaza, I. Sensing Systems for Respiration Monitoring: A Technical Systematic Review. Sensors 2020, 20, 5446. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, S.; Li, T.; Jin, S.; Shao, Y.; Yang, H.; Wu, D.; Dai, S.; Lu, Y.; Chen, S. Flexible Capacitive Humidity Sensors Based on Ionic Conductive Wood-Derived Cellulose Nanopapers. ACS Appl. Mater. Interfaces 2020, 12, 41896–41904. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, R.; Patil, A.; Zhu, S.; Meng, Z.; Meng, H.; Hou, C.; Zhang, Y.; Liu, Q.; Yu, R.; et al. Full-Textile Wireless Flexible Humidity Sensor for Human Physiological Monitoring. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- ATmega328P; Datasheet; Atmel: San Jose, CA, USA, 2015.
- Available online: https://www.Sparkfun.Com/Products/15590 (accessed on 1 March 2022).
- Available online: https://www.Respelearning.Scot/Topic-1-Anatomy-and-Physiology/Normal-Breathing (accessed on 1 March 2022).
- Rajabi-Abhari, A.; Kim, J.-N.; Lee, J.; Tabassian, R.; Mahato, M.; Youn, H.J.; Lee, H.; Oh, I.-K. Diatom Bio-Silica and Cellulose Nanofibril for Bio-Triboelectric Nanogenerators and Self-Powered Breath Monitoring Masks. ACS Appl. Mater. Interfaces 2020, 13, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, G.; Chen, C.; Gong, Q.; Xie, G.; Yao, M.; Tai, H.; Jiang, Y.; Chen, J. Self-Powered Respiration Monitoring Enabled by a Triboelectric Nanogenerator. Adv. Mater. 2021, 33, 2101262. [Google Scholar] [CrossRef]
- Bošković, M.V.; Sarajlić, M.; Frantlović, M.; Smiljanić, M.M.; Randjelović, D.V.; Zobenica, K.C.; Radović, D.V. Aluminum-Based Self-Powered Hyper-Fast Miniaturized Sensor for Breath Humidity Detection. Sens. Actuators B Chem. 2020, 321, 128635. [Google Scholar] [CrossRef]
- Zhou, Z.; Padgett, S.; Cai, Z.; Conta, G.; Wu, Y.; He, Q.; Zhang, S.; Sun, C.; Liu, J.; Fan, E. Single-Layered Ultra-Soft Washable Smart Textiles for All-around Ballistocardiograph, Respiration, and Posture Monitoring during Sleep. Biosens. Bioelectron. 2020, 155, 112064. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Jin, Y.M.; Ouyang, H.; Zou, Y.; Wang, X.X.; Xie, L.X.; Li, Z. Flexible Piezoelectric Nanogenerator in Wearable Self-Powered Active Sensor for Respiration and Healthcare Monitoring. Semicond. Sci. Technol. 2017, 32, 064004. [Google Scholar] [CrossRef]
- Kano, S.; Yamamoto, A.; Ishikawa, A.; Fujii, M. Respiratory Rate on Exercise Measured by Nanoparticle-Based Humidity Sensor. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3567–3570. [Google Scholar]
- Hoffmann, T.; Eilebrecht, B.; Leonhardt, S. Respiratory Monitoring System on the Basis of Capacitive Textile Force Sensors. IEEE Sens. J. 2010, 11, 1112–1119. [Google Scholar] [CrossRef]








| Reference, Year | Type of the Sensor | Mounting Details | Reported Limitations |
|---|---|---|---|
| [10], 2008 | Piezo-resistive sensor | Respiratory belt | Relatively complex readout circuit; large size; change in conditions between skin and electrodes over time causes changes in sensor response; the sensor positioning is very restricted due to sensitivity to the chest and abdominal breathing; limited sampling rate. |
| [11], 2015 | Weft-knitted resistive strain sensor | ||
| [12], 2006 | An inductive fiber meshed strain sensor | ||
| [13], 2014 | Textile capacitive force sensor | ||
| [17], 2013 | Piezoresistive sensor | ||
| [16], 2013 | Textile wearable capacitive sensor | Two electrodes are fixed on the inner anterior and posterior sides of a T-shirt. | The sensor positioning is very restricted due to sensitivity to chest and abdominal breathing; limited sampling rate. |
| [18], 2016 | Paper-based moisture sensor | Sensor embedded in the facemask. | Linear response in a very narrow humidity range, fast response and recovery speed, and non-toxicity are still not fully reached. |
| [19], 2021 | Flexible pressure sensor | Sensor embedded in the facemask. | Lack of ability to allow air to pass through the sensor and complicated fabrication process. |
| [20], 2020 | Differential MEMS pressure sensor and Venturi tube | Sensor embedded in the facemask with a robust plastic case. | Complex mechanical structure; total weight of the mask device is 160 g; solution based on the commercial sensors has limited level of innovation. |
| Nominal Value | Z (Ω) | θ (deg) | Cs (pF) |
|---|---|---|---|
| 27 pF ± 10% | 539.027 | −89.931 | 29.5264 |
| 47 pF ± 10% | 324.034 | −89.958 | 49.1168 |
| Nominal Capacitance | Measured with HIOKI | Average ADC Value | SD of ADC Value | Cs (pF) |
|---|---|---|---|---|
| 27 pF ± 10% | 29.53 pF | 671.94 | 0.24 | 25.99 |
| 47 pF ± 10% | 49.12 pF | 543.86 | 0.35 | 25.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simić, M.; Stavrakis, A.K.; Sinha, A.; Premčevski, V.; Markoski, B.; Stojanović, G.M. Portable Respiration Monitoring System with an Embroidered Capacitive Facemask Sensor. Biosensors 2022, 12, 339. https://doi.org/10.3390/bios12050339
Simić M, Stavrakis AK, Sinha A, Premčevski V, Markoski B, Stojanović GM. Portable Respiration Monitoring System with an Embroidered Capacitive Facemask Sensor. Biosensors. 2022; 12(5):339. https://doi.org/10.3390/bios12050339
Chicago/Turabian StyleSimić, Mitar, Adrian K. Stavrakis, Ankita Sinha, Velibor Premčevski, Branko Markoski, and Goran M. Stojanović. 2022. "Portable Respiration Monitoring System with an Embroidered Capacitive Facemask Sensor" Biosensors 12, no. 5: 339. https://doi.org/10.3390/bios12050339
APA StyleSimić, M., Stavrakis, A. K., Sinha, A., Premčevski, V., Markoski, B., & Stojanović, G. M. (2022). Portable Respiration Monitoring System with an Embroidered Capacitive Facemask Sensor. Biosensors, 12(5), 339. https://doi.org/10.3390/bios12050339








