Nanozymes with Multiple Activities: Prospects in Analytical Sensing
Abstract
:1. Introduction
2. Types of Enzyme-like Catalytic Activities
2.1. Peroxidase Mimics
2.2. Catalase Mimics
2.3. Oxidase Mimics
2.4. Superoxide Dismutase Mimics
2.5. Hydrolase Mimics
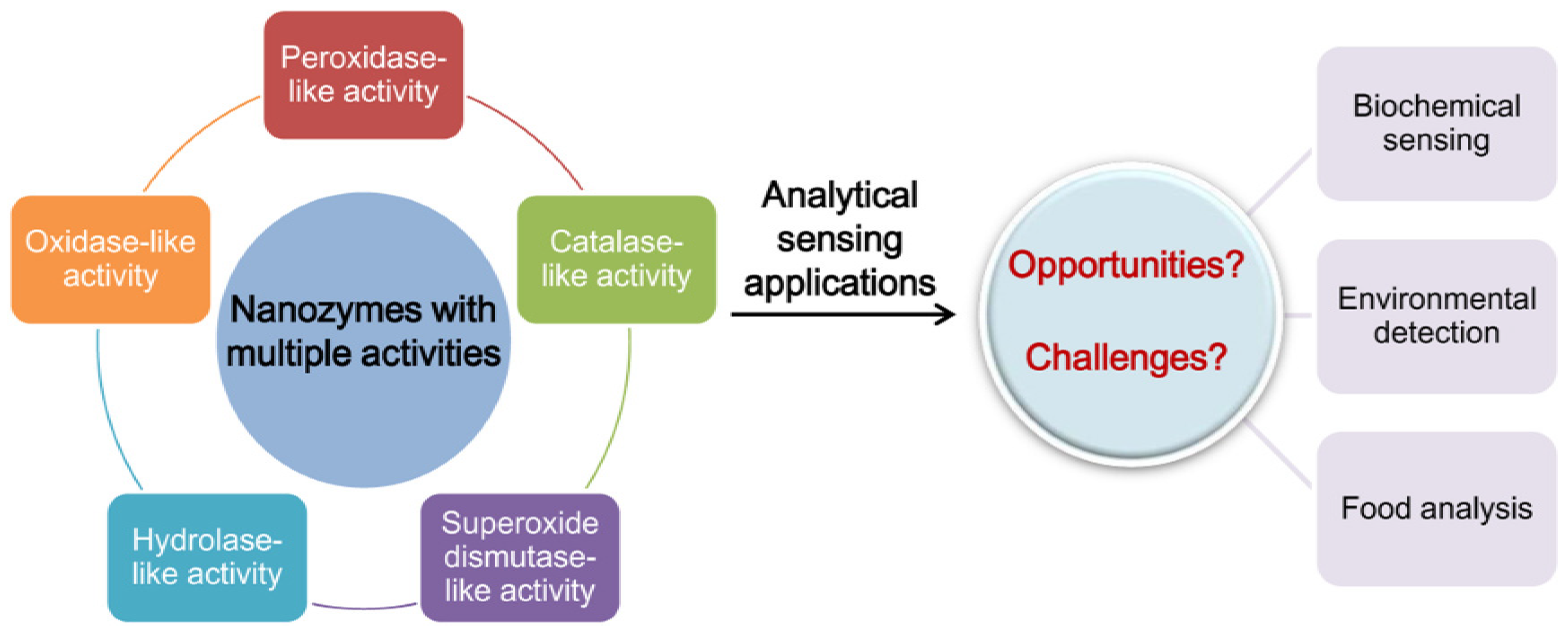
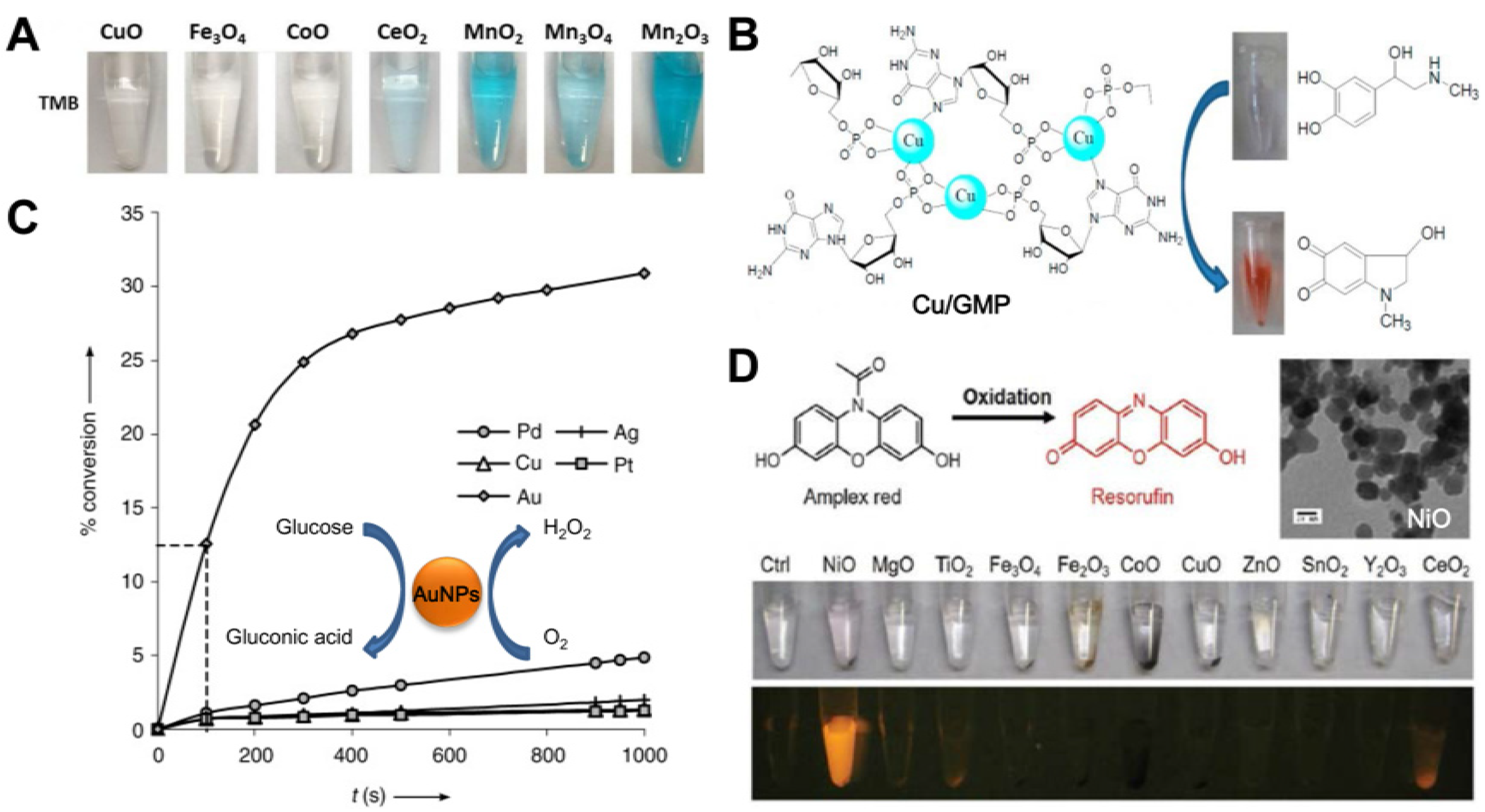
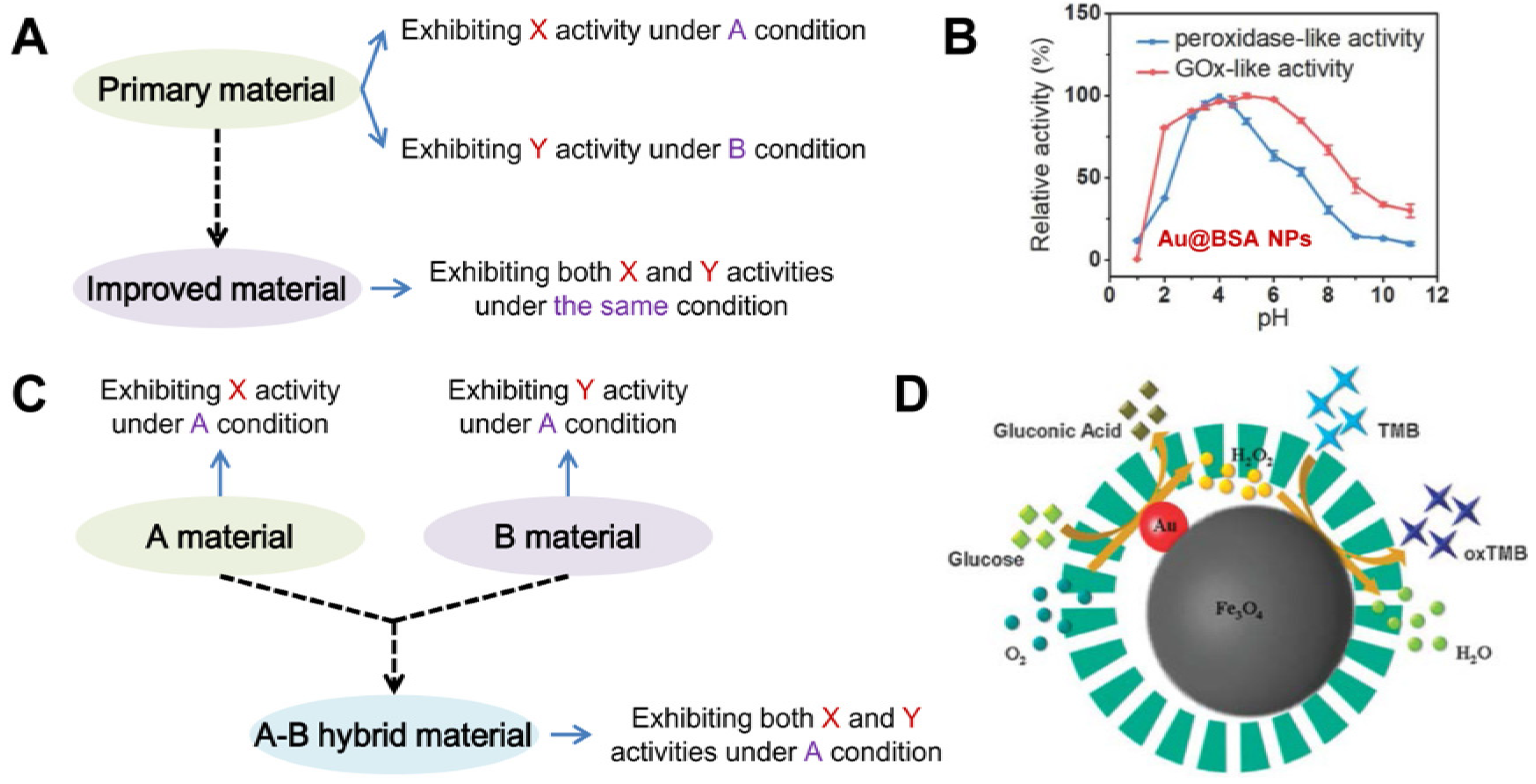
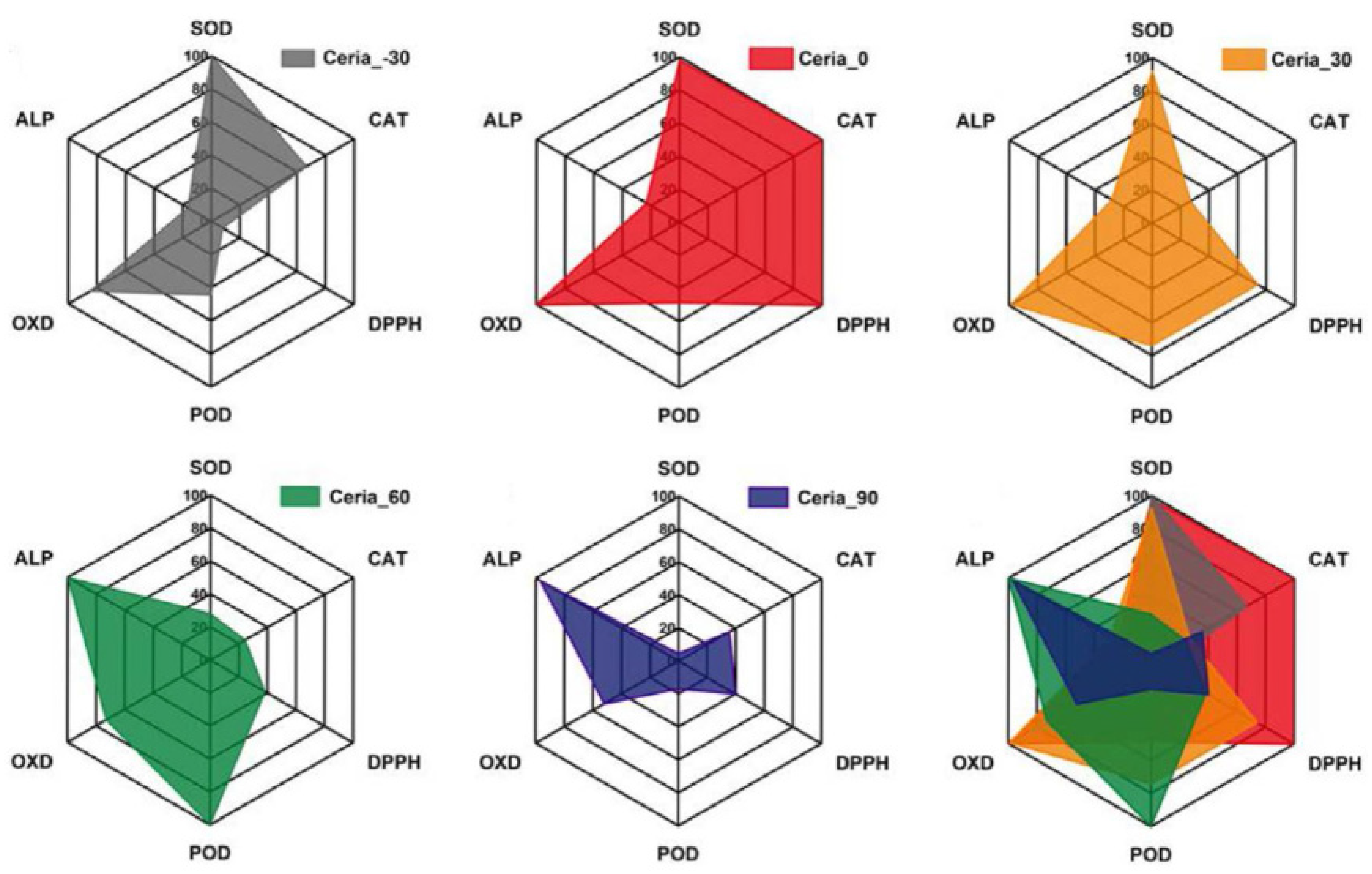
3. Nanozymes with Multiple Activities
3.1. Strategies to Design Nanozymes with Multiple Activities
3.2. Typical Nanozymes with Multiple Activities
4. Applications of Nanozymes with Multiple Activities in Analytical Sensing
5. Conclusions
- (1)
- Types of multi-activity materials. Although the materials that can be used as enzyme mimics have been expanded to various transition metals, noble metals and carbon-based materials from initial Fe3O4 particles, the types of multi-activity nanozyme materials developed are still very limited currently. To expand their potential applications and better employ them in biochemical sensing, more materials with multiple enzyme-like activities are expected to be designed and developed in future;
- (2)
- Types of multiple activities. Currently, most applications of multi-activity nanozymes are performed on the basis of their oxidoreductase-like activities, including POD, OXD, CAT and SOD. In fact, there are several other types of enzyme activities that artificial materials can simulate according to bionics. Integrating other types of enzyme-like activities with these oxidoreductase-like activities will break new ground for various applications, not limited to analytical sensing.
- (3)
- Rational regulation of the interactions between multiple activities. Rational design of hybrid nanozymes with multi-enzyme-mimetic activities and accurate regulation of the interactions between these multiple activities, including proximity effect, confinement effect and mass diffusion path, are significant in thedevelopment of high-efficiency cascade catalytic and sensing systems. Strictly, only materials featuring multi-enzyme-like activities can be served as “all-in-one” catalysts, thus providing high-performance cascade reaction systems for biomedical and sensing applications.
- (4)
- Development of new analytical methods and sensors using the multiple activities of nanozymes. As can be seen from Table 1, although a number of materials have been reported to have multi-enzyme-like activities, their applications in the analytical sensing field is still very limited. More seriously, a majority of these reported activities are not rationally utilized to serve analytical applications. In this regard, further effort is necessary to design and develop more detection methods and sensors to advance the nanozyme-involved sensing field.
- (5)
- Integration of multiple activities with multiple functions in a nanozyme. Because of the nature of the nanoscale material, nanozymes not only present the enzyme-like catalytic feature but can also provide other optical, electrical and magnetic characteristics. For instance, metal nanoclusters not only show a fluorescent property, but have also been demonstrated to present enzyme-like behaviors [116,117,118]. Combining the catalytic activity of these nanoclusters with their optical features will open up a new window for nanozyme-based analytical sensing.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-mIM | 2-methylimidazolate |
| AAO | ascorbic acid oxidase |
| ABTS | 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ACP | acid phosphatase |
| ALP | alkaline phosphatase |
| AuNP@AuNCs | Au nanoparticles encapsulated by Au nanoclusters |
| AuNPs | gold nanoparticles |
| BA | benzoic anhydride |
| BrPE | 2-bromo-1-phenylethanone |
| BSA | bovine serum albumin |
| CAT | catalase |
| DAB | di-azo-amino-benzene |
| DMNP | dimethyl 4-nitrophenyl phosphate |
| EMSN | expanded mesoporous silica support |
| Fe3O4-Au@MS | mesoporous silica-coated Fe3O4-Au |
| GMP | guanosine monophosphate |
| GO-COOH | carboxyl-modified graphene oxide |
| GODs | graphene quantum dots |
| GOx | glucose oxidase |
| GPx | glutathione peroxidase |
| hCAII | human carbonic anhydrase II |
| HRP | horseradish peroxidase |
| LAC | laccase |
| MNP | magnetic nanoparticles |
| MOF | metal-organic framework |
| OPD | o-phenyl-enediamine |
| OXD | oxidase |
| PH | phenyl-hydrazine |
| pNPA | p-nitro-phenyl acetate |
| POD | peroxidase |
| SOD | superoxide dismutase |
| TA | tereph-thalic acid |
| TCPP | tetra-carboxylic phenyl porphyrin |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| V-HPO | vanadium-dependent halo-peroxidase |
| ZIFs | zeolitic imidazolate frameworks |
References
- Murakami, Y.; Kikuchi, J.; Hisaeda, Y.; Hayashida, O. Artificial Enzymes. Chem. Rev. 1996, 96, 721–758. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Luo, Q.; Liu, J. Artificial Enzymes Based on Supramolecular Scaffolds. Chem. Soc. Rev. 2012, 41, 7890–7908. [Google Scholar] [CrossRef] [PubMed]
- Motherwell, W.B.; Bingham, M.J.; Six, Y. Recent Progress in the Design and Synthesis of Artificial Enzymes. Tetrahedron 2001, 57, 4663–4686. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Lyu, Z.; Pan, J.; Ding, S.; Ruan, X.; Zhu, W.; Du, D.; Lin, Y. Metal–Organic Framework Based Nanozymes: Promising Materials for Biochemical Analysis. Chem. Commun. 2020, 56, 11338–11353. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Liu, P.; Wang, M.; Pan, J.; Qiu, F.; Ni, L.; Niu, X. Realizing Selective Detection with Nanozymes: Strategies and Trends. TrAC Trends Anal. Chem. 2021, 143, 116379. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New Horizons for Responsive Biomedical Applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Liu, F.; Wang, Y.; Li, N.; Ding, B. Enzyme Mimic Nanomaterials and Their Biomedical Applications. ChemBioChem 2020, 21, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.L.S.; Vuong, K.Q.; Chow, E. Nanozymes for Environmental Pollutant Monitoring and Remediation. Sensors 2021, 21, 408. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.A.P.; Leonel, A.G.; Krambrock, K.; Mansur, H.S. Bifunctional Oxidase-Peroxidase Inorganic Nanozyme Catalytic Cascade for Wastewater Remediation. Catal. Today 2021. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Du, D.; Ni, L.; Pan, J.; Niu, X. Emerging Applications of Nanozymes in Environmental Analysis: Opportunities and Trends. TrAC Trends Anal. Chem. 2019, 120, 115653. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Zhou, X.; Yu, Y.; Liu, J.; Hu, N.; Wang, H.; Li, G.; Wu, Y. Recent Progress in the Construction of Nanozyme-Based Biosensors and Their Applications to Food Safety Assay. TrAC Trends Anal. Chem. 2019, 121, 115668. [Google Scholar] [CrossRef]
- Huang, L.; Sun, D.; Pu, H.; Wei, Q. Development of Nanozymes for Food Quality and Safety Detection: Principles and Recent Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1496–1513. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, Y.; Wang, Q.; Lin, A.; Wei, H. Nanozyme-Enabled Analytical Chemistry. Anal. Chem. 2022, 94, 312–323. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Niu, X.; Ye, K.; Ni, L.; Du, D.; Pan, J.; Lin, Y. Tri-Functional Fe–Zr Bi-metal–Organic Frameworks Enable High-Performance Phosphate Ion Ratiometric Fluorescent Detection. Nanoscale 2020, 12, 19383–19389. [Google Scholar] [CrossRef]
- Cai, X.; Jiao, L.; Yan, H.; Wu, Y.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Nanozyme-Involved Biomimetic Cascade Catalysis for Biomedical Applications. Mater. Today 2021, 44, 211–228. [Google Scholar] [CrossRef]
- Niu, X.; Cheng, N.; Ruan, X.; Du, D.; Lin, Y. Review—Nanozyme-Based Immunosensors and Immunoassays: Recent Developments and Future Trends. J. Electrochem. Soc. 2020, 167, 037508. [Google Scholar] [CrossRef]
- Chatterjee, B.; Das, S.J.; Anand, A.; Sharma, T.K. Nanozymes and Aptamer-Based Biosensing. Mater. Sci. Energy Technol. 2020, 3, 127–135. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, J.; Liu, W.; Qi, L. Boosting the Peroxidase-like Activity of Gold Nanoclusters for the Colorimetric Detection of Oxytetracycline in Rat Serum. Analyst 2021, 146, 5061–5066. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Bain, D.; Chakraborty, S.; Kolay, S.; Patra, A. Copper Nanocluster (Cu23 NC)-Based Biomimetic System with Peroxidase Activity. ACS Sustain. Chem. Eng. 2019, 8, 18335–18344. [Google Scholar] [CrossRef]
- Xue, Q.; Li, X.; Peng, Y.; Liu, P.; Peng, H.; Niu, X. Polyethylenimine-Stabilized Silver Nanoclusters Act as an Oxidoreductase Mimic for Colorimetric Determination of Chromium(VI). Mikrochim. Acta 2020, 187, 263. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; He, Y.; Li, X.; Zhao, H.; Pan, J.; Qiu, F.; Lan, M. A Peroxidase-Mimicking Nanosensor with Hg2+-Triggered Enzymatic Activity of Cysteine-Decorated Ferromagnetic Particles for Ultrasensitive Hg2+ Detection in Environmental and Biological Fluids. Sens. Actuators B Chem. 2019, 281, 445–452. [Google Scholar] [CrossRef]
- Li, X.; Niu, X.; Liu, P.; Xu, X.; Du, D.; Lin, Y. High-Performance Dual-Channel Ratiometric Colorimetric Sensing of Phosphate Ion Based on Target-Induced Differential Oxidase-like Activity Changes of Ce-Zr Bimetal-Organic Frameworks. Sensors Actuators B Chem. 2020, 321, 128546. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Liu, P.; Wang, M.; Niu, X.; Pan, J. A Single-Nanozyme Colorimetric Array Based on Target-Induced Differential Surface Passivation for Quantification and Discrimination of Cl-, Br- and I- Ions. Anal. Chim. Acta 2021, 1160, 338451. [Google Scholar] [CrossRef]
- Niu, X.; Wang, M.; Zhu, H.; Liu, P.; Pan, J.; Liu, B. Nanozyme Catalysis-Assisted Ratiometric Multicolor Sensing of Heparin Based on Target-Specific Electrostatic-Induced Aggregation. Talanta 2022, 238, 123003. [Google Scholar] [CrossRef]
- Gao, S.; Lin, H.; Zhang, H.; Yao, H.; Chen, Y.; Shi, J. Nanocatalytic Tumor Therapy by Biomimetic Dual Inorganic Nanozyme-Catalyzed Cascade Reaction. Adv. Sci. 2018, 6, 1801733. [Google Scholar] [CrossRef]
- Mu, X.; Wang, J.; Li, Y.; Xu, F.; Long, W.; Ouyang, L.; Liu, H.; Jing, Y.; Wang, J.; Dai, H.; et al. Redox Trimetallic Nanozyme with Neutral Environment Preference for Brain Injury. ACS Nano 2019, 13, 1870–1884. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qing, Y.; Jing, L.; Zou, W.; Guo, R. Platinum–Copper Bimetallic Colloid Nanoparticle Cluster Nanozymes with Multiple Enzyme-like Activities for Scavenging Reactive Oxygen Species. Langmuir 2021, 37, 7364–7372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Younis, M.R.; Liu, H.; Lei, S.; Wan, Y.; Qu, J.; Lin, J.; Huang, P. Multi-Enzyme Mimetic Ultrasmall Iridium Nanozymes as Reactive Oxygen/Nitrogen Species Scavengers for Acute Kidney Injury Management. Biomaterials 2021, 271, 120706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, W.; Cheng, J.; Qu, Y.; Dai, Y.; Liu, M.; Yu, J.; Wang, C.; Wang, H.; Wang, S.; et al. Stimuli-Responsive Manganese Single-Atom Nanozyme for Tumor Therapy via Integrated Cascade Reactions. Angew. Chem. Int. Ed. 2021, 60, 9480–9488. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Tan, X.; Li, T.; Liu, S.; Li, Y.; Li, H. Norepinephrine-Induced AuPd Aerogels with Peroxidase- and Glucose Oxidase-like Activity for Colorimetric Determination of Glucose. Mikrochim. Acta 2021, 188, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, X.; Han, L.; Li, F. “Non-naked” Gold with Glucose Oxidase-like Activity: A Nanozyme for Tandem Catalysis. Small 2018, 14, 1803256. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, W.; Huang, L.; Ma, Q.; Dong, S. Self-Indicative Gold Nanozyme for H2O2 and Glucose Sensing. Chem. Eur. J. 2019, 25, 11940–11944. [Google Scholar] [CrossRef]
- He, X.; Tan, L.; Chen, D.; Wu, X.; Ren, X.; Zhang, Y.; Meng, X.; Tang, F. Fe3O4–Au@mesoporous SiO2 Microspheres: An Ideal Artificial Enzymatic Cascade System. Chem. Commun. 2013, 49, 4643–4645. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, H.; Qi, F.; Niu, X.; Xu, X.; Qiu, F.; He, Y.; Pan, J.; Ni, L. Three Hidden Talents in One Framework: A Terephthalic Acid-Coordinated Cupric Metal–Organic Framework with Cascade Cysteine Oxidase- And Peroxidase-Mimicking Activities and Stimulus-Responsive Fluorescence for Cysteine Sensing. J. Mater. Chem. B 2018, 6, 6207–6211. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An Emerging Alternative to Natural Enzyme for Biosensing and Immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Song, W.; Zhao, B.; Wang, C.; Ozaki, Y.; Lu, X. Functional Nanomaterials with Unique Enzyme-like Characteristics for Sensing Applications. J. Mater. Chem. B 2019, 7, 850–875. [Google Scholar] [CrossRef] [PubMed]
- Ragg, R.; Tahir, M.N.; Tremel, W. Solids Go Bio: Inorganic Nanoparticles as Enzyme Mimics. Eur. J. Inorg. Chem. 2016, 2016, 1906–1915. [Google Scholar] [CrossRef]
- André, R.; Natálio, F.; Humanes, M.; Leppin, J.; Heinze, K.; Wever, R.; Schröder, H.-C.; Müller, W.E.G.; Tremel, W. V2O5 Nanowires with an Intrinsic Peroxidase-Like Activity. Adv. Funct. Mater. 2011, 21, 501–509. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, A.; Gao, N.; Li, K.; Ren, J.; Qu, X. Deciphering a Nanocarbon-Based Artificial Peroxidase: Chemical Identification of the Catalytically Active and Substrate-Binding Sites on Graphene Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 7176–7180. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yang, D.; Han, X.; Cai, J.; Liu, H.; He, W. Peroxidase-like Activity of the Co3O4 Nanoparticles Used for Biodetection and Evaluation of Antioxidant Behavior. Nanoscale 2016, 8, 5938–5945. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.; Chen, L.; Li, X.; Li, L.; Ai, S. Peroxidase-like Activity of Manganese Selenide Nanoparticles and Its Analytical Application for Visual Detection of Hydrogen Peroxide and Glucose. Sens. Actuators B Chem. 2014, 193, 255–262. [Google Scholar] [CrossRef]
- Lin, T.; Zhong, L.; Guo, L.; Fu, F.; Chen, G. Seeing Diabetes: Visual Detection of Glucose Based on the Intrinsic Peroxidase-like Activity of MoS2 Nanosheets. Nanoscale 2014, 6, 11856–11862. [Google Scholar] [CrossRef]
- Tripathi, A.; Harris, K.D.; Elias, A.L. Peroxidase-Like Behavior of Ni Thin Films Deposited by Glancing Angle Deposition for Enzyme-Free Uric Acid Sensing. ACS Omega 2020, 5, 9123–9130. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; He, Y.; Pan, J.; Li, X.; Qiu, F.; Yan, Y.; Shi, L.; Zhao, H.; Lan, M. Uncapped Nanobranch-Based CuS Clews Used as an Efficient Peroxidase Mimic Enable the Visual Detection of Hydrogen Peroxide and Glucose with Fast Response. Anal. Chim. Acta 2016, 947, 42–49. [Google Scholar] [CrossRef]
- He, Y.; Qi, F.; Niu, X.; Zhang, W.; Zhang, X.; Pan, J. Uricase-Free On-Demand Colorimetric Biosensing of Uric Acid Enabled by Integrated CoP Nanosheet Arrays as a Monolithic Peroxidase Mimic. Anal. Chim. Acta 2018, 1021, 113–120. [Google Scholar] [CrossRef]
- Cui, M.; Zhou, J.; Zhao, Y.; Song, Q. Facile Synthesis of Iridium Nanoparticles with Superior Peroxidase-like Activity for Colorimetric Determination of H2O2 and Xanthine. Sens. Actuators B Chem. 2017, 243, 203–210. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, Y.; Gu, N. Peroxidase-like Catalytic Activity of Cubic Pt Nanocrystals. Colloids Surf. A Physicochem. Eng. Asp. 2011, 373, 6–10. [Google Scholar] [CrossRef]
- He, W.; Wu, X.; Liu, J.; Hu, X.; Zhang, K.; Hou, S.; Zhou, W.; Xie, S. Design of AgM Bimetallic Alloy Nanostructures (M = Au, Pd, Pt) with Tunable Morphology and Peroxidase-Like Activity. Chem. Mater. 2010, 22, 2988–2994. [Google Scholar] [CrossRef]
- Zhang, W.; Niu, X.; Li, X.; He, Y.; Song, H.; Peng, Y.; Pan, J.; Qiu, F.; Zhao, H.; Lan, M. A Smartphone-Integrated Ready-To-Use Paper-Based Sensor with Mesoporous Carbon-Dispersed Pd Nanoparticles as a Highly Active Peroxidase Mimic for H2O2 Detection. Sens. Actuators B Chem. 2018, 265, 412–420. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, L.; Liang, Q.; Xi, J.; Zhao, H.; Jin, Y.; Gao, X.; Yan, X.; Gao, L.; Fan, K. Unveiling the Active Sites on Ferrihydrite with Apparent Catalase-like Activity for Potentiating Radiotherapy. Nano Today 2021, 41, 101317. [Google Scholar] [CrossRef]
- Hu, M.; Korschelt, K.; Daniel, P.; Landfester, K.; Tremel, W.; Bannwarth, M.B. Fibrous Nanozyme Dressings with Catalase-like Activity for H2O2 Reduction to Promote Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 38024–38031. [Google Scholar] [CrossRef]
- Aliaga, M.E.; Andrade-Acuña, D.; López-Alarcón, C.; Sandoval-Acuña, C.; Speisky, H. Cu(II)–Disulfide Complexes Display Simultaneous Superoxide Dismutase- And Catalase-like Activities. J. Inorg. Biochem. 2013, 129, 119–126. [Google Scholar] [CrossRef]
- Mu, J.; Wang, Y.; Zhao, M.; Zhang, L. Intrinsic Peroxidase-like Activity and Catalase-like Activity of Co3O4 Nanoparticles. Chem. Commun. 2012, 48, 2540–2542. [Google Scholar] [CrossRef]
- Zhen, W.; Liu, Y.; Lin, L.; Bai, J.; Jia, X.; Tian, H.; Jiang, X. BSA-IrO2: Catalase-like Nanoparticles with High Photothermal Conversion Efficiency and a High X-Ray Absorption Coefficient for Anti-inflammation and Antitumor Theranostics. Angew. Chem. 2018, 130, 10466–10470. [Google Scholar] [CrossRef]
- Liu, C.-P.; Wu, T.-H.; Liu, C.-Y.; Chen, K.-C.; Chen, Y.-X.; Chen, G.-S.; Lin, S.-Y. Self-Supplying O2 through the Catalase-Like Activity of Gold Nanoclusters for Photodynamic Therapy against Hypoxic Cancer Cells. Small 2017, 13, 1700278. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, W.; Wu, X.; Gao, X. Mechanism of pH-Switchable Peroxidase and Catalase-like Activities of Gold, Silver, Platinum and Palladium. Biomaterials 2015, 48, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Song, Y.; Wu, X.; Zhu, C.; Su, X.; Du, D.; Lin, Y. Oxidase-Mimicking Activity of Ultrathin MnO2 Nanosheets in Colorimetric Assay of Acetylcholinesterase Activity. Nanoscale 2017, 9, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Shang, C.; Gu, W.; Wang, E.; Dong, S. Introducing Ratiometric Fluorescence to MnO2 Nanosheet-Based Biosensing: A Simple, Label-Free Ratiometric Fluorescent Sensor Programmed by Cascade Logic Circuit for Ultrasensitive GSH Detection. ACS Appl. Mater. Interfaces 2017, 9, 25870–25877. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, Z.; Sun, Y.; Xu, Z.; Liu, J. The Most Active Oxidase-Mimicking Mn2O3 Nanozyme for Biosensor Signal Generation. Chem. Eur. J. 2021, 27, 9597–9604. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; Binks, B.P.; He, Z. Construction of a Bioinspired Laccase-Mimicking Nanozyme for the Degradation and Detection of Phenolic Pollutants. Appl. Catal. B Environ. 2019, 254, 452–462. [Google Scholar] [CrossRef]
- Liang, H.; Lin, F.; Zhang, Z.; Liu, B.; Jiang, S.; Yuan, Q.; Liu, J. Multicopper Laccase Mimicking Nanozymes with Nucleotides as Ligands. ACS Appl. Mater. Interfaces 2017, 9, 1352–1360. [Google Scholar] [CrossRef]
- Li, D.; Liu, B.; Huang, P.-J.J.; Zhang, Z.; Liu, J. Highly Active Fluorogenic Oxidase-Mimicking NiO Nanozymes. Chem. Commun. 2018, 54, 12519–12522. [Google Scholar] [CrossRef]
- Wang, T.; Su, P.; Li, H.; Yang, Y.; Yang, Y. Triple-enzyme Mimetic Activity of Co3O4 Nanotubes and Their Applications in Colorimetric Sensing of Glutathione. New J. Chem. 2016, 40, 10056–10063. [Google Scholar] [CrossRef]
- Zhu, M.; Wen, Y.; Song, S.; Zheng, A.; Li, J.; Sun, W.; Dai, Y.; Yin, K.; Sun, L. Synergistic Effects between Polyvinylpyrrolidone and Oxygen Vacancies on Improving the Oxidase-Mimetic Activity of Flower-like CeO2 Nanozymes. Nanoscale 2020, 12, 19104–19111. [Google Scholar] [CrossRef]
- Yang, H.; Xiao, J.; Su, L.; Feng, T.; Lv, Q.; Zhang, X. Oxidase-Mimicking Activity of the Nitrogen-Doped Fe3C@C Composites. Chem. Commun. 2017, 53, 3882–3885. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, K.; Pan, J.; Song, H.; Li, X.; Niu, X. A Catalytic Reaction-Based Colorimetric Assay of Alkaline Phosphatase Activity Based on Oxidase-like MnO2 Microspheres. Anal. Methods 2019, 11, 5472–5477. [Google Scholar] [CrossRef]
- Comotti, M.; Della Pina, C.; Matarrese, R.; Rossi, M. The Catalytic Activity of "Naked" Gold Particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhu, C.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C. Self-Catalyzed, Self-Limiting Growth of Glucose Oxidase-Mimicking Gold Nanoparticles. ACS Nano 2010, 4, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, Q.; Li, M.; Chao, D.; Huang, L.; Wu, W.; Fang, Y.; Dong, S. Glucose-Oxidase Like Catalytic Mechanism of Noble Metal Nanozymes. Nat. Commun. 2021, 12, 3375. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.; Yin, J.-J.; Ma, Y.; Zhang, P.; Li, J.; Ding, Y.; Zhang, J.; Zhao, Y.; Chai, Z.; et al. Acquired Superoxide-Scavenging Ability of Ceria Nanoparticles. Angew. Chem. 2014, 127, 1852–1855. [Google Scholar] [CrossRef]
- Naganuma, T. Shape Design of Cerium Oxide Nanoparticles for Enhancement of Enzyme Mimetic Activity in Therapeutic Applications. Nano Res. 2017, 10, 199–217. [Google Scholar] [CrossRef]
- Korschelt, K.; Ragg, R.; Metzger, C.S.; Kluenker, M.; Oster, M.; Barton, B.; Panthöfer, M.; Strand, D.; Kolb, U.; Mondeshki, M.; et al. Glycine-Functionalized Copper(II) Hydroxide Nanoparticles with High Intrinsic Superoxide Dismutase Activity. Nanoscale 2017, 9, 3952–3960. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, Y.; Wang, Z.; Cao, F.; Sang, Y.; Dong, K.; Pu, F.; Ren, J.; Qu, X. Constructing Metal–Organic Framework Nanodots as Bio-Inspired Artificial Superoxide Dismutase for Alleviating Endotoxemia. Mater. Horizons 2019, 6, 1682–1687. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.J.; Isley, W.C., III; Ghosh, P.; Liao, P.; Bury, W.; Wagner, G.W.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W.; et al. Destruction of Chemical Warfare Agents Using Metal–Organic Frameworks. Nat. Mater. 2015, 14, 512–516. [Google Scholar] [CrossRef]
- Li, P.; Klet, R.C.; Moon, S.-Y.; Wang, T.C.; Deria, P.; Peters, A.W.; Klahr, B.M.; Park, H.-J.; Al-Juaid, S.S.; Hupp, J.T.; et al. Synthesis of Nanocrystals of Zr-Based Metal–Organic Frameworks with Csq-Net: Significant Enhancement in the Degradation of a Nerve Agent Simulant. Chem. Commun. 2015, 51, 10925–10928. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.Y.; Wagner, G.W.; Mondloch, J.E.; Peterson, G.W.; DeCoste, J.B.; Hupp, J.T.; Farha, O.K. Effective, Facile, and Selective Hydrolysis of the Chemical Warfare Agent VX Using Zr6-Based Metal−Organic Frameworks. Inorg. Chem. 2015, 54, 10829–10833. [Google Scholar] [CrossRef]
- Park, H.J.; Jang, J.K.; Kim, S.-Y.; Ha, J.-W.; Moon, D.; Kang, I.-N.; Bae, Y.-S.; Kim, S.; Hwang, D.-H. Synthesis of a Zr-Based Metal–Organic Framework with Spirobifluorenetetrabenzoic Acid for the Effective Removal of Nerve Agent Simulants. Inorg. Chem. 2017, 56, 12098–12101. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liao, P.; Mendonca, M.L.; Snurr, R.Q. Insights into Catalytic Hydrolysis of Organophosphate Warfare Agents by Metal–Organic Framework NU-1000. J. Phys. Chem. C 2018, 122, 12362–12368. [Google Scholar] [CrossRef]
- Chen, J.; Huang, L.; Wang, Q.; Wu, W.; Zhang, H.; Fang, Y.; Dong, S. Bio-Inspired Nanozyme: A Hydratase Mimic in a Zeolitic Imidazolate Framework. Nanoscale 2019, 11, 5960–5966. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Tian, Z.; Zhang, Y.; Qu, Y. Phosphatase-like Activity of Porous Nanorods of CeO2 for the Highly Stabilized Dephosphorylation under Interferences. ACS Appl. Mater. Interfaces 2019, 11, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, J. Self-limited Phosphatase-Mimicking CeO2 Nanozymes. ChemNanoMat 2020, 6, 947–952. [Google Scholar] [CrossRef]
- Li, B.; Chen, D.; Wang, J.; Yan, Z.; Jiang, L.; Duan, D.; He, J.; Luo, Z.; Zhang, J.; Yuan, F. MOFzyme: Intrinsic Protease-like Activity of Cu-MOF. Sci. Rep. 2014, 4, 6759. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Chen, D.; Zhao, J.; Yang, D.; Ma, D.; Jiang, L.; Yang, Y.; Li, Y.; Wang, J. MOFzyme: FJU-21 with Intrinsic High Protease-Like Activity for Hydrolysis of Proteins. J. Biosci. Med. 2019, 7, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Savanur, M.A.; Srivastava, S.; D’Silva, P.; Mugesh, G. A Redox Modulatory Mn3O4 Nanozyme with Multi-Enzyme Activity Provides Efficient Cytoprotection to Human Cells in a Parkinson’s Disease Model. Angew. Chem. 2017, 129, 14455–14459. [Google Scholar] [CrossRef]
- Yin, X.; Liu, P.; Xu, X.; Pan, J.; Li, X.; Niu, X. Breaking the pH Limitation of Peroxidase-like CoFe2O4 Nanozyme via Vitriolization for One-Step Glucose Detection at Physiological pH. Sens. Actuators B Chem. 2021, 328, 129033. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Xu, X.; Pan, J.; Niu, X. A Cobalt-Based Polyoxometalate Nanozyme with High Peroxidase-Mimicking Activity at Neutral pH for One-Pot Colorimetric Analysis of Glucose. J. Mater. Chem. B 2018, 6, 5750–5755. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Xu, X.; Li, X.; Pan, J.; Qiu, F.; Zhao, H.; Lan, M. Surface Charge Engineering of Nanosized CuS via Acidic Amino Acid Modification Enables High Peroxidase-Mimicking Activity at Neutral pH for One-Pot Detection of Glucose. Chem. Commun. 2018, 54, 13443–13446. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pan, Y.; Yang, J.; Gao, Y.; Huang, T.; Luan, X.; Wang, Y.; Song, Y. Gold Nanoparticles Doped Metal-Organic Frameworks as Near-Infrared Light-Enhanced Cascade Nanozyme against Hypoxic Tumors. Nano Res. 2020, 13, 653–660. [Google Scholar] [CrossRef]
- Bhagat, S.; Vallabani, N.V.S.; Shutthanandan, V.; Bowden, M.; Karakoti, A.S.; Singh, S. Gold Core/Ceria Shell-Based Redox Active Nanozyme Mimicking the Biological Multienzyme Complex Phenomenon. J. Colloid Interface Sci. 2018, 513, 831–842. [Google Scholar] [CrossRef]
- Sun, M.; He, M.; Jiang, S.; Wang, Y.; Wang, X.; Liu, T.; Song, C.; Wang, S.; Rao, H.; Lu, Z. Multi-enzyme Activity of Three Layers FeOx@ZnMnFeOy@Fe-Mn Organogel for Colorimetric Detection of Antioxidants and Norfloxacin with Smartphone. Chem. Eng. J. 2021, 425, 131823. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Xu, J.; Zhou, H.; Gao, Z.; Song, Y. Pt Nanoparticle-Coupled WO2.72 Nanoplates as Multi-Enzyme Mimetics for Colorimetric Detection and Radical Elimination. Anal. Bioanal. Chem. 2020, 412, 521–530. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Y.; Zhang, Q.; Zou, W.; Jin, L.; Guo, R. Arginine-Rich Peptide/Platinum Hybrid Colloid Nanoparticle Cluster: A Single Nanozyme Mimicking Multi-Enzymatic Cascade Systems in Peroxisome. J. Colloid Interface Sci. 2021, 600, 37–48. [Google Scholar] [CrossRef]
- Tran, V.-K.; Gupta, P.K.; Park, Y.; Son, S.E.; Hur, W.; Lee, H.B.; Park, J.Y.; Kim, S.N.; Seong, G.H. Functionalized Bimetallic IrPt Alloy Nanoparticles: Multi-Enzyme Mimics for Colorimetric and Fluorometric Detection of Hydrogen Peroxide and Glucose. J. Taiwan Inst. Chem. Eng. 2021, 120, 336–343. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Peng, M.; Ren, G.; Guan, L.; Li, K.; Lin, Y. ZIF-67 as a Template Generating and Tuning “Raisin Pudding”-Type Nanozymes with Multiple Enzyme-like Activities: Toward Online Electrochemical Detection of 3,4-Dihydroxyphenylacetic Acid in Living Brains. ACS Appl. Mater. Interfaces 2020, 12, 29631–29640. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, D.; Cho, A.; Weon, S.; Lee, S.; Lee, J.; Han, J.W.; Kim, D.-P.; Choi, W. Modified Carbon Nitride Nanozyme as Bifunctional Glucose Oxidase-Peroxidase for Metal-Free Bioinspired Cascade Photocatalysis. Nat. Commun. 2019, 10, 91–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, S.; Wang, J.; Cai, Y.; Niu, L.; Liu, X.; Liu, C.; Qi, H.; Liu, A. Copper Sulfide Nanoclusters with Multi-Enzyme-like Activities and Its Application in Acid Phosphatase Sensing Based on Enzymatic Cascade Reaction. Talanta 2021, 233, 122594. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chang, Q.; Li, N.; Xue, C.; Liu, H.; Yang, J.; Hu, S.; Wang, H. Carbon Dots-stabilized Cu4O3 for a Multi-Responsive Nanozyme with Exceptionally High Activity. Chem. Eng. J. 2020, 394, 125045. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; Qu, X. Nano-Gold as Artificial Enzymes: Hidden Talents. Adv. Mater. 2014, 26, 4200–4217. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Aloui, H.; Kim, B.S. Novel Biogenic Gold Nanoparticles Catalyzing Multienzyme Cascade Reaction: Glucose Oxidase and Peroxidase Mimicking Activity. Chem. Eng. J. 2021, 421, 127859. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Z.; Chen, Z.; Ren, J.; Qu, X. Mesoporous Silica-Encapsulated Gold Nanoparticles as Artificial Enzymes for Self-Activated Cascade Catalysis. Biomaterials 2013, 34, 2600–2610. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, Y.; Zhang, J.; Chou, J.-P.; Hu, A.; Peng, Y. Nanoisozymes: The Origin behind Pristine CeO2 as Enzyme Mimetics. Chem. Eur. J. 2020, 26, 10598–10606. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Liu, Q.; Lin, A.; Li, S.; Zhang, Y.; Wang, Q.; Li, T.; An, X.; Zhou, Z.; et al. Synthesis-Temperature-Regulated Multi-Enzyme-Mimicking Activities of Ceria Nanozymes. J. Mater. Chem. B 2021, 9, 7238–7245. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Gu, Z.; Miao, X.; Huang, Q.; Chang, B. Temperature-Responsive Iron Nanozymes Based on Poly(N-Vinylcaprolactam) with Multi-Enzyme Activity. RSC Adv. 2020, 10, 39954–39966. [Google Scholar] [CrossRef]
- Guo, S.; Guo, L. Unraveling the Multi-Enzyme-Like Activities of Iron Oxide Nanozyme via a First-Principles Microkinetic Study. J. Phys. Chem. C 2019, 123, 30318–30334. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Cheng, J.; Xu, Y.; Chen, W.; Li, Y. Facile Preparation of Four-in-One Nanozyme Catalytic Platform and the Application in Selective Detection of Catechol and Hydroquinone. Sens. Actuators B Chem. 2021, 337, 129763. [Google Scholar] [CrossRef]
- Guo, X.; Yang, F.; Jing, L.; Li, J.; Li, Y.; Ding, R.; Duan, B.; Zhang, X. In-situ Generation of Highly Active and Four-in-One CoFe2O4/H2PPOP Nanozyme: Mechanism and its Spplication for Fast Colorimetric Detection of Cr(VI). J. Hazard. Mater. 2022, 431, 128621. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, L.; Ren, H.; Cai, Y.; Liu, C.; Zeng, L.; Guo, J.; Liu, A. Facile Synthesis of Magnetic Hierarchical Flower-like Co3O4 Spheres: Mechanism, Excellent Tetra-Enzyme Mimics and Their Colorimetric Biosensing Applications. Biosens. Bioelectron. 2020, 165, 112342. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Y.; Zhong, M.; Yu, D.; Wang, C.; Lu, X. Fe(III)-Tannic Acid Complex Derived Fe3C Decorated Carbon Nanofibers for Triple-Enzyme Mimetic Activity and Their Biosensing Application. ACS Biomater. Sci. Eng. 2019, 5, 1238–1246. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, B.; Thakur, N.; Mishra, S.; Sarma, T.K. Copper Pyrovanadate Nanoribbons as Efficient Multienzyme Mimicking Nanozyme for Biosensing Applications. ACS Appl. Nano Mater. 2020, 3, 7917–7929. [Google Scholar] [CrossRef]
- Yan, Z.; Niu, Q.; Mou, M.; Wu, Y.; Liu, X.; Liao, S. A Novel Colorimetric Method Based on Copper Nanoclusters with Intrinsic Peroxidase-like for Detecting Xanthine in Serum Samples. J. Nanoparticle Res. 2017, 19, 235. [Google Scholar] [CrossRef]
- Hu, L.; Liao, H.; Feng, L.; Wang, M.; Fu, W. Accelerating the Peroxidase-Like Activity of Gold Nanoclusters at Neutral pH for Colorimetric Detection of Heparin and Heparinase Activity. Anal. Chem. 2018, 90, 6247–6252. [Google Scholar] [CrossRef]
- Tan, F.; Xie, X.; Xu, A.; Deng, K.; Zeng, Y.; Yang, X.; Huang, H. Fabricating and Regulating Peroxidase-like Activity of Eggshell Membrane-Templated Gold Nanoclusters for Colorimetric Detection of Staphylococcal Enterotoxin B. Talanta 2019, 194, 634–642. [Google Scholar] [CrossRef]




| Nanozyme | Potential Activity | Activity Used | Analyte | Detection Mode | Real Sample | Ref. |
|---|---|---|---|---|---|---|
| AuPd-NE | GOx, POD | GOx and POD | glucose | colorimetric | human serum | [35] |
| CuS | POD, CAT, AAO, SOD | AAO | ACP | fluorescence | human serum | [102] |
| Co1.5Mn1.5O4 | LAC, POD, OXD, CAT | LAC; OXD | catechol; hydroquinone | colorimetric | water samples | [111] |
| PVP/IrPt | POD, OXD, CAT | POD | glucose | colorimetric and fluorescence | none | [99] |
| CoFe2O4/H2PPOP | OXD, POD, CAT, SOD | OXD | Cr(Ⅵ) | colorimetric | water samples | [112] |
| Co3O4 | OXD, POD, CAT, SOD | OXD; POD | ACP; H2O2 | colorimetric | human serum | [113] |
| Pt/WO2.72 | POD, CAT | POD | H2O2 and glucose | colorimetric | human serum | [97] |
| FeOx@ZnMnFeOy@Fe-Mn | POD, OXD, CAT | POD; OXD | citric acid and norfloxacin; gallic acid | colorimetric | juice | [96] |
| AuNP@AuNCs | GOx, POD | GOx and POD | glucose | colorimetric and fluorescence | none | [37] |
| GNE-based AuNPs | GOx, POD | GOx and POD | glucose | colorimetric | none | [105] |
| ZIF-67/Cu0.76Co2.24O4 | POD, GPx, SOD, CAT | CAT | 3,4-dihydroxyphenylacetic acid | electrochemical | rat brain | [100] |
| Au@BSA-GO | GOx, POD | GOx and POD | glucose | colorimetric | none | [36] |
| Fe3O4-Au@MS | GOx, POD | GOx and POD | glucose | colorimetric | none | [38] |
| Fe3C/C | POD, OXD, CAT | OXD | glutathione | colorimetric | none | [114] |
| Cu3V2O7(OH)2 | POD, OXD, LAC | OXD | glutathione | colorimetric | human serum | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Liu, B.; Hu, P.; Zhu, H.; Wang, M. Nanozymes with Multiple Activities: Prospects in Analytical Sensing. Biosensors 2022, 12, 251. https://doi.org/10.3390/bios12040251
Niu X, Liu B, Hu P, Zhu H, Wang M. Nanozymes with Multiple Activities: Prospects in Analytical Sensing. Biosensors. 2022; 12(4):251. https://doi.org/10.3390/bios12040251
Chicago/Turabian StyleNiu, Xiangheng, Bangxiang Liu, Panwang Hu, Hengjia Zhu, and Mengzhu Wang. 2022. "Nanozymes with Multiple Activities: Prospects in Analytical Sensing" Biosensors 12, no. 4: 251. https://doi.org/10.3390/bios12040251
APA StyleNiu, X., Liu, B., Hu, P., Zhu, H., & Wang, M. (2022). Nanozymes with Multiple Activities: Prospects in Analytical Sensing. Biosensors, 12(4), 251. https://doi.org/10.3390/bios12040251






