Overview of Liquid Crystal Biosensors: From Basic Theory to Advanced Applications
Abstract
1. Introduction
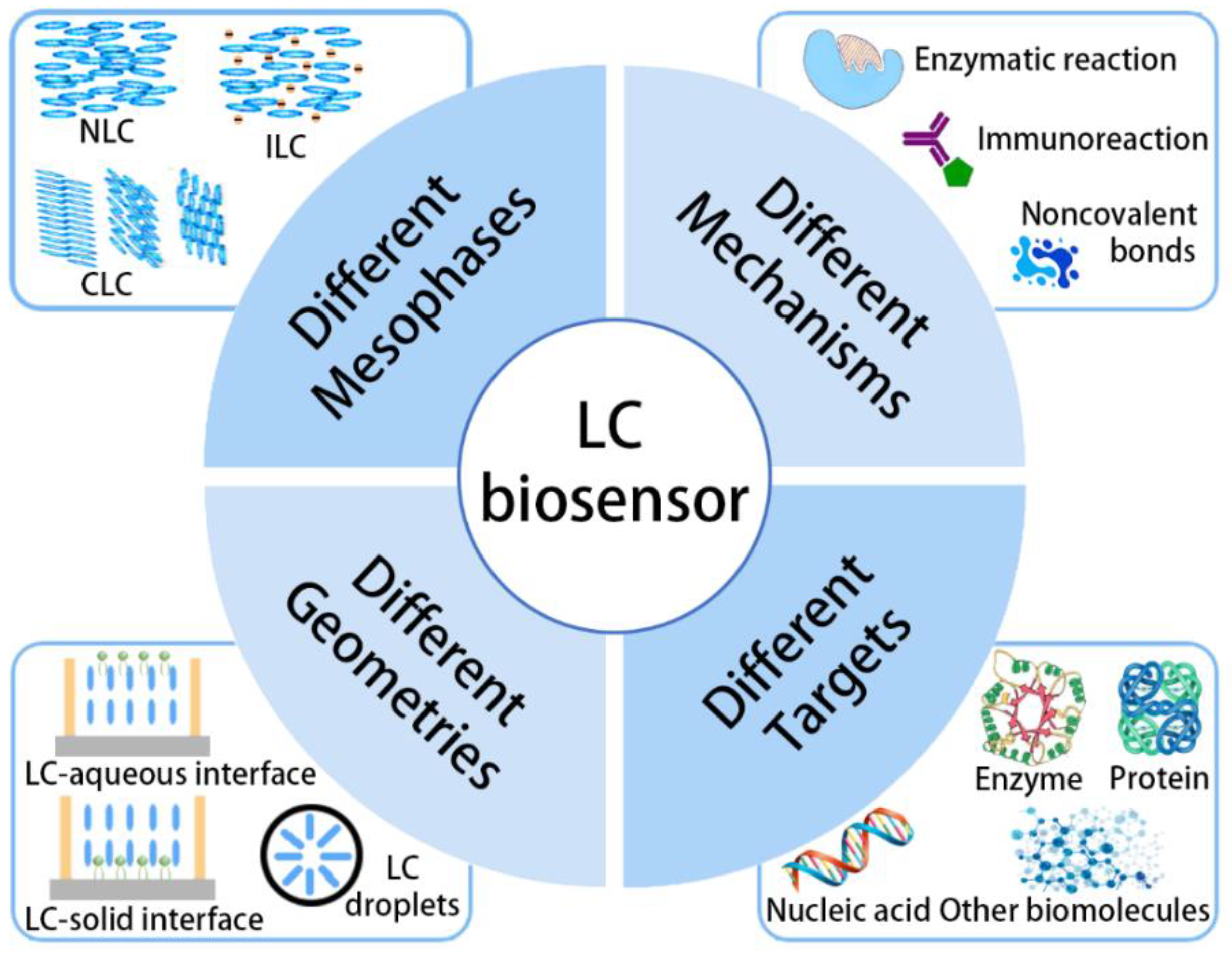
2. Different Sensor Structures
2.1. Mesophases
2.1.1. Nematic LCs
2.1.2. Chiral Nematic LCs
2.1.3. Smectic LCs and Blue Phase LCs
2.1.4. Ionic LCs
2.2. Geometries
2.2.1. LC–Solid Interface
2.2.2. LC-Aqueous Interface
2.2.3. LC Droplets
3. Different Sensing Mechanisms
3.1. Enzymatic Reactions
3.2. Immunoreactions
3.3. Hydrogen Bonds
3.4. Nonspecific Interactions
4. Different Sensing Targets
4.1. Enzyme
| Mesophase | Geometry | Receptor | Target | Sensing Mechanism | Refs. |
|---|---|---|---|---|---|
| NLC | LC droplets | Carboxylic acids | Urease/urea | The reaction between urea and urease produce ammonia to deprotonate carboxylic acids | [89] |
| NLC | LC–solid interface | Thrombin aptamer-functionalized AuNP | Thrombin | The specific combination between the thrombin and aptamer | [120] |
| NLC | LC–aqueous interface | Poly-L-lysine | Trypsin | The degradation of peptides under the catalysis of trypsin | [128] |
| NLC | LC–solid interface | Cholylglycine | Cholylglycine hydrolase | The degradation of cholylglycine under the catalysis of cholylglycine hydrolase | [131] |
| NLC | LC–solid interface | Casein | Protease | The degradation of casein under the catalysis of protease | [125] |
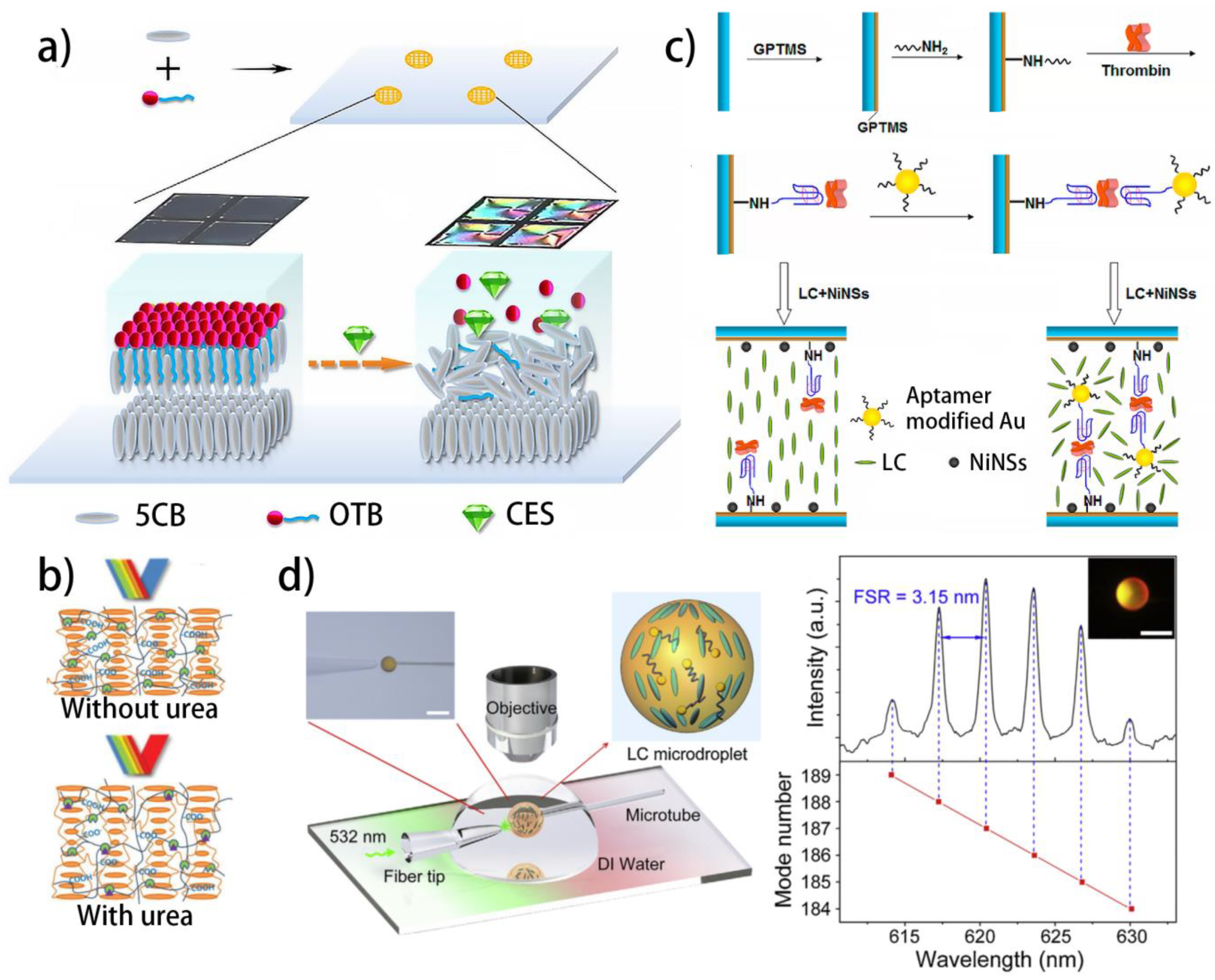
| NLC | LC–aqueous interface | OTB | Carboxylesterase | The degradation of OTB under the catalysis of carboxylesterase | [127] |
| NLC | LC–solid interface | Casein | Protease inhibition | Inhibiting the activity of protease by pefabloc | [126] |
| NLC | LC droplets | PBA | Penicillinase | The reaction between penicillinase and penicillin produce H+ to protonize PBA | [132] |
| CLC | CLC polymer | PAA | Urease | The reaction between urease and urea produce H+ to protonize PAA | [129] |
| CLC | CLC polymer | PAA | Urease/Glucose/ions | The reactions produce H+ to protonize PAA | [130] |
4.2. Nucleic Acid
| Mesophase | Geometry | Receptor | Target | Sensing Mechanism | Refs. |
|---|---|---|---|---|---|
| NLC | LC–solid interface | DNA aptamer | DNA | The hydrogen bond interaction between DNA single strands | [133] |
| NLC | LC–solid interface | DNA aptamer | DNA | The quantification of DNA concentrations through the interference colors of LCs | [134] |
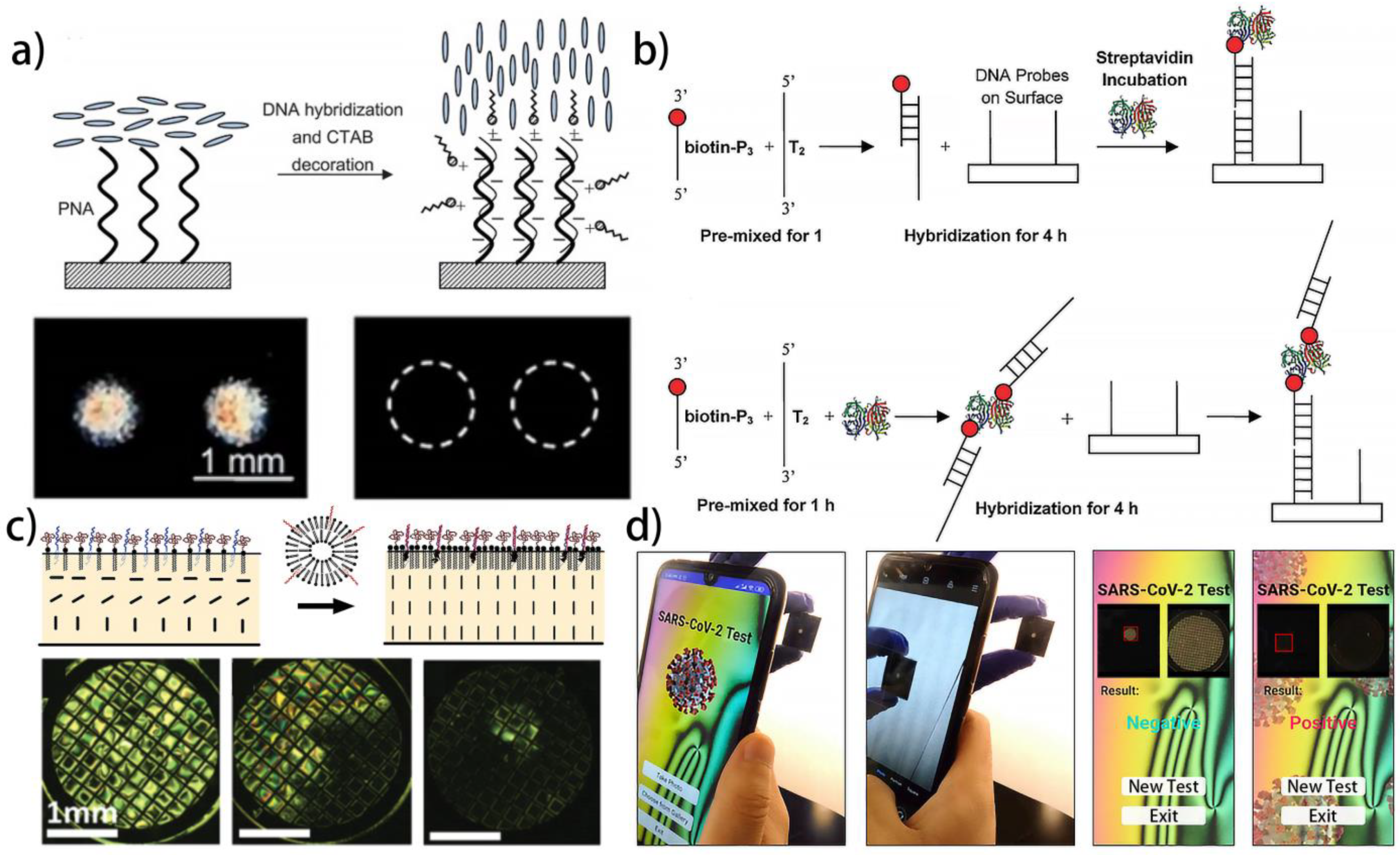
| NLC | LC–solid interface | CTAB/PNA | DNA | The electrostatic interaction between DNA and CTAB, and the hydrogen bond interaction between DNA single strands | [121] |
| NLC | LC–solid interface | DNA streptavidin complex | DNA | The hydrogen bond interaction between DNA and DNA streptavidin complex | [136] |
| NLC | LC–solid interface | DNA aptamer | DNA | The LC alignment is related to the concentration and chain length of the DNA | [135] |
| NLC | LC–aqueous interface | OTAB/DNA | DNA | The electrostatic interaction between DNA and OTAB, and the hydrogen bond interaction between DNA single strands | [118] |
| NLC | LC–aqueous interface | OTAB/DNA | DNA | The electrostatic interaction, hydrogen bond interaction, and hydrophobic interaction | [88] |
| NLC | LC–aqueous interface | PEG-lipid monolayer decorated with DNA | Bulk phase liposomes decorated with DNA | DNA hybridization-mediated liposome fusion | [137] |
| NLC | LC–aqueous interface | DNA-lipids | DNA | The hydrogen bond interaction between DNA single strands | [138] |
| NLC | LC–aqueous interface | DTAB/DNA | DNAs of bacterium Erwinia carotovora and fungi Rhazictonia solani | The electrostatic interaction between DNA and DTAB, and the hydrogen bond interaction between DNA single strands | [139] |
| NLC | LC–aqueous interface | OTAB | The Fenton reaction of DNA | The electrostatic interaction and Fenton reaction | [140] |
| NLC | LC–aqueous interface | DTAB | RNA of SARS-CoV-2 | The electrostatic interaction between DNA and DTAB, and the hydrogen bond interaction between DNA and RNA | [141] |
4.3. Protein and Amino Acid
| Mesophase | Geometry | Receptor | Target | Sensing Mechanism | Refs. |
|---|---|---|---|---|---|
| NLC | LC–aqueous interface | DOGS-NTA-Ni and histidine-tagged ubiquitin | Anti-ubiquitin antibody | Immunoreaction | [90] |
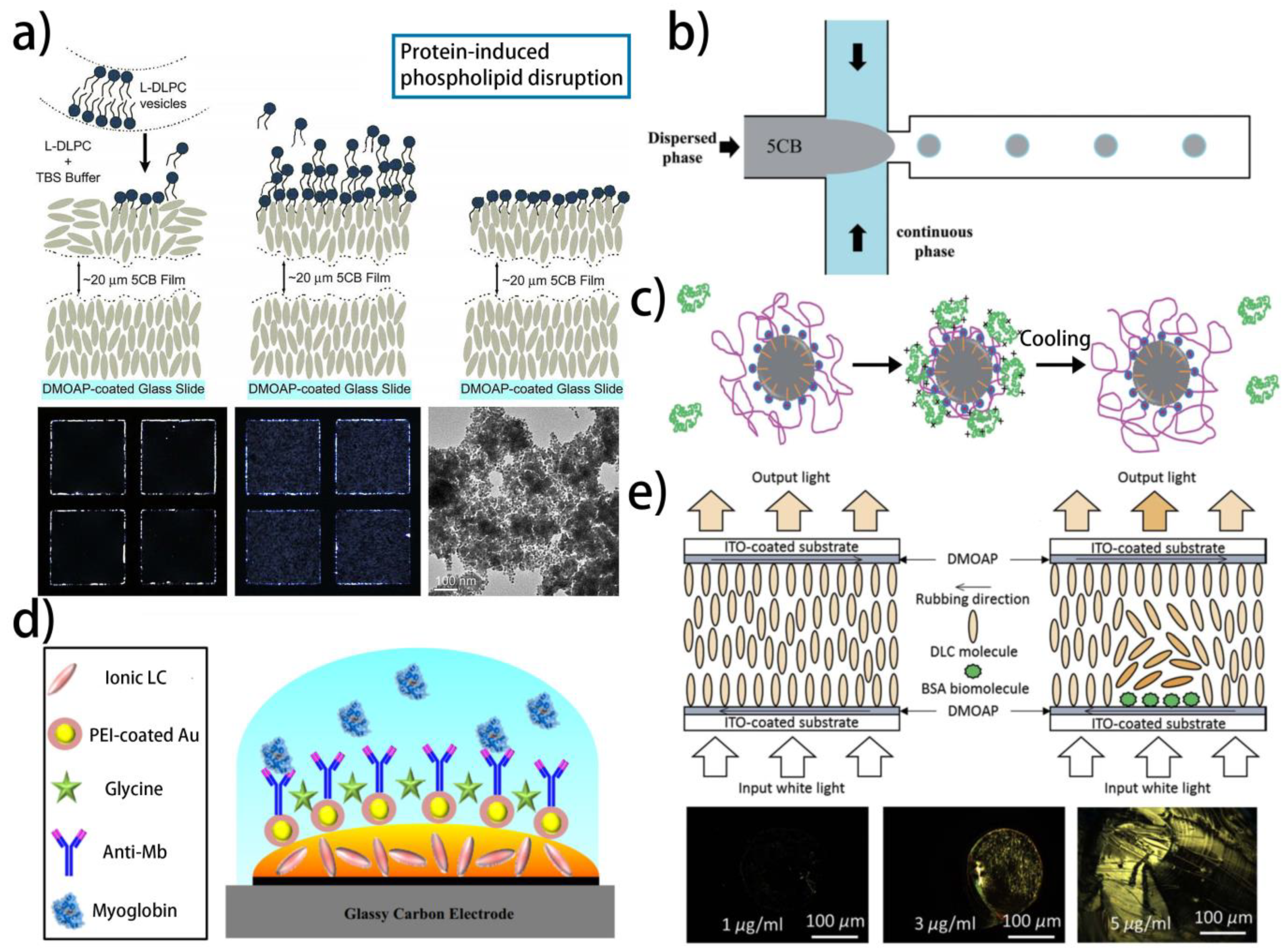
| NLC | LC–aqueous interface | Phospholipids | Protein-coated AuNPs | The hydrophobic interaction between phospholipids and protein | [142] |
| NLC | LC droplets | PAA-b-LCP | Lysozyme and BSA | The electrostatic interaction between proteins and PAA | [144] |
| NLC | LC–aqueous interface | PAA-b-LCP | Lysozyme, BSA, lactalbumin, and insulin | The electrostatic interaction between proteins and PAA | [145] |
| NLC | LC–aqueous interface | PNIPAAm-b-LCP | Lysozyme, BSA, hemoglobin, and chymotrypsinogen | The electrostatic interaction between proteins and PNIPAAm | [146] |
| NLC | LC droplets | PNIPAAm-b-LCP | BSA, lysozyme, hemoglobin, and chymotrypsinogen | The electrostatic interaction between proteins and PNIPAAm | [147] |
| NLC | LC–aqueous interface | Phospholipids | Antimicrobial peptides | The electrostatic interaction between phospholipids and antimicrobial peptides | [122] |
| ILC | LC–solid interface | AuNP-Si4Pic+Cl− and ab-cTnT | cTnT | Immunoreaction | [149] |
| ILC | LC–solid interface | AuNP-PEI and ab-Mb | Myoglobin | Immunoreaction | [150] |
| NLC | LC droplets | QP4VP and PSS | Hemoglobin and BSA | The hydrophobic interaction between polyelectrolytes and protein | [148] |
| NLC | LC–solid interface | HAuCl4 | Tyrosine | Enzymatic reaction | [143] |
| NLC | LC–aqueous interface | Lipopolysaccharide | Hemoglobin, BSA, and Lysozyme | The hydrophobic interaction between lipopolysaccharide and protein | [151] |
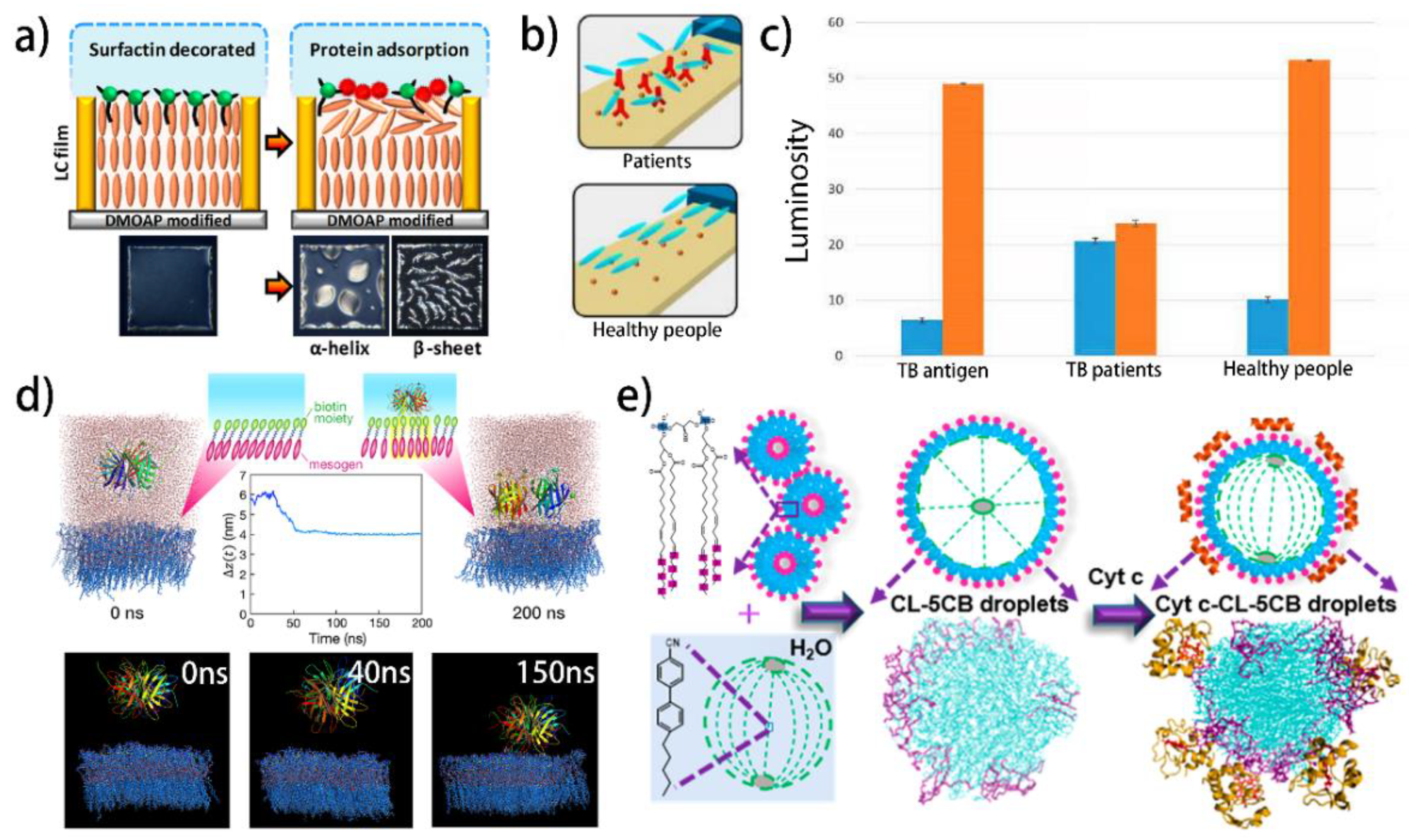
| NLC | LC–solid interface | Anti-TB | TB antigen | Immunoreaction | [159] |
| NLC | LC–solid interface | Anti-cecropin B | Cecropin B | Immunoreaction | [152] |
| DLC | LC–solid interface | No receptor | BSA | BSA directly changes the LC orientation | [154] |
| DFLC | LC–solid interface | No receptor | BSA | BSA directly changes the LC orientation | [155] |
| NLC | LC–aqueous interface | Biotin | Streptavidin | The combination of biotin and streptavidin | [160] |
| NLC | LC–aqueous interface | Surfactin | Proteins with different secondary conformations | The electrostatic interaction between surfactin and proteins | [158] |
| NLC | LC–solid interface | DNA aptamer | Parkinson’s Disease related alpha-synuclein | The specific binding of protein to DNA aptamer | [156] |
| NLC | LC–aqueous interface | CTAB/DNA aptamer | Parkinson’s Disease related alpha-synuclein | The specific binding of protein to DNA aptamer | [157] |
| NLC | LC droplets | Cardiolipin | Cytochrome c | The electrostatic interaction between cardiolipin and cytochrome c | [161] |
4.4. Other Target
4.4.1. Glucose
4.4.2. Bile Acids
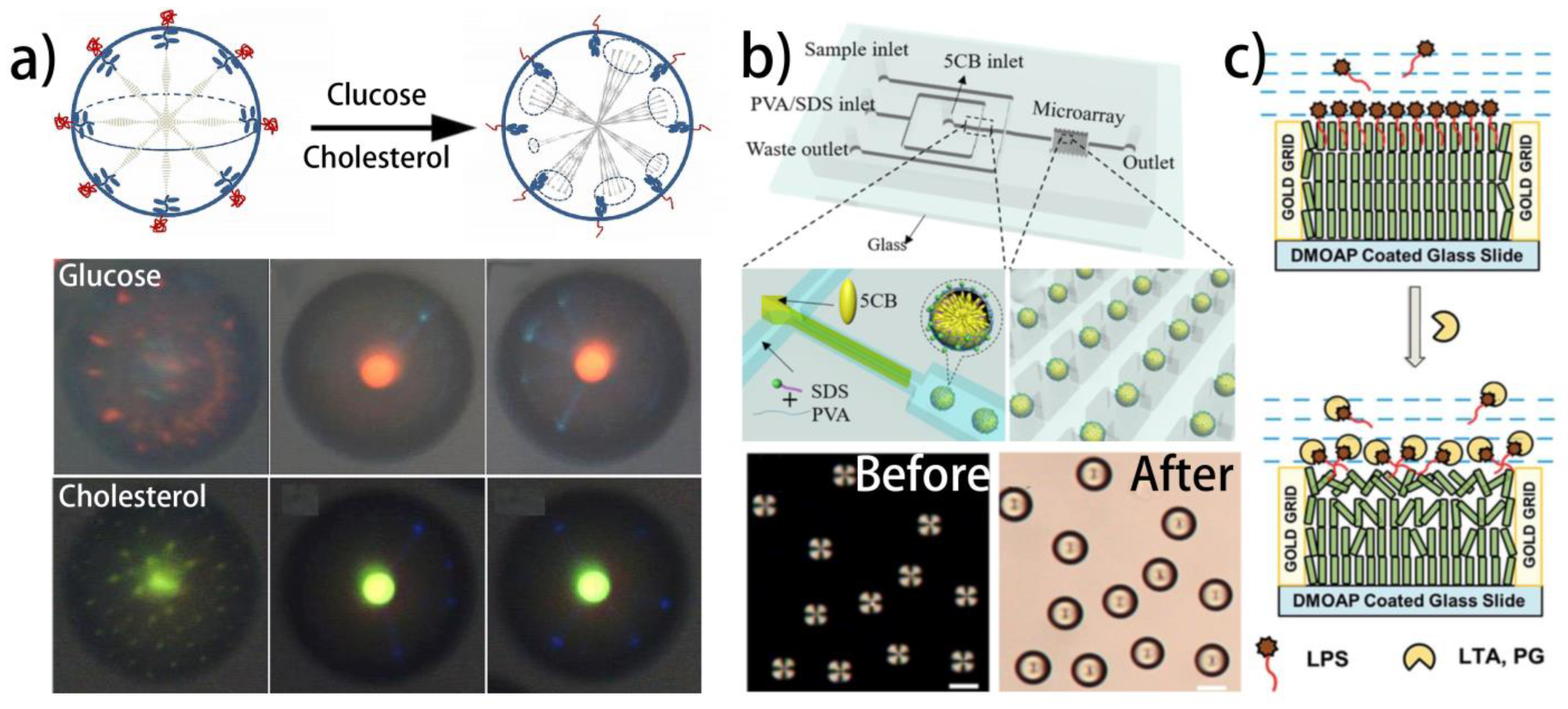
| Mesophase | Geometry | Receptor | Target | Sensing Mechanism | Refs. |
|---|---|---|---|---|---|
| NLC | LC droplets | Glucose oxidase modified PAA | Glucose | Enzymatic reaction | [162] |
| CLC | LC droplets | Glucose oxidase and cholesterol oxidase modified PAA | Glucose and cholesterol | Enzymatic reaction | [163] |
| NLC | LC–aqueous interface | Glucose oxidase | Glucose | Enzymatic reaction | [164] |
| NLC | LC–aqueous interface | Glucose oxidase-immobilized QP4VP | Glucose | Enzymatic reaction | [123] |
| CLC | CLC polymer | Glucose oxidase | Glucose | Enzymatic reaction | [165] |
| NLC | LC–aqueous interface | Glucose oxidase and horseradish peroxidase | Glucose | Enzymatic reaction | [166] |
| NLC | LC–aqueous interface | Cholesterol oxidase and horseradish peroxidase | Cholesterol | Enzymatic reaction | [167] |
| NLC | LC-aqueous interface | 3-aminophenyl boronic acid | Glucose | The specific reaction between 3-aminophenyl boronic acid and glucose | [168] |
| NLC | LC droplets | 3-aminophenyl boronic acid | Glucose | The specific reaction between 3-aminophenyl boronic acid and glucose | [169] |
| NLC | LC–aqueous interface | Peptidoglycan and lipoteichoic acid | bacterial endotoxin | HydrophobicInteraction | [174] |
| NLC | LC–solid interface | DNA aptamer | Lipopolysaccharides | The specific reorganization of lipopolysaccharides using DNA | [175] |
| NLC | LC droplets | SDS | Bile acid | Hydrophobic interaction | [171] |
| NLC | LC–aqueous interface | Surfactants with different chain lengths | Bile acid | Hydrophobic interaction | [172] |
| NLC | LC droplets | SDS | Bile acid | Hydrophobic interaction | [173] |
4.4.3. Lipopolysaccharides
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional Liquid Crystals towards the Next Generation of Materials. Angew. Chem. Int. Edit. 2018, 57, 4335–4371. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Urbas, A.M.; Li, Q. Nature-Inspired Emerging Chiral Liquid Crystal Nanostructures: From Molecular Self-Assembly to DNA Mesophase and Nanocolloids. Adv. Mater. 2020, 32, 1801335. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lv, J.; Zhu, C.; Qi, L.; Yu, Y. Photodeformable Azobenzene-Containing Liquid Crystal Polymers and Soft Actuators. Adv. Mater. 2019, 31, 1904224. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; George, T.F.; Li, G. Conjugation of Nanomaterials and Nematic Liquid Crystals for Futuristic Applications and Biosensors. Biosensors 2018, 8, 69. [Google Scholar] [CrossRef]
- Gutierrez-Cuevas, K.G.; Wang, L.; Zheng, Z.; Bisoyi, H.K.; Li, G.; Tan, L.S.; Vaia, R.A.; Li, Q. Frequency-Driven Self-Organized Helical Superstructures Loaded with Mesogen-Grafted Silica Nanoparticles. Angew. Chem. Int. Edit. 2016, 55, 13090–13094. [Google Scholar] [CrossRef]
- Akbari, A.; Sheath, P.; Martin, S.; Shinde, D.B.; Shaibani, M.; Banerjee, P.C.; Tkacz, R.; Bhattacharyya, D.; Majumder, M. Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide. Nat. Commun. 2016, 7, 10891. [Google Scholar] [CrossRef]
- Prevot, M.E.; Nemati, A.; Cull, T.R.; Hegmann, E. A Zero-Power Optical, ppt- to ppm-Level Toxic Gas and Vapor Sensor with Image, Text, and Analytical Capabilities. Adv. Mater. Technol. 2020, 5, 2000058. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, S.H. Controlled Encapsulation of Cholesteric Liquid Crystals Using Emulsion Templates. Macromol. Res. 2018, 26, 1054–1065. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, Y.; Bisoyi, H.K.; Wang, L.; Bunning, T. Three-dimensional control of the helical axis of a chiral nematic liquid crystal by light. Nature 2016, 531, 352–356. [Google Scholar] [CrossRef]
- Zheng, Z.; Yuan, C.; Hu, W.; Bisoyi, H.K.; Tang, M.; Liu, Z.; Sun, P.; Yang, W.; Wang, X.; Shen, D.; et al. Light-Patterned Crystallographic Direction of a Self-Organized 3D Soft Photonic Crystal. Adv. Mater. 2017, 29, 1703165. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.; Zhou, P.; Liu, S.; Lu, J.; Li, X.; Su, Y. Polymer-Stabilized Blue Phase Liquid Crystals for Photonic Applications. Adv. Mater. Technol. 2016, 1, 1600102. [Google Scholar] [CrossRef]
- Goossens, K.; Lava, K.; Bielawski, C.W.; Binnemans, K. Ionic Liquid Crystals: Versatile Materials. Chem. Rev. 2016, 116, 4643–4807. [Google Scholar] [CrossRef] [PubMed]
- Axenov, K.V.; Laschat, S. Thermotropic Ionic Liquid Crystals. Materials 2011, 4, 206–259. [Google Scholar] [CrossRef] [PubMed]
- Mitov, M. Liquid-Crystal Science from 1888 to 1922: Building a Revolution. Chemphyschem 2014, 15, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Reinitzer, F. Beitrage zur Kenntniss des Cholesterins. Monatsh. Chem. 1888, 9, 421–441. [Google Scholar] [CrossRef]
- Kristiansen, K.; Zeng, H.; Zappone, B.; Israelachvili, J.N. Simultaneous Measurements of Molecular Forces and Electro-Optical Properties of a Confined 5CB Liquid Crystal Film Using a Surface Forces Apparatus. Langmuir 2015, 31, 3965–3972. [Google Scholar] [CrossRef]
- Sahu, D.K.; Anjali, T.G.; Basavaraj, M.G.; Aplinc, J.; Copar, S.; Dhara, S. Orientation, elastic interaction and magnetic response of asymmetric colloids in a nematic liquid crystal. Sci. Rep. 2019, 9, 81. [Google Scholar] [CrossRef]
- Guillamat, P.; Ignes-Mullol, J.; Sagues, F. Control of active liquid crystals with a magnetic field. Proc. Natl. Acad. Sci. USA 2016, 113, 5498–5502. [Google Scholar] [CrossRef]
- Shah, A.A.; Kang, H.; Kohlstedt, K.L.; Ahn, K.H.; Glotzer, S.C.; Monroe, C.W.; Solomon, M.J. Liquid Crystal Order in Colloidal Suspensions of Spheroidal Particles by Direct Current Electric Field Assembly. Small 2012, 8, 1551–1562. [Google Scholar] [CrossRef]
- ul Amin, N.; Siddiqi, H.M.; Lin, Y.K.; Hussain, Z.; Majeed, N. Bovine Serum Albumin Protein-Based Liquid Crystal Biosensors for Optical Detection of Toxic Heavy Metals in Water. Sensor 2020, 20, 298. [Google Scholar] [CrossRef]
- Dabrowski, R.; Kula, P.; Herman, J. High Birefringence Liquid Crystals. Crystals 2013, 3, 443–482. [Google Scholar] [CrossRef]
- Xie, J.; Zeng, T.; Zhou, Y.; Yuan, Y.; Zhou, Y.; Lin, T.; Jiang, X.; Fan, F.; Wen, S. Optical edge imaging based on the birefringence property and electro-optic tunability characteristic of the liquid crystals. Liq. Cryst. 2021, 48, 1953–1958. [Google Scholar] [CrossRef]
- Geis, M.W.; Bos, P.J.; Liberman, V.; Rothschild, M. Broadband optical switch based on liquid crystal dynamic scattering. Opt. Express 2016, 24, 13812–13823. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, C.; Chao, C. Supramolecular Liquid-Crystal Gels Formed by Polyfluorene-Based pi-Conjugated Polymer for Switchable Anisotropic Scattering Device. ACS Appl. Mater. Interfaces 2014, 6, 6757–6764. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.; Tamaoki, N. Planar chiral azobenzenophanes as chiroptic switches for photon mode reversible reflection color control in induced chiral nematic liquid crystals. JACS 2008, 130, 11409–11416. [Google Scholar] [CrossRef]
- Gan, P.; Zhang, X.; Zhao, L.; Shi, W.; Cao, H.; Wang, H.; Yang, Z.; Wang, D.; He, W. Broadband reflection in polymer-stabilized cholesteric liquid crystal film with zinc oxide nanoparticles film thermal diffusion method. Liq. Cryst. 2021, 48, 1959–1968. [Google Scholar] [CrossRef]
- Zografopoulos, D.C.; Beccherelli, R.; Kriezis, E.E. Beam-splitter switches based on zenithal bistable liquid-crystal gratings. Phys. Rev. 2014, 90, 042503. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhang, L.; Jiang, Y.; Ma, J.; Sun, W. Temperature-controllable beam splitter and optical filter based on filling liquid crystal into both ends of photonic crystal fibers. Liq. Cryst. 2016, 43, 61–65. [Google Scholar] [CrossRef]
- Li, G. Adaptive Lens. Prog. Optics. 2010, 55, 199–283. [Google Scholar]
- Li, G.; Mathine, D.L.; Valley, P.; Ayras, P.; Haddock, J.; Giridhar, M.; Williby, G.; Schwiegerling, J.; Meredith, G.; Kippelen, B. Switchable electro-optic diffractive lens with high efficiency for ophthalmic applications. Proc. Natl. Acad. Sci. USA 2006, 103, 6100–6104. [Google Scholar] [CrossRef]
- Chen, H.; Lee, J.; Lin, B.; Chen, S.; Wu, S. Liquid crystal display and organic light-emitting diode display: Present status and future perspectives. Light-Sci. Appl. 2018, 7, 17168. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Rao, L.; Jiao, M.; Li, Y.; Cheng, H.; Wu, S. Polymer-stabilized optically isotropic liquid crystals for next-generation display and photonics applications. J. Mater. Chem. 2011, 21, 7870–7877. [Google Scholar] [CrossRef]
- Liu, Y.; Si, G.; Leong, E.; Xiang, N.; Danner, A.; Teng, J. Light-Driven Plasmonic Color Filters by Overlaying Photoresponsive Liquid Crystals on Gold Annular Aperture Arrays. Adv. Mater. 2012, 24, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, H.; Hu, H.; Gan, Q.; Cartwright, A.N. One-Step Fabrication of Graded Rainbow-Colored Holographic Photopolymer Reflection Gratings. Adv. Mater. 2012, 24, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, Y.; Wu, S. High-efficiency and fast-response tunable phase grating using a blue phase liquid crystal. Opt. Lett. 2011, 36, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Zola, R.S.; Bisoyi, H.; Wang, H.; Urbas, A.M.; Bunning, T.J.; Li, Q. Dynamic Control of Light Direction Enabled by Stimuli-Responsive Liquid Crystal Gratings. Adv. Mater. 2019, 31, 1806172. [Google Scholar] [CrossRef]
- Schwartz, M.; Lagerwall, J.P.F. Embedding Intelligence in Materials for Responsive Built Environment Using Liquid Crystal Elastomer Actuators and Sensors. arXiv 2021, arXiv:2103.11005. [Google Scholar]
- Liu, Q.; Tang, J.; Zhang, Y.; Martinez, A.; Wang, S.; He, S.; White, T.; Smalyukh, I.I. Shape-dependent dispersion and alignment of nonaggregating plasmonic gold nanoparticles in lyotropic and thermotropic liquid crystals. Phys. Rev. E 2014, 89, 052505. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, T.; Noel, A.; Chen, Y.; Liu, T. Applications of liquid crystals in biosensing. Soft Matter 2021, 17, 4675–4702. [Google Scholar] [CrossRef]
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal materials find a new order in biomedical applications. Nat. Mater. 2007, 6, 929–938. [Google Scholar] [CrossRef]
- Esteves, C.; Ramou, E.; Porteira, A.R.P.; Barbosa, A.J.M.; Roque, A.C.A. Seeing the Unseen: The Role of Liquid Crystals in Gas-Sensing Technologies. Adv. Opt. Mater. 2020, 8, 1902117. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kim, W.; Kim, K.; Ansari, M.A.; Mehmood, M.Q.; Badloe, T.; Kim, Y.; Gwak, J.; Lee, H.; Kim, Y.; et al. Holographic metasurface gas sensors for instantaneous visual alarms. Sci. Adv. 2021, 7, eabe9943. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; George, T.F.; Li, G. Development in liquid crystal microcapsules: Fabrication, optimization and applications. J. Mater. Chem. C 2022, 10, 413–432. [Google Scholar] [CrossRef]
- Duan, R.; Li, Y.; Li, H.; Yang, J. Detection of heavy metal ions using whispering gallery mode lasing in functionalized liquid crystal microdroplets. Biomed. Opt. Express 2019, 10, 6073–6083. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Rather, A.M.; Yao, Y.; Fang, J.; Mamtani, R.S.; Bennett, R.K.A.; Atta, R.G.; Adera, S.; Tkalec, U.; Wang, X. Liquid crystal-based open surface microfluidics manipulate liquid mobility and chemical composition on demand. Sci. Adv. 2021, 7, eabi7607. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, B.; Kim, S.K.; Won, J.C.; Kim, Y.H. Robust Microfluidic Encapsulation of Cholesteric Liquid Crystals toward Photonic Ink Capsules. Adv. Mater. 2015, 27, 627–633. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, J.; Liu, X.; Sun, W.; Wang, F.; Liu, Y. High-sensitivity fiber liquid crystals temperature sensor with tiny size and simple tapered structure. Chin. Opt. Lett. 2020, 18, 101202. [Google Scholar] [CrossRef]
- Guan, Y.; Agra-Kooijman, D.M.; Fu, S.; Jákli, A.; West, J.L. Responsive liquid-crystal-clad fibers for advanced textiles and wearable sensors. Adv. Mater. 2019, 31, 1902168. [Google Scholar] [CrossRef]
- Arya, S.; Wong, C.; Jeon, Y.; Barisal, T.; Park, M. Advances in Complementary-Metal-Oxide-Semiconductor-Based Integrated Biosensor Arrays. Chem. Rev. 2015, 115, 5116–5158. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Y.; Lei, L.; Xu, Z.; Zhang, W. Photoelectrochemical biosensor for acetylcholinesterase activity study based on metal oxide semiconductor nanocomposites. J. Electroanal. Chem. 2016, 781, 377–382. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Calas-Blanchard, C.; Catanante, G.; Noguer, T. Electrochemical Sensor and Biosensor Strategies for ROS/RNS Detection in Biological Systems. Electroanalysis 2014, 26, 1277–1286. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, N.; Zhang, F.; Wang, H.; Ye, W.; Wang, P. Neurosecretory cell-based biosensor: Monitoring secretion of adrenal chromaffin cells by local extracellular acidification using light-addressable potentiometric sensor. Biosens. Bioelectron. 2012, 35, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shi, X.; Feng, H.; Chen, M.; Li, W.; Lai, J.; Hu, W.; Li, G. 1,5-anhydroglucitol biosensor based on light-addressable potentiometric sensor with RGO-CS-Fc/Au NPs nanohybrids. Bioelectrochemistry 2021, 142, 107938. [Google Scholar] [CrossRef] [PubMed]
- Carlton, R.J.; Hunter, J.T.; Miller, D.S.; Abbasi, R.; Mushenheim, P.C.; Tan, L.N.; Abbott, N.L. Chemical and biological sensing using liquid crystals. Liq. Cryst. Rev. 2013, 1, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Nayani, K.; Yang, Y.; Yu, H.; Jani, P.; Mavrikakis, M.; Abbott, N. Areas of opportunity related to design of chemical and biological sensors based on liquid crystals. Liq. Cryst. Today 2020, 29, 24–35. [Google Scholar] [CrossRef]
- Popov, P.; Mann, E.K.; Jakli, A. Thermotropic liquid crystal films for biosensors and beyond. J. Mater. Chem. B 2017, 5, 5061–5078. [Google Scholar] [CrossRef]
- Pathak, G.; Katiyar, R.; Agrahari, K.; Srivastava, A.; Dabrowski, R.; Garbat, K.; Manohar, R. Analysis of birefringence property of three different nematic liquid crystals dispersed with TiO2 nanoparticles. Opto-Electron. Rev. 2018, 26, 11–18. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Chen, Y.; Li, C.; Hou, G.; Liu, X.; Zhang, X.; He, W.; Yang, H. Broadband reflection of polymer-stabilized chiral nematic liquid crystals induced by a chiral azobenzene compound. Chem. Commun. 2014, 50, 691–694. [Google Scholar] [CrossRef]
- Serra, F.; Gharbi, M.A.; Luo, Y.; Liu, I.B.; Bade, N.D.; Kamien, R.D.; Yang, S.; Stebe, K.J. Curvature-Driven, One-Step Assembly of Reconfigurable Smectic Liquid Crystal “Compound Eye” Lenses. Adv. Opt. Mater. 2015, 3, 1287–1292. [Google Scholar] [CrossRef]
- Hyman, R.M.; Lorenz, A.; Morris, S.M.; Wilkinson, T.D. Polarization-independent phase modulation using a blue-phase liquid crystal over silicon device. Appl. Opt. 2014, 53, 6925–6929. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, Y.; Chang, H.; Lee, A. Ligand-Doped Liquid Crystal Sensor System for Detecting Mercuric Ion in Aqueous Solutions. Anal. Chem. 2015, 87, 4546–4551. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Nandi, R.; Mishra, K.; Singh, H.K.; Singh, R.K.; Singh, B. Liquid crystal based sensor system for the real time detection of mercuric ions in water using amphiphilic dithiocarbamate. Sensor Actuat B-Chem. 2016, 226, 381–387. [Google Scholar] [CrossRef]
- Yang, S.; Wu, C.; Tan, H.; Wu, Y.; Liao, S.; Wu, Z.; Shen, G.; Yu, R. Label-Free Liquid Crystal Biosensor Based on Specific Oligonucleotide Probes for Heavy Metal Ions. Anal. Chem. 2013, 85, 14–18. [Google Scholar] [CrossRef]
- Ul Amin, N.; Yang, K.; Majeed, N.; Siddiqi, H.M. Fabrication of a Fluorophore/Liquid-Crystal-Based Oligopeptide Biosensor for the Detection of Cu (II) Ions. Chemistryselect 2021, 6, 6607–6618. [Google Scholar] [CrossRef]
- Duong, T.D.S.; Jang, C. A label-free liquid crystal droplet-based sensor used to detect lead ions using single-stranded DNA zyme. Colloid Surf. A 2020, 604, 125304. [Google Scholar] [CrossRef]
- Lin, P.; Yan, Q.; Wei, Z.; Chen, Y.; Chen, S.; Wang, H.; Huang, Z.; Wang, X.; Cheng, Z. Chiral Photonic Crystalline Microcapsules with Strict Monodispersity, Ultrahigh Thermal Stability, and Reversible Response. ACS Appl. Mater. Interface 2018, 10, 18298–18299. [Google Scholar] [CrossRef]
- Hyeon, S.; Lee, J.; Kim, D.; Jeong, H.; Oh, B.; Han, J.; Lee, J.; Seo, D. Free residual DC voltage for nematic liquid crystals on solution-derived lanthanum tin oxide film. Liq. Cryst. 2017, 44, 1421–1428. [Google Scholar] [CrossRef]
- Goodby, J.W. The nanoscale engineering of nematic liquid crystals for displays. Liq. Cryst. 2011, 38, 11–12. [Google Scholar] [CrossRef]
- Mori, T.; Sharma, A.; Hegmann, T. Significant Enhancement of the Chiral Correlation Length in Nematic Liquid Crystals by Gold Nanoparticle Surfaces Featuring Axially Chiral Binaphthyl Ligands. ACS Nano 2016, 10, 1552–1564. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; He, Z.; Chen, G.; Wang, D.; Zhang, H.; Yang, H. Photoinduced polymer-stabilised chiral nematic liquid crystal films reflecting both right- and left-circularly polarised light. Liq. Cryst. 2015, 42, 1120–1123. [Google Scholar] [CrossRef]
- Wang, C.; Chen, C.; Yang, T.; Nys, I.; Li, C.; Lin, T.; Neyts, K.; Beeckman, J. Electrically assisted bandedge mode selection of photonic crystal lasing in chiral nematic liquid crystals. Appl. Phys. Lett. 2018, 112, 043301. [Google Scholar] [CrossRef]
- Mitov, M. Cholesteric liquid crystals in living matter. Soft Matter 2017, 13, 4176–4209. [Google Scholar] [CrossRef] [PubMed]
- Mitov, M. Cholesteric Liquid Crystals with a Broad Light Reflection Band. Adv. Mater. 2012, 24, 6260–6276. [Google Scholar] [CrossRef]
- Roberts, J.C.; Kapernaum, N.; Song, Q.; Nonnenmacher, D.; Ayub, K.; Giesselmann, F.; Lemieux, R.P. Design of Liquid Crystals with “de Vries-like” Properties: Frustration between SmA- and SmC-Promoting Elements. JACS 2010, 132, 364–370. [Google Scholar] [CrossRef]
- Song, Q.; Nonnenmacher, D.; Giesselmann, F.; Lemieux, R.P. Tuning the frustration between SmA- and SmC-promoting elements in liquid crystals with ‘de Vries-like’ properties. Chem. Commun. 2011, 47, 4781–4783. [Google Scholar] [CrossRef][Green Version]
- Macary, M.H.; Krasinski, F.; Abboud, N.; Lin, Y.; Douali, R.; Zaouk, D.; Legrand, C. Electronic and ionic ambipolar transports in the isotropic, SmA, SmB and crystalline phases of a liquid crystal. J. Mol. Liq. 2017, 240, 564–569. [Google Scholar] [CrossRef]
- Coles, H.J.; Pivnenko, M.N. Liquid crystal ‘blue phases’ with a wide temperature range. Nature 2005, 436, 997–1000. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yokota, M.; Hisakado, Y.; Yang, H.; Kajiyama, T. Polymer-stabilized liquid crystal blue phases. Nat. Mater. 2002, 1, 64–68. [Google Scholar] [CrossRef]
- Fernandez, A.A.; Kouwer, P.H.J. Key Developments in Ionic Liquid Crystals. Int. J. Mol. Sci. 2002, 1, 64–68. [Google Scholar]
- Bi, X.; Yang, K. Real-time liquid crystal-based glutaraldehyde sensor. Sens. Actuators B Chem. 2008, 134, 432–437. [Google Scholar] [CrossRef]
- Espinoza, L.A.T.; Schumann, K.R.; Luk, Y.Y.; Israel, B.A.; Abbott, N.L. Orientational behavior of thermotropic liquid crystals on surfaces presenting electrostatically bound vesicular stomatitis virus. Langmuir 2004, 20, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, M.; Kim, S.; Shin, J.; Moon, C.; Hwang, J.; Choi, J.; Park, H.; Kim, H.; Jang, J. Electrical Broad Tuning of Plasmonic Color Filter Employing an Asymmetric-Lattice Nanohole Array of Metasurface Controlled by Polarization Rotator. ACS Photonics 2017, 4, 1954–1966. [Google Scholar] [CrossRef]
- Gupta, S.K.; Singh, D.P.; Manohar, R.; Kumar, S. Tuning phase retardation behaviour of nematic liquid crystal using quantum dot. Curr. Appl. Phys. 2018, 267, 456–458. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Wang, F.; Luo, D. Highly sensitive and selective optical sensor for lead ion detection based on liquid crystal decorated with DNAzyme. Opt. Express 2019, 27, 30421–30428. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q.; Qi, L.; Lin, J.; Yu, L. Liquid crystal-based sensing platform for detection of Pb2+ assisted by DNAzyme and rolling circle amplification. J. Hazard. Mater. 2020, 400, 123218. [Google Scholar] [CrossRef]
- Verma, I.; Devi, M.; Sharma, D.; Nandi, R.; Pal, S.K. Liquid Crystal based Detection of Pb(II) Ions Using Spinach RNA as Recognition Probe. Langmuir 2019, 35, 7816–7823. [Google Scholar] [CrossRef]
- McUmber, A.C.; Noonan, P.S.; Schwartz, D.K. Surfactant-DNA interactions at the liquid crystal-aqueous interface. Soft Matter 2012, 8, 4335–4342. [Google Scholar] [CrossRef]
- Liu, D.; Jang, C. A new strategy for imaging urease activity using liquid crystal droplet patterns formed on solid surfaces. Sens. Actuat.-B Chem. 2014, 193, 770–773. [Google Scholar] [CrossRef]
- Hartono, D.; Xue, C.; Yang, K.; Yung, L.L. Decorating Liquid Crystal Surfaces with Proteins for Real-Time Detection of Specific Protein-Protein Binding. Adv. Funct. Mater. 2009, 19, 3574–3579. [Google Scholar] [CrossRef]
- Iglesias, W.; Abbott, N.L.; Mann, E.K.; Jakli, A. Improving liquid-crystal-based biosensing in aqueous phases. ACS Appl. Mater. Interfaces 2012, 4, 6884–6890. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo, S.; Ueta, M.; Uddin, M.A.; Kato, Y. Comparison of Oil-in-Water Emulsion between Ultrasonic Irradiation and Mechanical Stirring. Chem. Eng. Technol. 2019, 42, 381–387. [Google Scholar] [CrossRef]
- Joscelyne, S.M.; Tragardh, G. Membrane emulsification-a literature review. J. Membrane Sci. 2000, 169, 107–117. [Google Scholar] [CrossRef]
- Yu, W.; Li, B.; Liu, X.; Chen, Y. Hydrodynamics of triple emulsion droplet generation in a flow-focusing microfluidic device. Chem. Eng. Sci. 2021, 243, 116648. [Google Scholar] [CrossRef]
- Dubtsov, A.; Pasechnik, S.; Shmeliova, D.; Semerenko, D.; Iglic, A.; Kralj, S. Influence of polar dopant on internal configuration of azoxybenzene nematic-in-water droplets. Liq. Cryst. 2018, 45, 388–400. [Google Scholar] [CrossRef]
- Fernandez-Nieves, A.; Link, D.R.; Marquez, M.; Weitz, D.A. Topological changes in bipolar nematic droplets under flow. Phys. Rev. Lett. 2007, 98, 087801. [Google Scholar] [CrossRef]
- Verma, I.; Sidiq, S.; Pal, S.K. Protein triggered ordering transitions in poly (L-lysine)-coated liquid crystal emulsion droplets. Liq. Cryst. 2019, 46, 1318–1326. [Google Scholar] [CrossRef]
- Krakhalev, M.N.; Prishchepa, O.O.; Sutormin, V.S.; Zyryanov, V.Y. Director configurations in nematic droplets with tilted surface anchoring. Liq. Cryst. 2017, 44, 355–363. [Google Scholar] [CrossRef]
- James, R.; Fukuda, J. Twist transition of nematic hyperbolic hedgehogs. Phys. Rev. E 2014, 89, 042501. [Google Scholar] [CrossRef]
- Paterson, D.A.; Du, X.; Bao, P.; Parry, A.A.; Peyman, S.A.; Sandoe, J.A.T.; Evans, S.D.; Luo, D.; Bushby, R.J.; Jones, J.C.; et al. Chiral nematic liquid crystal droplets as a basis for sensor systems. Mol. Syst. Des. Eng. 2022; in press. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, L.; Wang, D.; West, J.L.; Fu, S. Preparation of thermochromic liquid crystal microcapsules for intelligent functional fiber. Mater. Des. 2018, 147, 28–34. [Google Scholar] [CrossRef]
- Sheng, M.; Zhang, L.; Jiang, S.; Yang, L.; Zaaboul, F.; Fu, S. Bioinspired Electro-Responsive Multispectral Controllable Dye-Doped Liquid Crystal Yolk-Shell Microcapsules for Advanced Textiles. ACS Appl. Mater. Interface 2021, 13, 13586–13595. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Zhang, L.; Wang, D.; Li, M.; Li, L.; West, J.L.; Fu, S. Fabrication of dye-doped liquid crystal microcapsules for electro-stimulated responsive smart textiles. Dyes Pigm. 2018, 158, 1–11. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, B.; Han, S.W.; Seo, M.; Choi, S.E.; Yang, H. 2-Dimensional colloidal micropatterning of cholesteric liquid crystal microcapsules for temperature-responsive color displays. J. Ind. Eng. Chem. 2018, 68, 393–398. [Google Scholar] [CrossRef]
- Wang, J.; Kolacz, J.; Chen, Y.; Jákli, A.; Kawalec, J.; Benitez, M.; West, J.L. Smart Fabrics Functionalized by Liquid Crystals. SID Symp. Dig. Tech. Pap. 2017, 48, 147–149. [Google Scholar] [CrossRef]
- Kuchler, A.; Yoshimoto, M.; Luginbuhl, S.; Mavelli, F.; Walde, P. Enzymatic reactions in confined environments. Nat. Technol. 2016, 11, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Masterson, J.E.; Schwartz, S.D. The enzymatic reaction catalyzed by lactate dehydrogenase exhibits one dominant reaction path. Chem. Phys. 2014, 442, 132–136. [Google Scholar] [CrossRef][Green Version]
- Netto, L.E.S.; de Oliveira, M.A.; Tairum, C.A.; da Silva Neto, J.F. Conferring specificity in redox pathways by enzymatic thiol/disulfide exchange reactions. Free Radic. Res. 2016, 50, 206–245. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, Z.; Zhu, Y.; Chen, F.; Lu, B.; Cao, W.; Zhang, Y.; Cai, Z.; Chen, F. Enzymatic Reaction Generates Biomimic Nanominerals with Superior Bioactivity. Small 2018, 14, 1804321. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, S.; Yoo, P.; Park, J.H.; Lee, J.Y. Glucose Sensing by Glucose Oxidase/PEDOT Thin Film Electrode. Mol. Cryst. Liq. Cryst. 2013, 580, 22–28. [Google Scholar] [CrossRef]
- Grigorenko, V.G.; Andreeva, I.; Rubtsova, M.Y.; Egorov, A.M. Recombinant horseradish peroxidase: Production and analytical applications. Biochemistry 2015, 80, 408–416. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Waller, M.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014, 42, D503–D509. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, B.; Wen, Q.; Yang, T.; Yang, Z. Deep eutectic solvents can be viable enzyme activators and stabilizers. J. Chem. Technol. Biot. 2014, 89, 1975–1981. [Google Scholar] [CrossRef]
- Ramon-Azcon, J.; Yasukawa, T.; Mizutani, F. Sensitive and Spatially Multiplexed Detection System Based on Dielectrophoretic Manipulation of DNA-Encoded Particles Used as Immunoreactions Platform. Anal. Chem. 2011, 83, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Hirowatari, A.; Ikeda, T.; Fukuyama, M.; Amemiya, Y.; Kuroda, A.; Yokoyama, S. Detection of antibody-antigen reaction by silicon nitride slot-ring biosensors using protein G. Opt. Commun. 2016, 365, 16–23. [Google Scholar] [CrossRef]
- Jeong, H.; Erdene, N.; Lee, S.; Jeong, D.; Park, J. Fabrication of fiber-optic localized surface plasmon resonance sensor and its application to detect antibody-antigen reaction of interferon-gamma. Opt. Eng. 2011, 50, 124405. [Google Scholar]
- Sonmezoglu, S.; Sonmezoglu, O. Optical and dielectric properties of double helix DNA thin films. Mat. Sci. Eng. C-Mater. 2011, 31, 1619–1624. [Google Scholar] [CrossRef]
- Price, A.D.; Schwartz, D.K. DNA Hybridization-Induced Reorientation of Liquid Crystal Anchoring at the Nematic Liquid Crystal/Aqueous Interface. JACS 2008, 130, 8118–8194. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, W.; Cui, X.; Tian, Y.; Guo, L.; Yang, H. Alignment of Liquid Crystals Doped with Nickel Nanoparticles Containing Different Morphologies. Adv. Mater. 2011, 23, 5779–5784. [Google Scholar] [CrossRef]
- Zhao, D.; Peng, Y.; Xu, L.; Zhou, W.; Wang, Q.; Guo, L. Liquid-Crystal Biosensor Based on Nickel-Nanosphere-Induced Homeotropic Alignment for the Amplified Detection of Thrombin. ACS Appl. Mater. Interface 2015, 7, 23418–23422. [Google Scholar] [CrossRef]
- Lai, S.L.; Yang, K.L. Detecting DNA targets through the formation of DNA/CTAB complex and its interactions with liquid crystals. Analyst 2011, 136, 3329–3334. [Google Scholar] [CrossRef]
- Hu, Q.; Jang, C. Using liquid crystals to report molecular interactions between cationic antimicrobial peptides and lipid membranes. Analyst 2012, 137, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Park, S. Liquid crystal-based glucose biosensor functionalized with mixed PAA and QP4VP brushes. Biosens. Bioelectron. 2015, 68, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Fu, H.; Fryar, K.L.; Landua, J.; Trevino, S.R.; Shirley, B.A.; Hendricks, M.M.; Iimura, S.; Gajiwala, K.; Scholtz, J.M.; et al. Contribution of Hydrophobic Interactions to Protein Stability. J. Mol. Biol. 2011, 408, 514–528. [Google Scholar] [CrossRef]
- Jannat, M.; Yang, K. Continuous protease assays using liquid crystal as a reporter. Sens. Actuators B Chem. 2018, 269, 8–14. [Google Scholar] [CrossRef]
- Jannat, M.; Yang, K. Liquid crystal-enabled protease inhibition assays developed in a millifluidic device. Sens. Actuators B Chem. 2019, 296, 126595. [Google Scholar] [CrossRef]
- Zhou, L.; Kang, Q.; Fang, M.; Yu, L. Label-free, rapid, and sensitive detection of carboxylesterase using surfactant-doped liquid crystal sensor. J. Mol. Liq. 2019, 296, 111921. [Google Scholar] [CrossRef]
- Kim, H.; Jang, C. Micro-capillary sensor for imaging trypsin activity using confined nematic liquid crystals. J. Mol. Liq. 2016, 222, 596–600. [Google Scholar] [CrossRef]
- Noh, K.; Park, S. Biosensor Array of Interpenetrating Polymer Network with Photonic Film Templated from Reactive Cholesteric Liquid Crystal and Enzyme-Immobilized Hydrogel Polymer. Adv. Funct. Mater. 2018, 28, 1707562. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S. Optical Multisensor Array with Functionalized Photonic Droplets by an Interpenetrating Polymer Network for Human Blood Analysis. ACS Appl. Mater. Interfaces 2020, 12, 47342–47354. [Google Scholar] [CrossRef]
- Wei, Y.; Jang, C. Visualization of cholylglycine hydrolase activities through nickel nanoparticle-assisted liquid crystal cells. Sens. Actuators B Chem. 2017, 239, 1268–1274. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Xu, A.; Wang, L.; Zhang, L.; Liu, S.; Liu, Y.; Li, H. Detecting enzymatic reactions in penicillinase via liquid crystal microdroplet-based pH sensor. Sens. Actuators B Chem. 2018, 258, 1090–1098. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Kim, T.; Oh, S.; Choi, E. Optical detection of deoxyribonucleic acid hybridization using an anchoring transition of liquid crystal alignment. Appl. Phys. Lett. 2005, 87, 143901. [Google Scholar] [CrossRef]
- Lai, S.K.; Huang, S.; Bi, X.; Yang, K. Optical Imaging of Surface-Immobilized Oligonucleotide Probes on DNA Microarrays Using Liquid Crystals. Langmuir 2009, 25, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; He, F.; Chen, L.; Ding, L.; Liu, H.; Wang, Y.; Xiong, X. Liquid crystal-based detection of DNA hybridization using surface immobilized single-stranded DNA. Microchim. Acta 2017, 184, 3137–3144. [Google Scholar] [CrossRef]
- Lai, S.L.; Tan, W.L.; Yang, K.L. Detection of DNA Targets Hybridized to Solid Surfaces Using Optical Images of Liquid Crystals. ACS Appl. Mater. Interfaces 2011, 3, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Noonan, P.S.; Mohan, P.; Goodwin, A.P.; Schwartz, D.K. DNA Hybridization-Mediated Liposome Fusion at the Aqueous Liquid Crystal Interface. Adv. Funct. Mater. 2014, 24, 3206–3212. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, Y.; Zhang, Y.; Liu, D.; Yang, Z. The Assembly of DNA Amphiphiles at Liquid Crystal-Aqueous Interface. Nanomaterials 2016, 6, 229. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.R.; Shin, J.; Park, S. A liquid-crystal-based DNA biosensor for pathogen detection. Sci. Rep. 2016, 6, 22676. [Google Scholar] [CrossRef]
- Kim, H.; Jang, C. Imaging DNA single-strand breaks generated by reactive oxygen species using a liquid crystal-based sensor. Anal. Biochem. 2018, 556, 1–6. [Google Scholar] [CrossRef]
- Xu, Y.; Rather, A.M.; Song, S.; Fang, J.; Dupont, R.L.; Kara, U.I.; Chang, Y.; Paulson, J.A.; Qin, R.; Bao, X.; et al. Ultrasensitive and Selective Detection of SARS-CoV-2 Using Thermotropic Liquid Crystals and Image-Based Machine Learning. Cell Rep. Phys. Sci. 2020, 1, 100276. [Google Scholar] [CrossRef]
- Hartono, D.; Qin, W.; Yang, K.; Yung, L.L. Imaging the disruption of phospholipid monolayer by protein-coated nanoparticles using ordering transitions of liquid crystals. Biomaterials 2009, 30, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, G.; Yang, M.; Chen, L.; Xiong, X. Gold nanoparticle based signal enhancement liquid crystal biosensors for tyrosine assays. Sens. Actuators B Chem. 2015, 215, 152–158. [Google Scholar] [CrossRef]
- Khan, W.; Park, S. Configuration change of liquid crystal microdroplets coated with a novel polyacrylic acid block liquid crystalline polymer by protein adsorption. Lab. Chip. 2012, 12, 4553–4559. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Khan, W.; Park, S. Protein detection using aqueous/LC interfaces decorated with a novel polyacrylic acid block liquid crystalline polymer. Soft Matter 2012, 8, 198–203. [Google Scholar] [CrossRef]
- Jung, Y.; Park, S. Protein detection using aqueous/LC interfaces decorated with a novel poly(N-isopropyl acrylamide) block liquid crystalline polymer. RSC Adv. 2013, 3, 17930–17937. [Google Scholar] [CrossRef]
- Jung, Y.; Khan, M.; Park, S. Fabrication of temperature- and pH-sensitive liquid-crystal droplets with PNIPAM-b-LCP and SDS coatings by microfluidics. J. Mater. Chem. B 2014, 2, 4922–4928. [Google Scholar] [CrossRef]
- Yang, L.; Khan, M.; Park, S. Liquid crystal droplets functionalized with charged surfactant and polyelectrolyte for non-specific protein detection. RSC Adv. 2015, 5, 97264–97271. [Google Scholar] [CrossRef]
- Zapp, E.; Silva, P.S.; Westphal, E.; Gallardo, H.; Spinelli, A.; Vieira, I.C. Troponin T Immunosensor Based on Liquid Crystal and Silsesquioxane-Supported Gold Nanoparticles. Bioconjug. Chem. 2014, 25, 1638–1643. [Google Scholar] [CrossRef]
- Zapp, E.; Westphal, E.; Gallardo, H.; Souza, B.; Vieira, I.C. Liquid crystal and gold nanoparticles applied to electrochemical immunosensor for cardiac biomarker. Biosens. Bioelectron. 2014, 59, 127–133. [Google Scholar] [CrossRef]
- Das, D.; Sidiq, S.; Pal, S.K. A Simple Quantitative Method to Study Protein-Lipopolysaccharide Interactions by Using Liquid Crystals. Chem. Phys. Chem. 2015, 16, 753–760. [Google Scholar] [CrossRef]
- Zhang, J.; Su, X.; Yang, D.; Luan, C. Label-free liquid crystal biosensor for cecropin B detection. Talanta 2018, 186, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Concellón, A.; Fong, D.; Swager, T. Complex liquid crystal emulsions for biosensing. J. Am. Chem. Soc. 2021, 143, 9177–9182. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Karn, A.; Lee, M.; Lee, W.; Chen, C. Dye-liquid-crystal-based biosensing for quantitative protein assay. Dyes Pigm. 2018, 150, 73–78. [Google Scholar] [CrossRef]
- Lin, C.; Wu, P.; Lee, M.; Lee, W. Label-free protein quantitation by dielectric spectroscopy of dual-frequency liquid crystal. Sens. Actuat. B-Chem. 2019, 282, 158–163. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Zhao, X.; Liao, W.; Zhang, C.; Yang, Z. A novel, label-free liquid crystal biosensor for Parkinson’s disease related alpha-synuclein. Chem. Commun. 2020, 56, 5441–5444. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, X.; Lu, F.; Li, H.; Zhang, C.; Yang, Z. Simple, rapid and sensitive detection of Parkinson’s disease related alpha-synuclein using a DNA aptamer assisted liquid crystal biosensor. Soft Matter 2021, 17, 4842–4847. [Google Scholar] [CrossRef]
- Verma, I.; Selvakumar, S.L.V.; Pal, S.K. Surfactin-Laden Aqueous-Liquid Crystal Interface Enabled Identification of Secondary Structure of Proteins. J. Phys. Chem. C 2020, 124, 780–788. [Google Scholar] [CrossRef]
- Kim, H.J.; Rim, J.; Jang, C. Liquid-Crystal-Based Immunosensor for Diagnosis of Tuberculosis in Clinical Specimens. ACS Appl. Mater. Interfaces 2017, 9, 21209–21215. [Google Scholar] [CrossRef]
- Watanabe, G.; Eimura, H.; Abbott, N.; Kato, T. Biomolecular Binding at Aqueous Interfaces of Langmuir Monolayers of Bioconjugated Amphiphilic Mesogenic Molecules: A Molecular Dynamics Study. Langmuir 2020, 36, 12281–12287. [Google Scholar] [CrossRef]
- Pani, I.; Nazreen, F.; Sharma, M.; Pal, S.K. Probing Nanoscale Lipid-Protein Interactions at the Interface of Liquid Crystal Droplets. Nano Lett. 2021, 21, 4546–4553. [Google Scholar] [CrossRef]
- Kim, J.; Khan, M.; Park, S. Glucose Sensor using Liquid-Crystal Droplets Made by Microfluidics. ACS Appl. Mater. Interfaces 2013, 5, 13135–13139. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Munir, S.; Park, S. Cholesteric Liquid Crystal Droplets for Biosensors. ACS Appl. Mater. Interfaces 2016, 8, 26407–26417. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Jang, C. Highly sensitive and selective glucose sensor based on ultraviolet-treated nematic liquid crystals. Biosens. Bioelectron. 2014, 59, 293–299. [Google Scholar] [CrossRef]
- Hussain, S.; Park, S. Optical glucose biosensor based on photonic interpenetrating polymer network with solid-state cholesteric liquid crystal and cationic polyelectrolyte. Sens. Actuat. B-Chem. 2020, 316, 128099. [Google Scholar] [CrossRef]
- Khan, M.; Park, S. Glucose biosensor based on GOx/HRP bienzyme at liquid-crystal/aqueous interface. J. Col. Int. Sci. 2015, 457, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Khan, M.; Park, S. Bienzyme liquid-crystal-based cholesterol biosensor. Sens. Actuat. B-Chem. 2015, 220, 508–515. [Google Scholar] [CrossRef]
- Munir, S.; Park, S. Label- and enzyme-free detection of glucose by boronic acid-coupled poly(styrene-b-acrylic acid) at liquid crystal/aqueous interfaces. Anal. Chim. Acta 2018, 1032, 122–129. [Google Scholar] [CrossRef]
- Munir, S.; Park, S. Liquid-crystal droplets functionalized with a non-enzymatic moiety for glucose sensing. Sens. Actuat. B-Chem. 2018, 257, 579–585. [Google Scholar] [CrossRef]
- Bunnett, N.W. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J. Physiol. 2014, 592, 2943–2950. [Google Scholar] [CrossRef]
- Niu, X.; Luo, D.; Chen, R.; Wang, F.; Sun, X.; Dai, H. Optical biosensor based on liquid crystal droplets for detection of cholic acid. Opt. Commun. 2016, 381, 286–291. [Google Scholar] [CrossRef]
- Kim, H.; Jang, C. Liquid crystal-based capillary sensory platform for the detection of bile acids. Chem. Phys. Lipids 2017, 204, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Han, D.; Zeng, J.; Deng, J.; Hu, N.; Yang, J. Fabrication and performance of monodisperse liquid crystal droplet-based microchips for the on-chip detection of bile acids. Microchem. J. 2020, 157, 105067. [Google Scholar] [CrossRef]
- Das, D.; Sidiq, S.; Pal, S.K. Design of bio-molecular interfaces using liquid crystals demonstrating endotoxin interactions with bacterial cell wall components. RSC Adv. 2015, 5, 66476–66486. [Google Scholar] [CrossRef]
- An, Z.; Jang, C. Simple and Label-Free Liquid Crystal-based Optical Sensor for Highly Sensitive and Selective Endotoxin Detection by Aptamer Binding and Separation. Chem. Sel. 2019, 4, 1416–1422. [Google Scholar] [CrossRef]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. 2017, 18, 485–497. [Google Scholar] [CrossRef]
- Hu, L.; Zou, L.; Qin, Z.; Fang, J.; Huang, L.; Wang, P. A novel label-free bioengineered cell-based biosensor for salicin detection. Sens. Actuat. B-Chem. 2017, 238, 1151–1158. [Google Scholar] [CrossRef]
- Chen, P.; Wang, B.; Cheng, G.; Wang, P. Taste receptor cell-based biosensor for taste specific recognition based on temporal firing. Biosens. Bioelectron. 2009, 25, 228–233. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, F.; Zhang, D.; Hu, N.; Hsia, K.; Wang, P. Extracellular potentials recording in intact taste epithelium by microelectrode array for a taste sensor. Biosens. Bioelectron. 2013, 43, 186–192. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, H.; Abbott, N.L.; Zavala, V.M. Machine learning algorithms for liquid crystal-based sensors. ACS Sens. 2018, 3, 2237–2245. [Google Scholar] [CrossRef]
- Smith, A.D.; Abbott, N.; Zavala, V.M. Convolutional network analysis of optical micrographs for liquid crystal sensors. J. Phys. Chem. C 2020, 124, 15152–15161. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, R.; Li, G. Overview of Liquid Crystal Biosensors: From Basic Theory to Advanced Applications. Biosensors 2022, 12, 205. https://doi.org/10.3390/bios12040205
Qu R, Li G. Overview of Liquid Crystal Biosensors: From Basic Theory to Advanced Applications. Biosensors. 2022; 12(4):205. https://doi.org/10.3390/bios12040205
Chicago/Turabian StyleQu, Ruixiang, and Guoqiang Li. 2022. "Overview of Liquid Crystal Biosensors: From Basic Theory to Advanced Applications" Biosensors 12, no. 4: 205. https://doi.org/10.3390/bios12040205
APA StyleQu, R., & Li, G. (2022). Overview of Liquid Crystal Biosensors: From Basic Theory to Advanced Applications. Biosensors, 12(4), 205. https://doi.org/10.3390/bios12040205






