Detection Efficiency of Ag Nanoparticle Labels for a Heart Failure Marker Using Linear and Square-Wave Anodic Stripping Voltammetry
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Instrumentation
2.3. Electrochemistry
2.4. Preparation of the AgNP-Ab Conjugates
2.5. Preparation of the MµB-Ab Conjugates
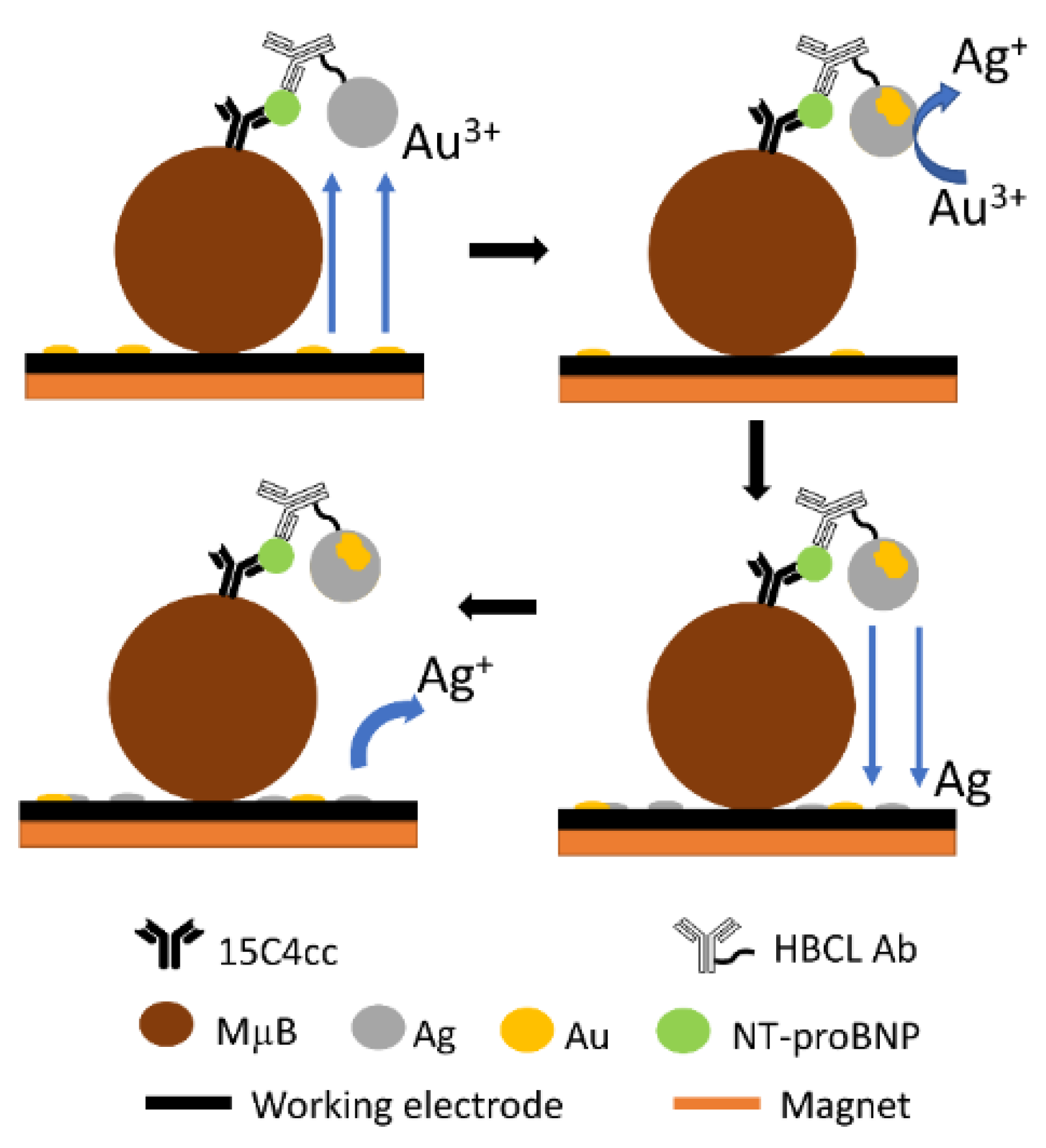
2.6. Formation of Metalloimmunoassays
3. Results and Discussion
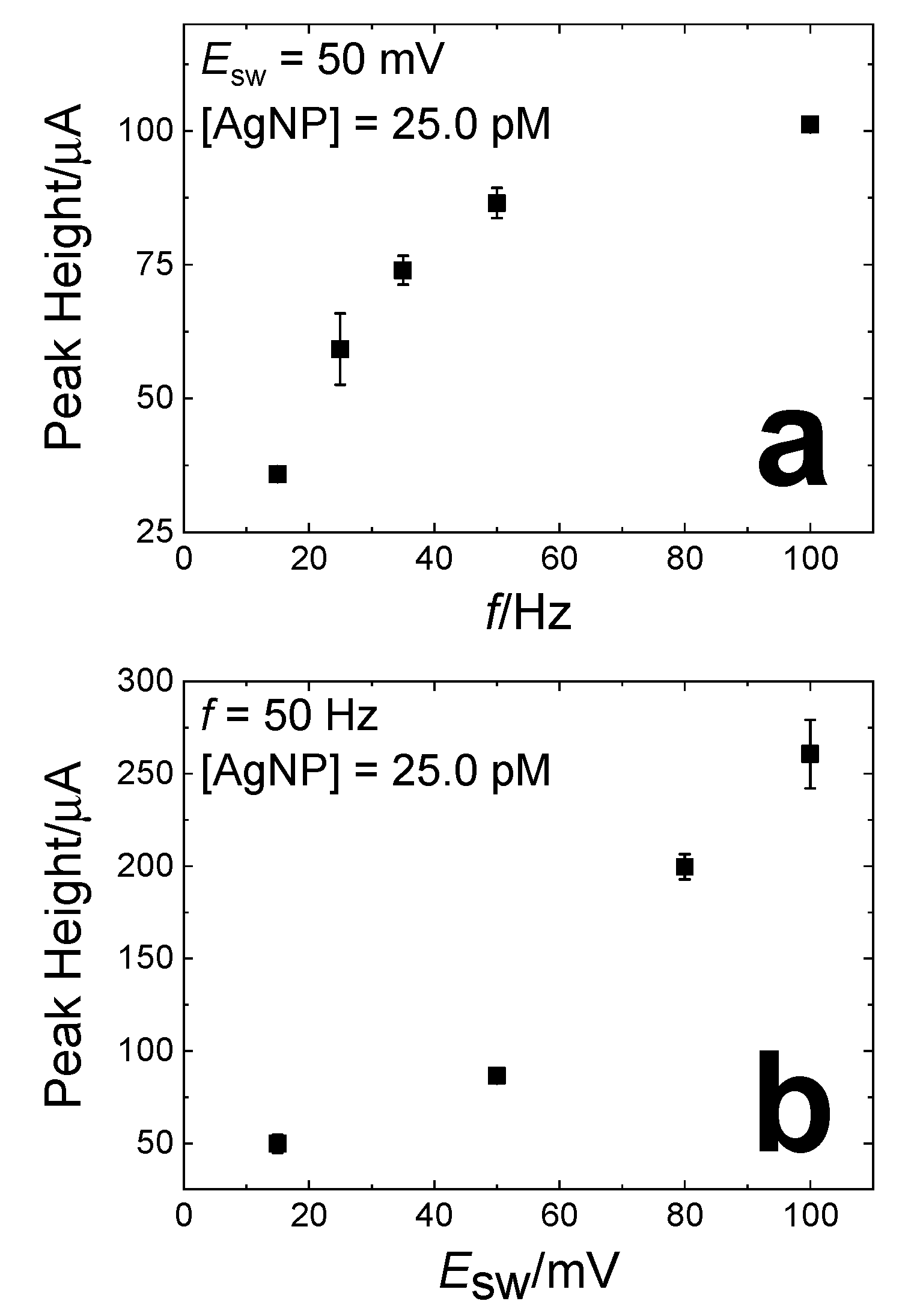
3.1. Electrochemical Analysis of the Current Signal as a Function of SWASV Parameters
3.2. Comparing Calibration Curves Obtained Using SWASV and LASV for the MC Assay
3.3. Using SWASV for the NT-proBNP Assay
3.4. Improving the Limit of Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef]
- Kim, H.N.; Januzzi, J.L. Natriuretic peptide testing in heart failure. Circulation 2011, 123, 2015–2019. [Google Scholar] [CrossRef] [Green Version]
- Januzzi, J.L.; Troughton, R. Are serial BNP measurements useful in heart failure management? Serial natriuretic peptide measurements are useful in heart failure management. Circulation 2013, 127, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [Green Version]
- Palazzuoli, A.; Gallotta, M.; Quatrini, I.; Nuti, R. Natriuretic peptides (BNP and NT-proBNP): Measurement and relevance in heart failure. Vasc. Health Risk Manag. 2010, 6, 411. [Google Scholar] [CrossRef] [Green Version]
- Richards, M.; Troughton, R.W. NT-proBNP in heart failure: Therapy decisions and monitoring. Eur. J. Heart Fail. 2004, 6, 351–354. [Google Scholar] [CrossRef] [Green Version]
- McCullough, P.A.; Kluger, A.Y. Interpreting the wide range of NT-proBNP concentrations in clinical decision making. J. Am. Coll. Cardiol. 2018, 71, 1201–1203. [Google Scholar] [CrossRef]
- Don-Wauchope, A.C.; McKelvie, R.S. Evidence based application of BNP/NT-proBNP testing in heart failure. Clin. Biochem. 2015, 48, 236–246. [Google Scholar] [CrossRef]
- Dequaire, M.; Degrand, C.; Limoges, B. An electrochemical metalloimmunoassay based on a colloidal gold label. Anal. Chem. 2000, 72, 5521–5528. [Google Scholar] [CrossRef]
- Kogan, M.R.; Pollok, N.E.; Crooks, R.M. Detection of silver nanoparticles by electrochemically activated galvanic exchange. Langmuir 2018, 34, 15719–15726. [Google Scholar] [CrossRef]
- Cunningham, J.C.; Kogan, M.R.; Tsai, Y.-J.; Luo, L.; Richards, I.; Crooks, R.M. Paper-based sensor for electrochemical detection of silver nanoparticle labels by galvanic exchange. ACS Sens. 2016, 1, 40–47. [Google Scholar] [CrossRef]
- Almeida, A.M.; Castel-Branco, M.M.; Falcao, A. Linear regression for calibration lines revisited: Weighting schemes for bioanalytical methods. J. Chromatogr. B 2002, 774, 215–222. [Google Scholar] [CrossRef]
- Odeh, A.A.; Al-Douri, Y.; Voon, C.; Ayub, R.M.; Gopinath, S.C.; Odeh, R.A.; Ameri, M.; Bouhemadou, A. A needle-like Cu2CdSnS4 alloy nanostructure-based integrated electrochemical biosensor for detecting the DNA of Dengue serotype 2. Microchim. Acta 2017, 184, 2211–2218. [Google Scholar] [CrossRef]
- Westgard, J.O.; Carey, R.N.; Wold, S. Criteria for judging precision and accuracy in method development and evaluation. Clin. Chem. 1974, 20, 825–833. [Google Scholar] [CrossRef]
- Mirceski, V.; Gulaboski, R.; Lovric, M.; Bogeski, I.; Kappl, R.; Hoth, M. Square-wave voltammetry: A review on the recent progress. Electroanalysis 2013, 25, 2411–2422. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Shah, B. Electrochemical sensing and biosensing based on square wave voltammetry. Anal. Methods 2013, 5, 2158–2173. [Google Scholar] [CrossRef]
- Mirčeski, V.; Stojanov, L.; Skrzypek, S. Recent advances and prospects of square-wave voltammetry. Sec. Nat. Math. Biotech. Sci. 2018, 39, 103–121. [Google Scholar] [CrossRef]
- Dou, J.; Wang, Y.; Ding, A.; Xie, E.; Fan, F. The Optimization of Square Wave Voltammetry’s Parameters for the Determination of Copper Using Screen-Printed Eletrochemical Sensor. Sens. Lett. 2013, 11, 2114–2116. [Google Scholar] [CrossRef]
- Gulaboski, R.; Mirčeski, V.; Bogeski, I.; Hoth, M. Protein film voltammetry: Electrochemical enzymatic spectroscopy. A review on recent progress. J. Solid State Electrochem. 2012, 16, 2315–2328. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Vicente-Beckett, V. Glassy carbon electrodes modified with multiwalled carbon nanotubes for the determination of ascorbic acid by square-wave voltammetry. Beilstein. J. Nanotechnol. 2012, 3, 388–396. [Google Scholar] [CrossRef]
- Nsabimana, J.; Wang, Y.; Ruan, Q.; Li, T.; Shen, H.; Yang, C.J.; Zhu, Z. An electrochemical method for rapid and sensitive immunoassay on digital microfluidics with integrated indium tin oxide electrodes coated on PET film. Analyst 2021, 146, 4473–4479. [Google Scholar] [CrossRef] [PubMed]
- Ardila, J.A.; Oliveira, G.G.; Medeiros, R.A.; Fatibello-Filho, O. Determination of gemfibrozil in pharmaceutical and urine samples by square-wave adsorptive stripping voltammetry using a glassy carbon electrode modified with multi-walled carbon nanotubes within a dihexadecyl hydrogen phosphate film. J. Electroanal. Chem. 2013, 690, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Naggar, A.H.; Saleh, G.A.; Omar, M.A.; Haredy, A.M.; Derayea, S.M. Square wave adsorptive anodic stripping voltammetric determination of antidiabetic drug linagliptin in pharmaceutical formulations and biological fluids using pencil graphite electrode. Anal. Sci. 2020, 36, 1031–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beardsley, C.A.; Fuller, K.Z.; Reilly, T.H.; Henry, C.S. Method for analysis of environmental lead contamination in soils. Analyst 2021, 146, 7520–7527. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Maier, C.S.; Koley, D. Anodic stripping voltammetry on a carbon-based ion-selective electrode. Electrochim. Acta 2021, 390, 138855. [Google Scholar] [CrossRef]
- de Souza, A.P.R.; Foster, C.W.; Kolliopoulos, A.V.; Bertotti, M.; Banks, C.E. Screen-printed back-to-back electroanalytical sensors: Heavy metal ion sensing. Analyst 2015, 140, 4130–4136. [Google Scholar] [CrossRef] [Green Version]
- Pollok, N.E.; Rabin, C.; Walgama, C.T.; Smith, L.; Richards, I.; Crooks, R.M. Electrochemical detection of NT-proBNP using a metalloimmunoassay on a paper electrode platform. ACS Sens. 2020, 5, 853–860. [Google Scholar] [CrossRef]
- Peng, Y.; Rabin, C.; Walgama, C.T.; Pollok, N.E.; Smith, L.; Richards, I.; Crooks, R.M. Silver Nanocubes as Electrochemical Labels for Bioassays. ACS Sens. 2021, 6, 1111–1119. [Google Scholar] [CrossRef]
- Degregory, P.R.; Tapia, J.; Wong, T.; Villa, J.; Richards, I.; Crooks, R.M. Managing heart failure at home with point-of-care diagnostics. IEEE J. Transl. Eng. Health Med. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Pollok, N.E.; Rabin, C.; Smith, L.; Crooks, R.M. Orientation-Controlled Bioconjugation of Antibodies to Silver Nanoparticles. Bioconjug. Chem. 2019, 30, 3078–3086. [Google Scholar] [CrossRef]
- Thermofisher. Dynabeads MyOne Streptavidin T1 Protocol. Available online: https://www.fishersci.com/shop/products/invitrogen-dynal-myone-dynabeads-streptavidin-t1-2/65601 (accessed on 1 June 2021).
- Thermofisher. Pierce™ Antibody Biotinylation Kit for IP. Available online: https://www.thermofisher.cn/order/catalog/product/cn/en/90407 (accessed on 1 June 2021).
- Jelen, F.; Tomschik, M.; Paleček, E. Adsorptive stripping square-wave voltammetry of DNA. J. Electroanal. Chem. 1997, 423, 141–148. [Google Scholar] [CrossRef]
- Westgard, Q.C. Desirable Biological Variation Database Specifications. Available online: https://www.westgard.com/biodatabase1.htm (accessed on 1 January 2022).
- Pollok, N.E.; Peng, Y.; Raj, N.; Walgama, C.; Crooks, R.M. Dual-shaped silver nanoparticle labels for electrochemical detection of bioassays. ACS Appl. Nano Mater. 2021, 4, 10764–10770. [Google Scholar] [CrossRef]
- Walgama, C.; Nguyen, M.P.; Boatner, L.M.; Richards, I.; Crooks, R.M. Hybrid paper and 3D-printed microfluidic device for electrochemical detection of Ag nanoparticle labels. Lab Chip 2020, 20, 1648–1657. [Google Scholar] [CrossRef] [PubMed]




| Technique | Calibration Sensitivity | COV (%) |
|---|---|---|
| LASV | 0.13 µC/pM | 9.5 |
| SWASV50 | 0.83–4.91 µA/pM | 3.6 |
| SWASV100 | 0.75–11.9 µA/pM | 6.2 |
| Technique | Calibration Sensitivity | COV (%) |
|---|---|---|
| LASV | 0.0022 µC/pM | 15.7 |
| SWASV50 | 0.041 µA/pM | 9.7 |
| SWASV100 | 0.083 µA/pM | 12.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, N.; Crooks, R.M. Detection Efficiency of Ag Nanoparticle Labels for a Heart Failure Marker Using Linear and Square-Wave Anodic Stripping Voltammetry. Biosensors 2022, 12, 203. https://doi.org/10.3390/bios12040203
Raj N, Crooks RM. Detection Efficiency of Ag Nanoparticle Labels for a Heart Failure Marker Using Linear and Square-Wave Anodic Stripping Voltammetry. Biosensors. 2022; 12(4):203. https://doi.org/10.3390/bios12040203
Chicago/Turabian StyleRaj, Nikhil, and Richard M. Crooks. 2022. "Detection Efficiency of Ag Nanoparticle Labels for a Heart Failure Marker Using Linear and Square-Wave Anodic Stripping Voltammetry" Biosensors 12, no. 4: 203. https://doi.org/10.3390/bios12040203
APA StyleRaj, N., & Crooks, R. M. (2022). Detection Efficiency of Ag Nanoparticle Labels for a Heart Failure Marker Using Linear and Square-Wave Anodic Stripping Voltammetry. Biosensors, 12(4), 203. https://doi.org/10.3390/bios12040203





