Ion-Imprinted Polymer-on-a-Sensor for Copper Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification and Preparation of Plasmonic Sensors
2.3. Characterization of Plasmonic Sensors
2.4. Kinetic Analysis of Plasmonic Sensors
3. Result and Discussions
3.1. Characterization of Plasmonic Sensors
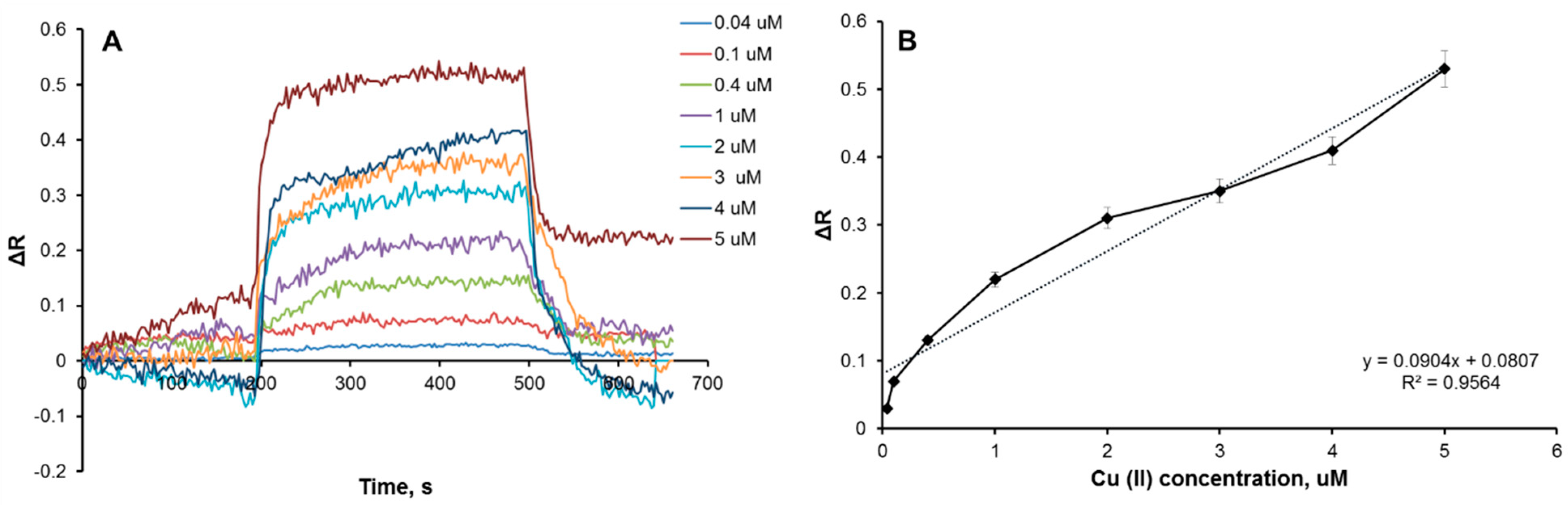
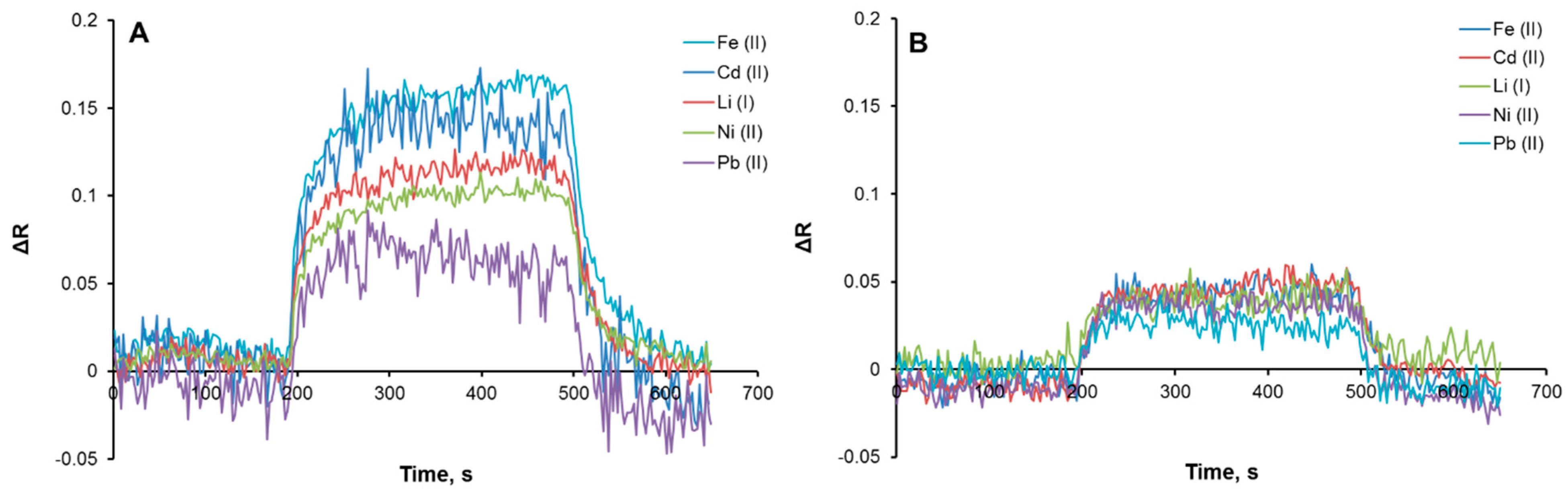
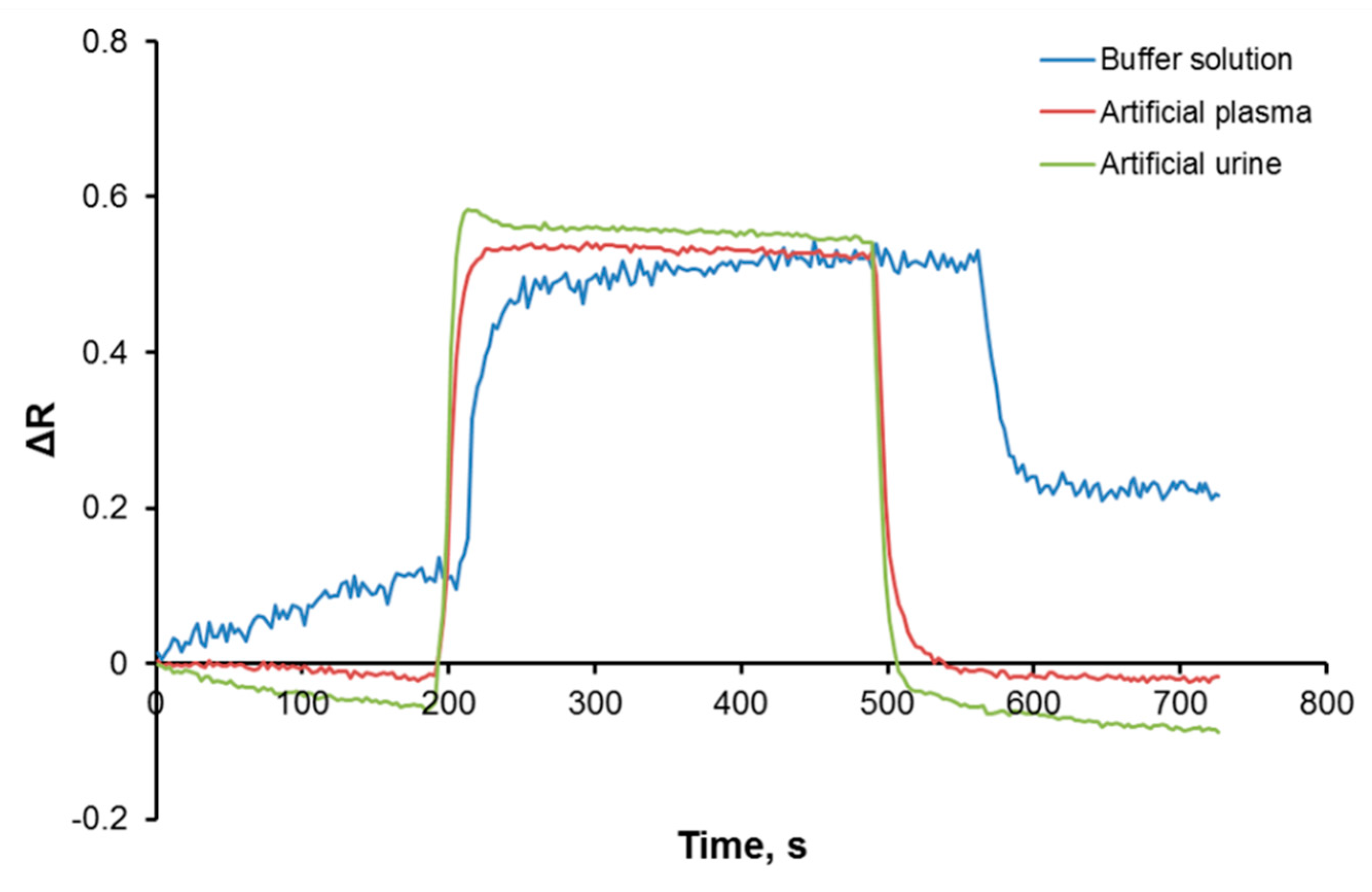
3.2. Kinetic Analysis of Plasmonic Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, H.P.; Lin, Z.; Tan, J.F.; Guo, L. Contents of fluoride, lead, copper, chromium, arsenic and cadmium in Chinese Pu-erh tea. Food Res. Int. 2013, 53, 938–944. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Lin, M.; Li, R.; Zhang, Q.; Liu, H.; Wang, J.; Huang, C. Aggregation-induced emission enhancement of yellow photoluminescent carbon dots for highly selective detection of environmental and intracellular copper(II) ions. Chin. Chem. Lett. 2019, 30, 1410–1414. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Yang, W.; Wang, F.; Liu, M.; Zhu, X.; Zhang, Y.; Yao, S. Exploitation of a turn-on photoelectrochemical sensing platform based on Au/BiOI for determination of copper (II) ions in food samples. J. Electroanal. Chem. 2021, 895, 115536. [Google Scholar] [CrossRef]
- Lou, T.; Chen, L.; Chen, Z.; Wang, Y.; Chen, L.; Li, J. Colorimetric detection of trace copper ions based on catalytic leaching of silver-coated gold nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 4215–4220. [Google Scholar] [CrossRef] [PubMed]
- Mohr, I.; Weiss, K.H. Biochemical markers for the diagnosis and monitoring of Wilson disease. Clin. Biochem. Rev. 2019, 40, 59–77. [Google Scholar]
- Zhao, Z.; Chen, H.; Zhang, H.; Ma, L.; Wang, Z. Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for rapid detection and removal of copper (II) ions. Biosens. Bioelectron. 2017, 91, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Mezzaroba, L.; Alfieri, D.F.; Simão, A.N.C.; Reiche, E.M.V. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Kong, T.; Liu, G.W.; Li, X.B.; Wang, Z.; Zhang, Z.G.; Xie, G.H.; Zhang, Y.; Sun, J.; Xu, C. Synthesis and identification of artificial antigens for cadmium and copper. Food Chem. 2010, 123, 1204–1209. [Google Scholar] [CrossRef]
- Safran, V.; Göktürk, I.; Derazshamshir, A.; Yılmaz, F.; Sağlam, N.; Denizli, A. Rapid sensing of Cu+2 in water and biological samples by sensitive molecularly imprinted based plasmonic biosensor. Microchem. J. 2019, 148, 141–150. [Google Scholar] [CrossRef]
- Normaya, E.; Baharu, N.A.; Ahmad, M.N. Synthesis of thiosemicarbazone-based colorimetric chemosensor for Cu2+ ions’ recognition in aqueous medium: Experimental and theoretical studies. J. Mol. Struct. 2020, 1212, 128094. [Google Scholar] [CrossRef]
- Inci, F.; Saylan, Y.; Kojouri, A.M.; Öğüt, M.G.; Denizli, A.; Demirci, U. A disposable microfluidic-integrated hand-held plasmonic platform for protein detection. Appl. Mater. Today 2020, 18, 100478. [Google Scholar] [CrossRef]
- Erdem, Ö.; Cihangir, N.; Saylan, Y.; Denizli, A. Comparison of molecularly imprinted plasmonic nanosensor performances for bacteriophage detection. New J. Chem. 2020, 41, 17654–17663. [Google Scholar] [CrossRef]
- Akceoğlu, G.A.; Saylan, Y.; Inci, F. A snapshot of microfluidics in point-of-care diagnostics: Multifaceted integrity with materials and sensors. Adv. Mater. Technol. 2021, 6, 2100049. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar] [CrossRef] [PubMed]
- Bakhshpour, M.; Denizli, A. Highly sensitive detection of Cd(II) ions using ion-imprinted surface plasmon resonance sensors. Microchem. J. 2020, 159, 105572. [Google Scholar] [CrossRef]
- Li, P.; Lee, G.H.; Kim, S.Y.; Kwan, S.Y.; Kim, H.R.; Park, S. From diagnosis to treatment: Recent advances in patient-friendly biosensors and implantable devices. ACS Nano 2021, 15, 1960–2004. [Google Scholar] [CrossRef]
- Saylan, Y.; Denizli, A. Molecular fingerprints of hemoglobin on a nanofilm chip. Sensors 2018, 18, 3016. [Google Scholar] [CrossRef] [Green Version]
- Saylan, Y.; Erdem, Ö.; Inci, F.; Denizli, A. Advances in biomimetic systems for molecular recognition and biosensing. Biomimetics 2020, 5, 20. [Google Scholar] [CrossRef]
- Parlapiano, M.; Akyol, Ç.; Foglia, A.; Pisani, M.; Astolfi, P.; Eusebi, A.L.; Fatone, F. Selective removal of contaminants of emerging concern (CECs) from urban water cycle via molecularly imprinted polymers (MIPs): Potential of upscaling and enabling reclaimed water reuse. J. Environ. Chem. Eng. 2021, 9, 105051. [Google Scholar] [CrossRef]
- Paruli, E.; Soppera, O.; Haupt, K.; Gonzato, C. Photopolymerization and photostructuring of molecularly imprinted polymers. ACS Appl. Polym. Mater. 2021, 3, 4769–4790. [Google Scholar] [CrossRef]
- Yarman, A.; Kurbanoğlu, S.; Zebger, I.; Scheller, F.W. Simple and robust: The claims of protein sensing by molecularly imprinted polymers. Sens. Actuators B Chem. 2021, 330, 129369. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Li, J.; Wang, X.; Zhang, Z.; Choo, J.; Chen, L. Molecular imprinting: Green perspectives and strategies. Adv. Mater. 2021, 33, 2100543. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Men, C.; Tian, L.; Liu, Y.; Zhan, L.; Li, Y.F.; Huang, C.Z.; Zhen, S.J. Preparation of a molecularly imprinted test strip for point-of-care detection of thiodiglycol, a sulfur mustard poisoning metabolic marker. Talanta 2021, 234, 122701. [Google Scholar] [CrossRef]
- Soufi, G.J.; Iravani, S.; Varma, R.S. Molecularly imprinted polymers for the detection of viruses: Challenges and opportunities. Analyst 2021, 146, 3087–3100. [Google Scholar] [CrossRef]
- Bai, R.; Sun, Y.; Zhao, M.; Han, Z.; Zhang, J.; Sun, Y.; Dong, W.; Li, S. Preparation of IgG imprinted polymers by metal-free visible-light-induced ATRP and its application in biosensor. Talanta 2021, 226, 122160. [Google Scholar] [CrossRef]
- Tlili, A.; Attia, G.; Khaoulani, S.; Mazouz, Z.; Zerrouki, C.; Yaakoubi, N.; Othmane, A.; Fourati, N. Contribution to the understanding of the interaction between a polydopamine molecular imprint and a protein model: Ionic strength and pH effect investigation. Sensors 2021, 21, 619. [Google Scholar] [CrossRef]
- Pan, M.; Hong, L.; Xie, X.; Liu, K.; Yang, J.; Wang, S. Nanomaterials-based surface protein imprinted polymers: Synthesis and medical applications. Macromol. Chem. Phys. 2021, 222, 2000222. [Google Scholar] [CrossRef]
- Bai, J.; Beyer, S.; Trau, D. Conjugated polymers for biosensor devices. Compr. Biomater. 2011, 3, 529–556. [Google Scholar]
- Qiu, Z.; Hammer, B.A.G.; Müllen, K. Conjugated polymers–Problems and promises. Prog. Polym. Sci. 2020, 100, 101179. [Google Scholar] [CrossRef]
- Saylan, Y.; Göktürk, I.; Pospiskova, K.; Safarik, I.; Denizli, A. Magnetic bacterial cellulose nanofibers for nucleoside recognition. Cellulose 2020, 27, 9479–9492. [Google Scholar] [CrossRef]
- Uzun, L.; Türkmen, D.; Yılmaz, E.; Bektaş, S.; Denizli, A. Cysteine functionalized poly(hydroxyethyl methacrylate) monolith for heavy metal removal. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 161–167. [Google Scholar] [CrossRef]
- Langel, L.; Menken, L. Simulation of the interface between titanium oxide and amino acids in solution by first principles MD. Surf. Sci. 2003, 538, 1–9. [Google Scholar] [CrossRef]
- Ding, N.; Zhao, H.; Peng, W.; He, Y.; Zhou, Y.; Yuan, L.; Zhan, Y. A simple colorimetric sensor based on anti-aggregation of gold nanoparticles for Hg2+ detection. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 161–167. [Google Scholar] [CrossRef]
- Özgür, E.; Saylan, Y.; Bereli, N.; Türkmen, D.; Denizli, A. Molecularly imprinted polymer integrated plasmonic nanosensor for cocaine detection. J. Biomater. Sci. Polym. Ed. 2020, 31, 1211–1222. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Carlen, E.T.; Van Der Berg, A.; Scasfoort, R.B.M. Surface plasmon resonance imaging based multiplex biosensor: Integration of biomolecular screening detection and kinetics estimation. Sens. Actuators B Chem. 2010, 148, 511–521. [Google Scholar] [CrossRef]
- Saylan, Y.; Erdem, Ö.; Cihangir, N.; Denizli, A. Detecting fingerprints of waterborne bacteria on a sensor. Chemosensors 2019, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Bakhshpour, M.; Chiodi, E.; Celebi, I.; Saylan, Y.; Ünlü, N.L.; Ünlü, M.S.; Denizli, A. Sensitive and real-time detection of IgG using novel interferometric reflecting imaging sensor system. Biosens. Bioelectron. 2022, 201, 113961. [Google Scholar] [CrossRef]
- Öztürk, G.; Saylan, Y.; Denizli, A. Designing composite cryogel carriers for tyrosine adsorption. Sep. Purif. Technol. 2021, 254, 117622. [Google Scholar] [CrossRef]
- Hwang, J.; Hwang, M.P.; Choi, M.; Seo, Y.; Jo, Y.; Son, J.; Hong, J.; Choi, J. Sensitive detection of copper ions via ion-responsive fluorescence quenching of engineered porous silicon nanoparticles. Sci. Rep. 2016, 6, 35565. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Gao, W.; Zhou, X.; Ma, Y. Pristine graphene quantum dots for detection of copper ions. J. Mater. Res. 2014, 29, 1401–1407. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Saratale, R.G.; Kadam, A.A.; Saratale, G.D.; Syed, A.; Ameen, F.; Kim, D.Y. Colorimetric detection of Cu2+ based on the formation of peptide–copper complexes on silver nanoparticle surfaces. Beilstein J. Nanotechnol. 2018, 9, 1414–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Yu, J.; Zhao, P.; Li, J.; Han, S. Preparation and mechanism investigation of CdS quantum dots applied for copper ion rapid detection. J. Alloys Compd. 2021, 854, 157195. [Google Scholar] [CrossRef]
- Awad, F.S.; AbouZied, K.M.; Bakry, A.M.; Abou El-Maaty, W.M.; El-Wakil, A.M.; El-Shal, M.S. Highly fluorescent hematoporphyrin modified graphene oxide for selective detection of copper ions in aqueous solutions. Anal. Chim. Acta 2020, 1140, 111–121. [Google Scholar] [CrossRef]




| Ref. | Material | Detection Range | Limit of Detection | Selectivity | Real Sample |
|---|---|---|---|---|---|
| [40] | Silicon nanoparticles | 0.1–200 µM | 0.1 μM | Fe2+, Na+, K+, Mg2+, Mn2+, Ca2+ | Tap water |
| [41] | Graphene quantum dot | 0–0.20 mM | 0.33 μM | Cr+3, Ba+2, Ca+2, Cd+2, Co+2, K+, Mn+2,Ni+2, Pb+2, Zn+2, Fe+3, Ag+, Hg+2 | River water |
| [42] | Silver nanoparticles | 0.08–1.44 μM | 0.16 μM | Mn+2, Mo+3, Na+, Cr+3, Hg+2, Ni+2, Ca+2, K+, Cs+, Li+, As+, PO4−3, NH4, NO3- | Tap and pond water |
| [43] | CdS quantum dot | 1–100 mg/L | - | Zn+2, Mn+2, Ni+2, Fe+2, Fe+3, I, Pb+2, Al+3, Mg+2,Ca+2, K+, Na+ | Potatoes |
| [44] | Graphene oxide | 0–1.18 μM | 54 nM | Na+, K+, Ca+2, Mn+2,Co+2,Fe+2, Fe+3, Zn+2, Al+3, Cr+6,As+5, Cd+2, Zn+2, Pb+2, Hg+2 | Water |
| This study | Imprinted polymer | 0.04-5 μM | 0.027 μM | Fe+2,Cd+2, Li+1, Ni+2, Pb+2 | Artificial plasma and urine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerdan, Z.; Saylan, Y.; Uğur, M.; Denizli, A. Ion-Imprinted Polymer-on-a-Sensor for Copper Detection. Biosensors 2022, 12, 91. https://doi.org/10.3390/bios12020091
Gerdan Z, Saylan Y, Uğur M, Denizli A. Ion-Imprinted Polymer-on-a-Sensor for Copper Detection. Biosensors. 2022; 12(2):91. https://doi.org/10.3390/bios12020091
Chicago/Turabian StyleGerdan, Zeynep, Yeşeren Saylan, Mukden Uğur, and Adil Denizli. 2022. "Ion-Imprinted Polymer-on-a-Sensor for Copper Detection" Biosensors 12, no. 2: 91. https://doi.org/10.3390/bios12020091
APA StyleGerdan, Z., Saylan, Y., Uğur, M., & Denizli, A. (2022). Ion-Imprinted Polymer-on-a-Sensor for Copper Detection. Biosensors, 12(2), 91. https://doi.org/10.3390/bios12020091







